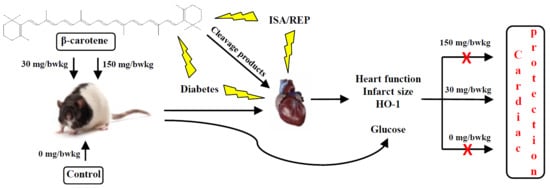
The Effects of Long-Term, Low- and High-Dose Beta-Carotene Treatment in Zucker Diabetic Fatty Rats: The Role of HO-1
Abstract
:1. Introduction
2. Results
2.1. The Effect of BC Treatment on the Cardiac Function in Isolated Hearts Subjected to ISA/REP
2.2. The BC Dose Effects on ISA/REP-Induced IS
2.3. The BC Effects on HO-1 Protein Expression
2.4. The Effects of BC on the Blood Glucose Level
3. Discussion
4. Materials and Methods
4.1. Animal Treatment
4.2. Isolated Working Heart Preparation
4.3. Induction of ISA/REP and Measurement of Cardiac Functions
4.4. The Determination of IS
4.5. Western Blot Analysis
4.6. Oral Glucose Tolerance Test (OGTT)
4.7. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Haines, D.D.; Bak, I.; Ferdinandy, P.; Mahmoud, F.F.; Al-Harbi, S.A.; Blasig, I.E.; Tosaki, A. Cardioprotective effects of the calcineurin inhibitor FK506 and the PAF receptor antagonist and free radical scavenger, EGb 761, in isolated ischemic/reperfused rat hearts. J. Cardiovasc. Pharmacol. 2000, 35, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Pataki, T.; Bak, I.; Kovacs, P.; Bagchi, D.; Das, D.K.; Tosaki, A. Grape seed proanthocyanidins improved cardiac recovery during reperfusion after ischemia in isolated rat hearts. Am. J. Clin. Nutr. 2002, 75, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Szabo, M.E.; Gallyas, E.; Bak, I.; Rakotovao, A.; Boucher, F.; de Leiris, J.; Nagy, N.; Varga, E.; Tosaki, A. Heme oxygenase-1-related carbon monoxide and flavonoids in ischemic/reperfused rat retina. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3727–3732. [Google Scholar] [CrossRef] [PubMed]
- Bak, I.; Lekli, I.; Juhasz, B.; Nagy, N.; Varga, E.; Varadi, J.; Gesztelyi, R.; Szabo, G.; Szendrei, L.; Bacskay, I. Cardioprotective mechanisms of Prunus cerasus (sour cherry) seed extract against ischemia-reperfusion-induced damage in isolated rat hearts. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H1329–H3236. [Google Scholar] [CrossRef] [PubMed]
- Bak, I.; Czompa, A.; Csepanyi, E.; Juhasz, B.; Kalantari, H.; Najm, K.; Aghel, N.; Varga, B.; Haines, D.D.; Tosaki, A. Evaluation of systemic and dermal toxicity and dermal photoprotection by sour cherry kernels. Phytother. Res. 2011, 25, 1714–1720. [Google Scholar] [CrossRef] [PubMed]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [PubMed]
- NCD Risk Factor Collaboration. Worldwide trends in diabetes since 1980: A pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 2016, 387, 1513–1530. [Google Scholar] [CrossRef]
- Berry, J.; Keebler, M.E.; McGuire, D.K. Diabetes mellitus and cardiovascular disease. Pandora’s box has been opened. Herz 2004, 29, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Szuszkiewicz-Garcia, M.M.; Davidson, J.A. Cardiovascular disease in diabetes mellitus: Risk factors and medical therapy. Endocrinol. Metab. Clin. N. Am. 2014, 43, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Cook, N.R.; Albert, C.M.; Van Denburgh, M.; Manson, J.E. Effects of vitamins C and E and β-carotene on the risk of type 2 diabetes in women at high risk of cardiovascular disease: A randomized controlled trial. Am. J. Clin. Nutr. 2009, 90, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Lekli, I.; Szabo, G.; Juhasz, B.; Das, S.; Das, M.; Varga, E.; Szendrei, L.; Gesztelyi, R.; Varadi, J.; Bak, I. Protective mechanisms of resveratrol against ischemia-reperfusion-induced damage in hearts obtained from Zucker obese rats: The role of GLUT-4 and endothelin. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H859–H866. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, G.; Geng, F.; Zhang, Z.; Li, J.; Yang, M.; Dong, L.; Gao, F. Berberine reduces ischemia/reperfusion-induced myocardial apoptosis via activating AMPK and PI3K-Akt signaling in diabetic rats. Apoptosis 2014, 19, 946–957. