Localized Intrathecal Delivery of Mesenchymal Stromal Cells Conditioned Medium Improves Functional Recovery in a Rat Model of Spinal Cord Injury
Abstract
:1. Introduction
2. Results
2.1. Functionality of CM
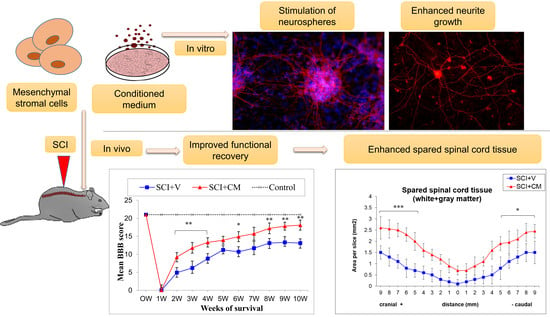
2.1.1. High-Density Plating Neurosphere-Like Structures Formation
2.1.2. Low-Density Plating
2.2. Locomotor Function Recovery
2.3. Spared Spinal Cord Tissue
2.4. Neurite Sprouting
2.5. Cytokines Profile
3. Discussion
4. Materials and Methods
4.1. MSc Culture and Conditioned Media (MScCM) Collection
4.2. Primary Cultures of Dorsal Root Ganglion Neurons (PCDRGs)
4.3. Intrathecal Implant Procedure
4.4. Spinal Cord Trauma
4.5. IT Delivery of MSCs Conditioned Medium (MScCM)
4.6. Behavioral Testing of Motor Function (BBB Scoring)
4.7. Cytokines Profile
4.8. Morphometric Analysis
4.9. Immunohistochemistry and Quantification Analysis
4.10. Quantification Analysis
4.11. Data and Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kou, Z.; Sun, D. New era of treatment and evaluation of traumatic brain injury and spinal cord injury. Neural Regen. Res. 2016, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Gerin, C.G.; Madueke, I.C.; Perkins, P.; Hill, S.; Smith, K.; Haley, B.; Allen, S.A.; Garcia, R.P.; Paunesku, T.; Woloschak, G. Combination strategies for repair, plasticity, and regeneration using regulation of gene expression during the chronic phase after spinal cord injury. Synapse 2011, 65, 1255–1281. [Google Scholar] [CrossRef] [PubMed]
- Pego, A.P.; Kubinova, S.; Cizkova, D.; Vanicky, I.; Mar, F.M.; Sousa, M.M.; Sykova, R. Regenerative medicine for the treatment of spinal cord injury: More than just promises? J. Cell. Mol. Med. 2012, 16, 2564–2582. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.M.; Khazaei, M.; Fehlings, M.G. Translating mechanisms of neuroprotection, regeneration, and repair to treatment of spinal cord injury. Prog. Brain Res. 2015, 218, 15–54. [Google Scholar] [CrossRef] [PubMed]
- Nagoshi, N.; Nakashima, H.; Fehlings, M.G. Riluzole as a neuroprotective drug for spinal cord injury: From bench to bedside. Molecules 2015, 20, 7775–7789. [Google Scholar] [CrossRef] [PubMed]
- Kakulas, B.A. Neuropathology: The foundation for new treatments in spinal cord injury. Spinal Cord 2004, 42, 549–563. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, W.D.; Atkins, C.M.; Bramlett, H.M. Protection in animal models of brain and spinal cord injury with mild to moderate hypothermia. J. Neurotrauma 2009, 26, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Nagoshi, N.; Fehlings, M.G. Investigational drugs for the treatment of spinal cord injury: Review of preclinical studies and evaluation of clinical trials from Phase I to II. Expert Opin. Investig. Drugs 2015, 24, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, J.W. Overcoming inhibition in the damaged spinal cord. J. Neurotrauma 2006, 23, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, J.W.; Asher, R.A. The glial scar and central nervous system repair. Brain Res. Bull. 1999, 49, 377–391. [Google Scholar] [CrossRef]
- Garcia-Alias, G.; Barkhuysen, S.; Buckle, M.; Fawcett, J.W. Chondroitinase ABC treatment opens a window of opportunity for task-specific rehabilitation. Nat. Neurosci. 2009, 12, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Galtrey, C.M.; Asher, R.A.; Nothias, F.; Fawcett, J.W. Promoting plasticity in the spinal cord with chondroitinase improves functional recovery after peripheral nerve repair. Brain 2007, 130 Pt 4, 926–939. [Google Scholar] [CrossRef]
