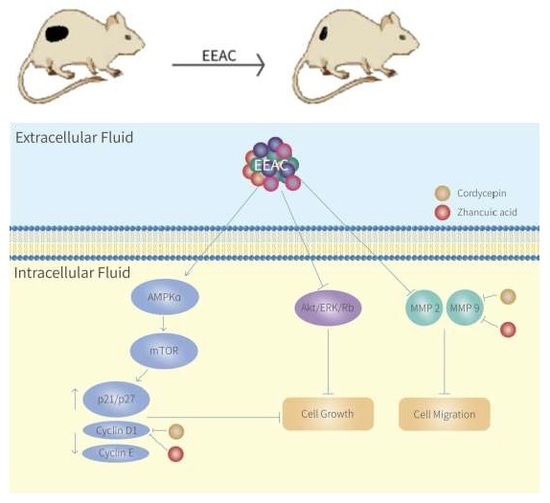
Suppression of Cell Growth, Migration and Drug Resistance by Ethanolic Extract of Antrodia cinnamomea in Human Lung Cancer A549 Cells and C57BL/6J Allograft Tumor Model
Abstract
:1. Introduction
2. Results
2.1. EEAC Induced Cell-Cycle Arrest and Reduced Cell Viability of A549 Cells
2.2. Regulation of EEAC on Cell Growth-Associated Proteins in A549 Cells
2.3. EEAC Suppressed Cell Migration of A549 Cells and Gelatinase Expression
2.4. Zhankuic Acid A and Cordycepin Decreased Cyclin D1 and MMP-9 Expressions in A549 Cells
2.5. Regulation of EEAC on Chemosensitivity of A549 Cells to Paclitaxel
2.6. Effect of EEAC on the Expressions of Cyclin D1 and MMP-9 in LLC Cells
2.7. EEAC Reduced Tumor Growth in the Lewis Lung Carcinoma Allograft Mode
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Ethanolic Extract of Antrodia cinnamomea (EEAC)
4.3. Cell Culture
4.4. Cell Viability Assay (MTT Assay)
4.5. Cell Cycle Analysis
4.6. Wound-Healing Analysis
4.7. Western Blot Analysis
4.8. Lewis Lung Cancer Allograft Model in C57BL/6J Mice
4.9. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ho, M.M.; Ng, A.V.; Lam, S.; Hung, J.Y. Side population in human lung cancer cell lines and tumors is enriched with stem-like cancer cells. Cancer Res. 2007, 67, 4827–4833. [Google Scholar] [CrossRef] [PubMed]
- Chellappan, S.P.; Giordano, A.; Fisher, P.B. Role of cyclin-dependent kinases and their inhibitors in cellular differentiation and development. Curr. Top. Microbiol. Immunol. 1998, 227, 57–103. [Google Scholar] [PubMed]
- Sherr, C.J. G1 phase progression: Cycling on cue. Cell 1994, 79, 551–555. [Google Scholar] [CrossRef]
- Motoshima, H.; Goldstein, B.J.; Igata, M.; Araki, E. AMPK and cell proliferation—AMPK as a therapeutic target for atherosclerosis and cancer. J. Physiol. 2006, 574, 63–71. [Google Scholar] [CrossRef] [PubMed]
- LoPiccolo, J.; Granville, C.A.; Gills, J.J.; Dennis, P.A. Targeting Akt in cancer therapy. Anticancer Drugs 2007, 18, 861–874. [Google Scholar] [PubMed]
- Yoon, S.O.; Park, S.J.; Yun, C.H.; Chung, A.S. Roles of matrix metalloproteinases in tumor metastasis and angiogenesis. J. Biochem. Mol. Biol. 2003, 36, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Roomi, M.W.; Monterrey, J.C.; Kalinovsky, T.; Rath, M.; Niedzwiecki, A. Patterns of MMP-2 and MMP-9 expression in human cancer cell lines. Oncol. Rep. 2009, 21, 1323–1333. [Google Scholar] [PubMed]
- Ho, C.C.; Kuo, S.H.; Huang, P.H.; Huang, H.Y.; Yang, C.H.; Yang, P.C. Caveolin-1 expression is significantly associated with drug resistance and poor prognosis in advanced non-small cell lung cancer patients treated with gemcitabine-based chemotherapy. Lung Cancer 2008, 59, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Bae, E.; Lee, C.; Yoon, S.S.; Chae, Y.S.; Ahn, K.S.; Won, N.H. RNA interference-directed caveolin-1 knockdown sensitizes SN12CPM6 cells to doxorubicin-induced apoptosis and reduces lung metastasis. Tumour Biol. 2010, 31, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.C.; Yang, R.M.; Chang, T.T.; Chou, J.C. Fructification of Antrodia cinnamomea was strain dependent in malt extract media and involved specific gene expression. J. Agric. Food Chem. 2010, 58, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Shang, K.M.; Su, T.H.; Lee, W.L.; Hsiao, W.W.; Chiou, C.Y.; Ho, B.Y.; Wang, S.Y.; Shyur, L.F. Novel effect and the mechanistic insights of fruiting body extract of medicinal fungus Antrodia cinnamomea against T47D breast cancer. Phytomedicine 2017, 24, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.Y.; Hung, Y.C.; Chen, Y.H.; Chen, Y.H.; Huang, Y.C.; Kao, C.W.; Su, Y.L.; Chiu, H.H.; Rau, K.M. A preliminary randomised controlled study of short-term Antrodia cinnamomea treatment combined with chemotherapy for patients with advanced cancer. BMC Complement. Altern. Med. 2016, 16. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Chou, P.Y.; Chien, Y.C.; Wu, C.H.; Wu, T.S.; Sheu, M.J. Ethanol extracts of fruiting bodies of Antrodia cinnamomea exhibit anti-migration action in human adenocarcinoma CL1–0 cells through the MAPK and PI3K/AKT signaling pathways. Phytomedicine 2012, 19, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.T.; Rao, Y.K.; Yao, C.J.; Yeh, C.F.; Li, C.H.; Chuang, S.E.; Luong, J.H.; Lai, G.M.; Tzeng, Y.M. Cytotoxic triterpenes from Antrodia camphorata and their mode of action in HT-29 human colon cancer cells. Cancer Lett. 2009, 285, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Chiang, P.C.; Lin, S.C.; Pan, S.L.; Kuo, C.H.; Tsai, I.L.; Kuo, M.T.; Wen, W.C.; Chen, P.; Guh, J.H. Antroquinonol displays anticancer potential against human hepatocellular carcinoma cells: A crucial role of AMPK and mTOR pathways. Biochem. Pharmacol. 2010, 79, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.W.; Pan, J.H.; Liu, R.H.; Kuo, Y.H.; Sheen, L.Y.; Chiang, B.H. The 4-acetylantroquinonol B isolated from mycelium of Antrodia cinnamomea inhibits proliferation of hepatoma cells. J. Sci. Food Agric. 2010, 90, 1739–1744. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, N.; Yamada, S.; Takeuchi, C.; Kagota, S.; Shinozuka, K.; Kunitomo, M.; Nakamura, K. Cordycepin (3′-deoxyadenosine) inhibits the growth of B16-BL6 mouse melanoma cells through the stimulation of adenosine A3 receptor followed by glycogen synthase kinase-3β activation and cyclin D1 suppression. Naunyn Schmiedebergs Arch. Pharmacol. 2008, 377, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Ernens, I.; Rouy, D.; Velot, E.; Devaux, Y.; Wagner, D.R. Adenosine inhibits matrix metalloproteinase-9 secretion by neutrophils: Implication of A2a receptor and cAMP/PKA/Ca2+ pathway. Circ. Res. 2006, 99, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.C.; Hsiao, J.R.; Lian, Y.Y.; Lin, C.Y.; Huang, B.M. The apoptotic effect of cordycepin on human OEC-M1 oral cancer cell line. Cancer Chemother. Pharmacol. 2007, 60, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Kim, S.K.; Choi, W.S.; Kim, W.J.; Moon, S.K. Cordycepin causes p21WAF1-mediated G2/M cell-cycle arrest by regulating c-Jun N-terminal kinase activation in human bladder cancer cells. Arch. Biochem. Biophys. 2009, 490, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Moon, G.S.; Jung, K.H.; Kim, W.J.; Moon, S.K. c-Jun N-terminal kinase 1 is required for cordycepin-mediated induction of G2/M cell-cycle arrest via p21WAF1 expression in human colon cancer cells. Food Chem. Toxicol. 2010, 48, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Yang, S.W.; Shen, Y.C. New steroid acids from Antrodia cinnamomea, a fungal parasite of Cinnamomum micranthum. J. Nat. Prod. 1995, 58, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Wang, S.; He, C.; Wang, S.; Liang, B.; Viollet, B.; Zou, M.H. AMPKα2 deletion exacerbates neointima formation by upregulating Skp2 in vascular smooth muscle cells. Circ. Res. 2011, 109, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Hseu, Y.C.; Chen, S.C.; Chen, H.C.; Liao, J.W.; Yang, H.L. Antrodia camphorata inhibits proliferation of human breast cancer cells in vitro and in vivo. Food Chem. Toxicol. 2008, 46, 2680–2688. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Hu, Y.D.; Li, N.; Zhu, Y. Inhibition of tumor growth and metastasis by non-small cell lung cancer cells transfected with cyclin D1-targeted siRNA. Oligonucleotides 2009, 19, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Wang, Y.; Dai, S.; Liu, Y.; Stoecker, M.; Wang, E.; Wang, E. P120-catenin isoforms 1 and 3 regulate proliferation and cell cycle of lung cancer cells via β-catenin and Kaiso respectively. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Lien, S.; Lowman, H. Therapeutic anti-VEGF antibodies. In Therapeutic Antibodies; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Deep, G.; Singh, R.P.; Agarwal, C.; Kroll, D.J.; Agarwal, R. Silymarin and silibinin cause G1 and G2-M cell cycle arrest via distinct circuitries in human prostate cancer PC3 cells: A comparison of flavanone silibinin with flavanolignan mixture silymarin. Oncogene 2006, 25, 1053–1069. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, A.B., 3rd; Chen, X.; Smeets, M.; Hengst, L.; Prives, C.; Reed, S.I. Effects of p21(Cip1/Waf1) at both the G1/S and the G2/M cell cycle transitions: pRb is a critical determinant in blocking DNA replication and in preventing endoreduplication. Mol. Cell. Biol. 1998, 18, 629–643. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Ren, H.; Oertel, M.; Sellers, R.S.; Zhu, L. Loss of p27Kip1 enhances tumor progression in chronic hepatocyte injury-induced liver tumorigenesis with widely ranging effects on Cdk2 or Cdc2 activation. Carcinogenesis 2007, 28, 1859–1866. [Google Scholar] [CrossRef] [PubMed]
- Carretero, J.; Medina, P.P.; Blanco, R.; Smit, L.; Tang, M.; Roncador, G.; Maestre, L.; Conde, E.; Lopez-Rios, F.; Clevers, H.C.; et al. Dysfunctional AMPK activity, signalling through mTOR and survival in response to energetic stress in LKB1-deficient lung cancer. Oncogene 2007, 26, 1616–1625. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.C.; Chiang, P.C.; Lu, P.H.; Kuo, M.T.; Wen, W.C.; Chen, P.; Guh, J.H. Antroquinonol, a natural ubiquinone derivative, induces a cross talk between apoptosis, autophagy and senescence in human pancreatic carcinoma cells. J. Nutr. Biochem. 2012, 23, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.Y.; Moon, A.; Duffin, R.; Barthet-Barateig, A.; Meijer, H.A.; Clemens, M.J.; de Moor, C.H. Cordycepin inhibits protein synthesis and cell adhesion through effects on signal transduction. J. Biol. Chem. 2010, 285, 2610–2621. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.M.; Shih, Y.T.; Chen, Y.S.; Liu, C.L.; Fang, W.K.; Tsai, C.H.; Tsai, F.J.; Kuo, W.W.; Lai, T.Y.; Huang, C.Y. Schwann cell migration induced by earthworm extract via activation of PAs and MMP2/9 mediated through ERK1/2 and p38. Evid.-Based Complement. Altern. Med. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.T.; Lin, C.C.; Wu, Y.C.; Yang, C.M. TNF-α induces matrix metalloproteinase-9 expression in A549 cells: Role of TNFR1/TRAF2/PKCα-dependent signaling pathways. J. Cell. Physiol. 2010, 224, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Vicent, S.; Lopez-Picazo, J.M.; Toledo, G.; Lozano, M.D.; Torre, W.; Garcia-Corchon, C.; Quero, C.; Soria, J.C.; Martin-Algarra, S.; Manzano, R.G.; et al. ERK1/2 is activated in non-small-cell lung cancer and associated with advanced tumours. Br. J. Cancer 2004, 90, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.C.; Shih, Y.W.; Chao, C.H.; Lee, X.Y.; Chiang, T.A. Involvement of the ERK signaling pathway in fisetin reduces invasion and migration in the human lung cancer cell line A549. J. Agric. Food Chem. 2009, 57, 8933–8941. [Google Scholar] [CrossRef] [PubMed]