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wei, J.; Xue, R.; Wu, J.D.; Zhao, W.; Wang, Z.Z.; Wang, S.K.; Zhou, Z.X.; Song, D.Q.; Wang, Y.M. Berberine lowers blood glucose in type 2 diabetes mellitus patients through increasing insulin receptor expression. Metabolism 2010, 59, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Dugoua, J.J. Nutritional supplements and their effect on glucose control. Curr. Diabetes Rep. 2011, 11, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Kataja-Tuomola, M.; Sundell, J.R.; Mannisto, S.; Virtanen, M.J.; Kontto, J.; Albanes, D.; Virtamo, J. Effect of α-tocopherol and β-carotene supplementation on the incidence of type 2 diabetes. Diabetologia 2008, 51, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Alija, A.J.; Bresgen, N.; Sommerburg, O.; Langhans, C.D.; Siems, W.; Eckl, P.M. Cyto- and genotoxic potential of β-carotene and cleavage products under oxidative stress. Biofactors 2005, 24, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Siems, W.; Salerno, C.; Crifo, C.; Sommerburg, O.; Wiswedel, I. β-carotene degradation products—Formation, toxicity and prevention of toxicity. Forum Nutr. 2009, 61, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Csepanyi, E.; Czompa, A.; Haines, D.; Lekli, I.; Bakondi, E.; Balla, G.; Tosaki, A.; Bak, I. Cardiovascular effects of low versus high-dose β-carotene in a rat model. Pharmacol. Res. 2015, 100, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Siems, W.; Wiswedel, I.; Salerno, C.; Crifo, C.; Augustin, W.; Schild, L.; Langhans, C.D.; Sommerburg, O. β-carotene breakdown products may impair mitochondrial functions--potential side effects of high-dose β-carotene supplementation. J. Nutr. Biochem. 2005, 16, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, R.; Ricordy, R.; Aglitti, T.; Gatta, V.; Perticone, P.; De Salvia, R. Ascorbic acid and β-carotene as modulators of oxidative damage. Carcinogenesis 1997, 18, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Van Helden, Y.G.; Keijer, J.; Knaapen, A.M.; Heil, S.G.; Briede, J.J.; van Schooten, F.J.; Godschalk, R.W. β-carotene metabolites enhance inflammation-induced oxidative DNA damage in lung epithelial cells. Free Radic. Biol. Med. 2009, 46, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Ndisang, J.F.; Jadhav, A. Heme oxygenase system enhances insulin sensitivity and glucose metabolism in streptozotocin-induced diabetes. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E829–E841. [Google Scholar] [CrossRef] [PubMed]
- Ptilovanciv, E.O.; Fernandes, G.S.; Teixeira, L.C.; Reis, L.A.; Pessoa, E.A.; Convento, M.B.; Simoes, M.J.; Albertoni, G.A.; Schor, N.; Borges, F.T. Heme oxygenase 1 improves glucoses metabolism and kidney histological alterations in diabetic rats. Diabetol. Metab. Syndr. 2013, 5, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ndisang, J.F.; Lane, N.; Jadhav, A. The heme oxygenase system abates hyperglycemia in Zucker diabetic fatty rats by potentiating insulin-sensitizing pathways. Endocrinology 2009, 150, 2098–2108. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ying, L.; Chen, Y.Y.; Shen, Y.L.; Guo, R.; Jin, K.K.; Wang, L.X. Induction of heme oxygenase-1 ameliorates vascular dysfunction in streptozotocin-induced type 2 diabetic rats. Vascul. Pharmacol. 2014, 61, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Khan, Z.A.; Barbin, Y.; Chakrabarti, S. Pro-oxidant role of heme oxygenase in mediating glucose-induced endothelial cell damage. Free Radic. Res. 2004, 38, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Farhangkhoee, H.; Khan, Z.A.; Mukherjee, S.; Cukiernik, M.; Barbin, Y.P.; Karmazyn, M.; Chakrabarti, S. Heme oxygenase in diabetes-induced oxidative stress in the heart. J. Mol. Cell. Cardiol. 2003, 35, 1439–1448. [Google Scholar] [CrossRef] [PubMed]