- Jendelova, P.; Kubinova, S.; Sandvig, I.; Erceg, S.; Sandvig, A.; Sykova, E. Current developments in cell- and biomaterial-based approaches for stroke repair. Expert Opin. Biol. Ther. 2016, 16, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Cizkova, D.; Devaux, S.; Le Marrec-Croq, F.; Franck, J.; Slovinska, L.; Blasko, J.; Rosocha, R.; Spakova, T.; Lefebvre, C.; Fournier, I.; et al. Modulation properties of factors released by bone marrow stromal cells on activated microglia: An in vitro study. Sci. Rep. 2014, 4, 7514. [Google Scholar] [CrossRef] [PubMed]
- Urdzíková, L.M.; Růžička, J.; LaBagnara, M.; Kárová, K.; Kubinová, Š.; Jiráková, K.; Murali, R.; Syková, E.; Jhanwar-Uniyal, M.; Jendelová, P. Human mesenchymal stem cells modulate inflammatory cytokines after spinal cord injury in rat. Int. J. Mol. Sci. 2014, 15, 11275–11293. [Google Scholar] [CrossRef] [PubMed]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 1995, 12, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Marote, A.; Teixeira, F.B.G.; Mendes-Pinheiro, B.R.; Salgado, A.J. MSCs-derived exosomes: Cell-secreted nanovesicles with regenerative potential. Front. Pharmacol. 2016, 7, 231. [Google Scholar] [CrossRef] [PubMed]
- Salgado, A.J.; Sousa, J.C.; Costa, B.M.; Pires, A.O.; Mateus-Pinheiro, A.; Teixeira, F.G.; Pinto, L.; Sousa, N. Mesenchymal stem cells secretome as a modulator of the neurogenic niche: Basic insights and therapeutic opportunities. Front. Cell. Neurosci. 2015, 9, 249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixeira, F.B.G.; Carvalho, M.M.; Neves-Carvalho, A.; Panchalingam, K.M.; Behie, L.A.; Pinto, L.; Sousa, N.; Salgado, A.J. Secretome of mesenchymal progenitors from the umbilical cord acts as modulator of neural/glial proliferation and differentiation. Stem Cell Rev. Rep. 2014, 11, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Cizkova, D.; Novotna, I.; Slovinska, L.; Vanicky, I.; Jergova, S.; Rosocha, J.; Radonak, J. Repetitive intrathecal catheter delivery of bone marrow mesenchymal stromal cells improves functional recovery in a rat model of contusive spinal cord injury. J. Neurotrauma 2011, 28, 1951–1961. [Google Scholar] [CrossRef] [PubMed]
- Cizkova, D.; Rosocha, J.; Vanicky, I.; Jergova, S.; Cizek, M. Transplants of human mesenchymal stem cells improve functional recovery after spinal cord injury in the rat. Cell. Mol. Neurobiol. 2006, 26, 1165–1178. [Google Scholar] [CrossRef] [PubMed]
- Grulova, I.; Slovinska, L.; Blasko, J.; Devaux, S.; Wisztorski, M.; Salzet, M.; Fournier, I.; Kryukov, O.; Cohen, S.; Cizkova, D. Delivery of alginate scaffold releasing two trophic factors for spinal cord injury repair. Sci. Rep. 2015, 5, 13702. [Google Scholar] [CrossRef] [PubMed]
- Kubinova, S.; Horak, D.; Hejcl, A.; Plichta, Z.; Kotek, J.; Proks, V.; Forostyak, S.; Sykova, E. SIKVAV-modified highly superporous PHEMA scaffolds with oriented pores for spinal cord injury repair. J. Tissue Eng. Regen. Med. 2015, 9, 1298–1309. [Google Scholar] [CrossRef] [PubMed]
- Ruzicka, J.; Machova-Urdzikova, L.; Gillick, J.; Amemori, T.; Romanyuk, N.; Karova, N.; Zaviskova, K.; Dubisova, J.; Kubinova, S.; Murali, R.; et al. A comparative study of three different types of stem cells for treatment of rat spinal cord injury. Cell Transplant. 2017, 26, 585–603. [Google Scholar] [CrossRef] [PubMed]
- Cizkova, D.; Le Marrec-Croq, F.; Franck, J.; Slovinska, J.; Grulova, I.; Devaux, S.; Lefebvre, C.; Fournier, I.; Salzet, M. Alterations of protein composition along the rostro-caudal axis after spinal cord injury: Proteomic, in vitro and in vivo analyses. Front. Cell. Neurosci. 2014, 8, 105. [Google Scholar] [CrossRef] [PubMed]