- Shajahan, A.N.; Wang, A.; Decker, M.; Minshall, R.D.; Liu, M.C.; Clarke, R. Caveolin-1 tyrosine phosphorylation enhances paclitaxel-mediated cytotoxicity. J. Biol. Chem. 2007, 282, 5934–5943. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ren, C.H.; Tahir, S.A.; Ren, C.; Thompson, T.C. Caveolin-1 maintains activated Akt in prostate cancer cells through scaffolding domain binding site interactions with and inhibition of serine/threonine protein phosphatases PP1 and PP2A. Mol. Cell. Biol. 2003, 23, 9389–9404. [Google Scholar] [CrossRef] [PubMed]
- Tirado, O.M.; MacCarthy, C.M.; Fatima, N.; Villar, J.; Mateo-Lozano, S.; Notario, V. Caveolin-1 promotes resistance to chemotherapy-induced apoptosis in Ewing’s sarcoma cells by modulating PKCα phosphorylation. Int. J. Cancer 2010, 126, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.S.; Chao, C.H.; Shen, D.Y.; Chan, H.H.; Chen, C.H.; Liao, Y.R.; Wu, S.J.; Leu, Y.L.; Shen, Y.C.; Kuo, Y.H.; et al. Biologically active constituents from the fruiting body of Taiwanofungus camphoratus. Bioorg. Med. Chem. 2011, 19, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.C.; Lai, M.T.; Chen, Y.Y.; Lin, W.H.; Chang, S.J.; Sheu, M.J.; Wu, C.H. Elucidating the inhibitory mechanisms of the ethanolic extract of the fruiting body of the mushroom Antrodia cinnamomea on the proliferation and migration of murine leukemia WEHI-3 cells and their tumorigenicity in a BALB/c allograft tumor model. Phytomedicine 2013, 20, 874–882. [Google Scholar] [CrossRef] [PubMed]








| Protein Name | EEAC (μg/mL) | |||
|---|---|---|---|---|
| 0 | 25 | 50 | 100 | |
| p-mTOR | 1 | 0.78 ± 0.36 | 0.85 ± 0.37 | 0.43 ± 0.10 ** |
| p-Rb | 1 | 0.87 ± 0.17 | 0.86 ± 0.01 * | 0.39 ± 0.06 ** |
| p-AMPK | 1 | 2.42 ± 0.02 ** | 2.49 ± 0.11 ** | 2.49 ± 0.51 * |
| AMPK | 1 | 1.89 ± 0.28 | 1.57 ± 0.35 | 1.51 ± 0.46 |
| p-Akt | 1 | 1.04 ± 0.23 | 1.00 ± 0.17 | 0.74 ± 0.06 * |
| Akt | 1 | 1.59 ± 0.27 | 1.72 ± 0.40 | 1.98 ± 0.72 |
| p-ERK1/2 | 1 | 1.07 ± 0.23 | 0.80 ± 0.10 | 0.50 ± 0.11 * |
| ERK1/2 | 1 | 1.13 ± 0.02 | 0.93 ± 0.17 | 0.89 ± 0.05 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.-H.; Liu, F.-C.; Pan, C.-H.; Lai, M.-T.; Lan, S.-J.; Wu, C.-H.; Sheu, M.-J. Suppression of Cell Growth, Migration and Drug Resistance by Ethanolic Extract of Antrodia cinnamomea in Human Lung Cancer A549 Cells and C57BL/6J Allograft Tumor Model. Int. J. Mol. Sci. 2018, 19, 791. https://doi.org/10.3390/ijms19030791
Wu C-H, Liu F-C, Pan C-H, Lai M-T, Lan S-J, Wu C-H, Sheu M-J. Suppression of Cell Growth, Migration and Drug Resistance by Ethanolic Extract of Antrodia cinnamomea in Human Lung Cancer A549 Cells and C57BL/6J Allograft Tumor Model. International Journal of Molecular Sciences. 2018; 19(3):791. https://doi.org/10.3390/ijms19030791
Chicago/Turabian StyleWu, Chi-Han, Fon-Chang Liu, Chun-Hsu Pan, Ming-Tsung Lai, Shou-Jen Lan, Chieh-Hsi Wu, and Ming-Jyh Sheu. 2018. "Suppression of Cell Growth, Migration and Drug Resistance by Ethanolic Extract of Antrodia cinnamomea in Human Lung Cancer A549 Cells and C57BL/6J Allograft Tumor Model" International Journal of Molecular Sciences 19, no. 3: 791. https://doi.org/10.3390/ijms19030791
APA StyleWu, C. -H., Liu, F. -C., Pan, C. -H., Lai, M. -T., Lan, S. -J., Wu, C. -H., & Sheu, M. -J. (2018). Suppression of Cell Growth, Migration and Drug Resistance by Ethanolic Extract of Antrodia cinnamomea in Human Lung Cancer A549 Cells and C57BL/6J Allograft Tumor Model. International Journal of Molecular Sciences, 19(3), 791. https://doi.org/10.3390/ijms19030791