- Meydani, M.; Azzi, A. Diabetes risk: Antioxidants or lifestyle? Am. J. Clin. Nutr. 2009, 90, 253–254. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.J.; Tappia, P.S.; Neki, N.S.; Dhalla, N.S. Prevention of diabetes-induced cardiovascular complications upon treatment with antioxidants. Heart Fail. Rev. 2014, 19, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Goszcz, K.; Deakin, S.J.; Duthie, G.G.; Stewart, D.; Leslie, S.J.; Megson, I.L. Antioxidants in Cardiovascular Therapy: Panacea or False Hope? Front. Cardiovasc. Med. 2015, 2, 29. [Google Scholar] [CrossRef] [PubMed]
- Da Rocha, R.F.; de Oliveira, M.R.; Schonhofen, P.; Schnorr, C.E.; Dal Pizzol, F.; Moreira, J.C. Long-term vitamin A supplementation at therapeutic doses induces mitochondrial electrons transfer chain (METC) impairment and increased mitochondrial membrane-enriched fraction (MMEF) 3-nitrotyrosine on rat heart. Free Radic. Res. 2010, 44, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Nagao, A. Oxidative conversion of carotenoids to retinoids and other products. J. Nutr. 2004, 134, 237S–240S. [Google Scholar] [CrossRef] [PubMed]
- Caballero, B. Guide to Nutritional Supplements; Elsevier/Academic Press: Oxford, UK, 2009; 548p. [Google Scholar]
- Sy, C.; Dangles, O.; Borel, P.; Caris-Veyrat, C. Iron-induced oxidation of (all-E)-β-carotene under model gastric conditions: Kinetics, products, and mechanism. Free Radic. Biol. Med. 2013, 63, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Furusho, T.; Kataoka, E.; Yasuhara, T.; Wada, M.; Innami, S. Administration of β-carotene suppresses lipid peroxidation in tissues and improves the glucose tolerance ability of streptozotocin-induced diabetic rats. Int. J. Vitam. Nutr. Res. 2002, 72, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.Y.; Fang, M.; Zheng, J.H.; Ren, D.F.; Lu, J. Optimised extraction of β-carotene from Spirulina platensis and hypoglycaemic effect in streptozotocin-induced diabetic mice. J. Sci. Food Agric. 2016, 96, 1783–1789. [Google Scholar] [CrossRef] [PubMed]
- Bak, I.; Varadi, J.; Nagy, N.; Vecsernyes, M.; Tosaki, A. The role of exogenous carbon monoxide in the recovery of post-ischemic cardiac function in buffer perfused isolated rat hearts. Cell. Mol. Biol. 2005, 51, 453–459. [Google Scholar] [CrossRef] [PubMed]





| Total Number of Type II Diabetic Rats: 30 | |||
|---|---|---|---|
| Experimental procedure | Vehicle control | Low dose BC treated | High dose BC treated |
| (C) | (LD-BC) | (HD-BC) | |
| 10 | 10 | 10 | |
| Oral Glucose Tolerance Test (OGTT) | 10 | 10 | 10 |
| Ischemia/Reperfusion (ISA/REP) | 8 | 8 | 8 |
| Cardiac function measurement | 8 | 8 | 8 |
| Infarct Size (IS) determination | 4 | 4 | 4 |
| Western Blot analysis | 5 | 5 | 5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Csepanyi, E.; Czompa, A.; Szabados-Furjesi, P.; Lekli, I.; Balla, J.; Balla, G.; Tosaki, A.; Bak, I. The Effects of Long-Term, Low- and High-Dose Beta-Carotene Treatment in Zucker Diabetic Fatty Rats: The Role of HO-1. Int. J. Mol. Sci. 2018, 19, 1132. https://doi.org/10.3390/ijms19041132
Csepanyi E, Czompa A, Szabados-Furjesi P, Lekli I, Balla J, Balla G, Tosaki A, Bak I. The Effects of Long-Term, Low- and High-Dose Beta-Carotene Treatment in Zucker Diabetic Fatty Rats: The Role of HO-1. International Journal of Molecular Sciences. 2018; 19(4):1132. https://doi.org/10.3390/ijms19041132
Chicago/Turabian StyleCsepanyi, Evelin, Attila Czompa, Peter Szabados-Furjesi, Istvan Lekli, Jozsef Balla, Gyorgy Balla, Arpad Tosaki, and Istvan Bak. 2018. "The Effects of Long-Term, Low- and High-Dose Beta-Carotene Treatment in Zucker Diabetic Fatty Rats: The Role of HO-1" International Journal of Molecular Sciences 19, no. 4: 1132. https://doi.org/10.3390/ijms19041132
APA StyleCsepanyi, E., Czompa, A., Szabados-Furjesi, P., Lekli, I., Balla, J., Balla, G., Tosaki, A., & Bak, I. (2018). The Effects of Long-Term, Low- and High-Dose Beta-Carotene Treatment in Zucker Diabetic Fatty Rats: The Role of HO-1. International Journal of Molecular Sciences, 19(4), 1132. https://doi.org/10.3390/ijms19041132