- Devaux, S.; Cizkova, D.; Quanico, J.; Franck, J.; Nataf, S.; Pays, L.; Hauberg-Lotte, L.; Maass, P.; Kobarg, J.H.; Kobeissy, F.; et al. Proteomic analysis of the spatio-temporal based molecular kinetics of acute spinal cord injury identifies a time- and segment-specific window for effective tissue repair. Mol. Cell. Proteom. 2016, 15, 2641–2670. [Google Scholar] [CrossRef] [PubMed]
- Cizkova, D.; Racekova, E.; Vanicky, I. The expression of B-50/GAP-43 and GFAP after bilateral olfactory bulbectomy in rats. Physiol. Res. 1997, 46, 487–495. [Google Scholar] [PubMed]
- Novotna, I.; Slovinska, L.; Vanicky, I.; Cizek, M.; Radonak, J.; Cizkova, D. IT delivery of ChABC modulates NG2 and promotes GAP-43 axonal regrowth after spinal cord injury. Cell. Mol. Neurobiol. 2011, 31, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, J.W.; Curt, A.; Steeves, J.D.; Coleman, W.P.; Tuszynski, M.H.; Lammertse, D.; Bartlett, P.F.; Blight, A.R.; Dietz, V.; Ditunno, J.; et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: Spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord 2007, 45, 190–205. [Google Scholar] [CrossRef] [PubMed]
- Hofer, H.R.; Tuan, R.S. Secreted trophic factors of mesenchymal stem cells support neurovascular and musculoskeletal therapies. Stem Cell Res. Ther. 2016, 7, 131. [Google Scholar] [CrossRef] [PubMed]
- Lankford, K.L.; Arroyo, E.J.; Nazimek, K.; Bryniarski, K.; Askenase, P.W.; Kocsis, J.D. Intravenously delivered mesenchymal stem cell-derived exosomes target M2-type macrophages in the injured spinal cord. PLoS ONE 2018, 13, e0190358. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ye, Y.; Su, X.; He, J.; Bai, W.; He, X. MSCs-derived exosomes and neuroinflammation, neurogenesis and therapy of traumatic brain injury. Front. Cell. Neurosci. 2017, 11. [Google Scholar] [CrossRef] [PubMed]
- Murgoci, A.-N.; Cizkova, D.; Majerova, P.; Petrovova, E.; Medvecky, L.; Fournier, I.; Salzet, M. Brain cortex microglia derived exosomes: Novel nanoparticles for glioma therapy. ChemPhysChem 2018. [Google Scholar] [CrossRef] [PubMed]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cizkova, D.; Cubinkova, V.; Smolek, T.; Murgoci, A.-N.; Danko, J.; Vdoviakova, K.; Humenik, F.; Cizek, M.; Quanico, J.; Fournier, I.; et al. Localized Intrathecal Delivery of Mesenchymal Stromal Cells Conditioned Medium Improves Functional Recovery in a Rat Model of Spinal Cord Injury. Int. J. Mol. Sci. 2018, 19, 870. https://doi.org/10.3390/ijms19030870
Cizkova D, Cubinkova V, Smolek T, Murgoci A-N, Danko J, Vdoviakova K, Humenik F, Cizek M, Quanico J, Fournier I, et al. Localized Intrathecal Delivery of Mesenchymal Stromal Cells Conditioned Medium Improves Functional Recovery in a Rat Model of Spinal Cord Injury. International Journal of Molecular Sciences. 2018; 19(3):870. https://doi.org/10.3390/ijms19030870
Chicago/Turabian StyleCizkova, Dasa, Veronika Cubinkova, Tomas Smolek, Adriana-Natalia Murgoci, Jan Danko, Katarina Vdoviakova, Filip Humenik, Milan Cizek, Jusal Quanico, Isabelle Fournier, and et al. 2018. "Localized Intrathecal Delivery of Mesenchymal Stromal Cells Conditioned Medium Improves Functional Recovery in a Rat Model of Spinal Cord Injury" International Journal of Molecular Sciences 19, no. 3: 870. https://doi.org/10.3390/ijms19030870
APA StyleCizkova, D., Cubinkova, V., Smolek, T., Murgoci, A. -N., Danko, J., Vdoviakova, K., Humenik, F., Cizek, M., Quanico, J., Fournier, I., & Salzet, M. (2018). Localized Intrathecal Delivery of Mesenchymal Stromal Cells Conditioned Medium Improves Functional Recovery in a Rat Model of Spinal Cord Injury. International Journal of Molecular Sciences, 19(3), 870. https://doi.org/10.3390/ijms19030870