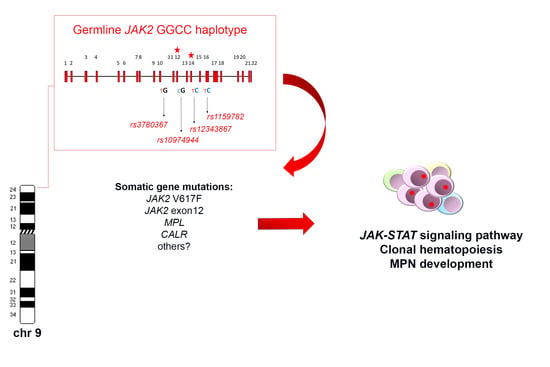
The JAK2 GGCC (46/1) Haplotype in Myeloproliferative Neoplasms: Causal or Random?
Abstract
:1. Introduction
2. Genomic Architecture of the JAK2 HaplotypeGGCC_46/1
3. Frequency of the HaplotypeGGCC_46/1 in MPN and Other Myeloid Neoplasms
4. The Role of the JAK2 HaplotypeGGCC_46/1 and Other Germ Line Variants in Familial and Sporadic MPNs
5. Clinical Implications of the JAK246/1_GGCC Haplotype
6. Correlation between the JAK2 HaplotypeGGCC_46/1 and JAK2 V617F Allele Burden
7. Potential Mechanisms Explaining the Association between the JAK2 HaplotypeGGCC_46/1 and JAK2 V617F Mutation
8. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| PV | polycythemia vera |
| PMF | primary myelofibrosis |
| ET | essential thrombocythemia |
| MPN | myeloproliferative neoplasms |
| SNP | single nucleotide polymorphisms |
| EPOR | receptor for erythropoietin |
| MPL | receptors for thrombopoietin |
| GCSFR | colony stimulating factor |
| INSL4 | insulin-like 4 |
| INSL6 | insulin-like 6 |
| WTCCC | Wellcome Trust Case Control Consortium |
| CALR | calreticulin |
| CML | chronic myeloid leukemia |
| AML | acute myeloid leukemia |
| NK | normal karyotype |
| TERT | telomerase reverse transcriptase |
| allo-HSCT | allogeneic hematopoietic stem cell transplantation |
References
- Baxter, E.J.; Scott, L.M.; Campbell, P.J.; East, C.; Fourouclas, N.; Swanton, S.; Vassiliou, G.S.; Bench, A.J.; Boyd, E.M.; Curtin, N.; et al. Cancer Genome Project Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 2005, 365, 1054–1061. [Google Scholar] [CrossRef]
- Kralovics, R.; Passamonti, F.; Buser, A.S.; Teo, S.-S.; Tiedt, R.; Passweg, J.R.; Tichelli, A.; Cazzola, M.; Skoda, R.C. A Gain-of-function mutation of JAK2 in myeloproliferative disorders. N. Engl. J. Med. 2005, 352, 1779–1790. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A.; Gilliland, D.G. Oncogenes in myeloproliferative disorders. Cell Cycle 2007, 6, 550–566. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.M.; Tong, W.; Levine, R.L.; Scott, M.A.; Beer, P.A.; Stratton, M.R.; Futreal, P.A.; Erber, W.N.; McMullin, M.F.; Harrison, C.N.; et al. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N. Engl. J. Med. 2007, 356, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.L. X-inactivation-based clonality analysis and quantitative JAK2V617F assessment reveal a strong association between clonality and JAK2V617F in PV but not ET/MMM, and identifies a subset of JAK2V617F-negative ET and MMM patients with clonal hematopoiesis. Blood 2006, 107, 4139–4141. [Google Scholar] [CrossRef] [PubMed]
- Pardanani, A.; Fridley, B.L.; Lasho, T.L.; Gilliland, D.G.; Tefferi, A. Host genetic variation contributes to phenotypic diversity in myeloproliferative disorders. Blood 2008, 111, 2785–2789. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.V.; Chase, A.; Silver, R.T.; Oscier, D.; Zoi, K.; Wang, Y.L.; Cario, H.; Pahl, H.L.; Collins, A.; Reiter, A.; et al. JAK2 haplotype is a major risk factor for the development of myeloproliferative neoplasms. Nat. Genet. 2009, 41, 446–449. [Google Scholar] [CrossRef] [PubMed]
- Olcaydu, D.; Harutyunyan, A.; Jäger, R.; Berg, T.; Gisslinger, B.; Pabinger, I.; Gisslinger, H.; Kralovics, R. A common JAK2 haplotype confers susceptibility to myeloproliferative neoplasms. Nat. Genet. 2009, 41, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Kilpivaara, O.; Mukherjee, S.; Schram, A.M.; Wadleigh, M.; Mullally, A.; Ebert, B.L.; Bass, A.; Marubayashi, S.; Heguy, A.; Garcia-Manero, G.; et al. A germline JAK2 SNP is associated with predisposition to the development of JAK2(V617F)-positive myeloproliferative neoplasms. Nat. Genet. 2009, 41, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Olcaydu, D.; Skoda, R.C.; Looser, R.; Li, S.; Cazzola, M.; Pietra, D.; Passamonti, F.; Lippert, E.; Carillo, S.; Girodon, F.; et al. The “GGCC” haplotype of JAK2 confers susceptibility to JAK2 exon 12 mutation-positive polycythemia vera. Leukemia 2009, 23, 1924–1926. [Google Scholar] [CrossRef] [PubMed]
- Hermouet, S.; Vilaine, M. The JAK2 46/1 haplotype: A marker of inappropriate myelomonocytic response to cytokine stimulation, leading to increased risk of inflammation, myeloid neoplasm, and impaired defense against infection? Haematologica 2011, 96, 1575–1579. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A.; Lasho, T.L.; Patnaik, M.M.; Finke, C.M.; Hussein, K.; Hogan, W.J.; Elliott, M.A.; Litzow, M.R.; Hanson, C.A.; Pardanani, A. JAK2 germline genetic variation affects disease susceptibility in primary myelofibrosis regardless of V617F mutational status: Nullizygosity for the JAK2 46/1 haplotype is associated with inferior survival. Leukemia 2010, 24, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Andrikovics, H.; Nahajevszky, S.; Koszarska, M.; Meggyesi, N.; Bors, A.; Halm, G.; Lueff, S.; Lovas, N.; Matrai, Z.; Csomor, J.; et al. JAK2 46/1 haplotype analysis in myeloproliferative neoplasms and acute myeloid leukemia. Leukemia 2010, 24, 1809–1813. [Google Scholar] [CrossRef] [PubMed]
- Soler, G.; Bernal-Vicente, A.; Antón, A.I.; Torregrosa, J.M.; Caparrós-Pérez, E.; Sánchez-Serrano, I.; Martínez-Pérez, A.; Sánchez-Vega, B.; Vicente, V.; Ferrer-Marin, F. The JAK2 46/1 haplotype does not predispose to CALR-mutated myeloproliferative neoplasms. Ann. Hematol. 2015, 94, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Stolyar, M.A.; Klimova, O.A.; Gorbenko, A.S.; Brenner, E.V.; Titov, S.E.; Ivanov, M.K.; Olkhovskiy, I.A. JAK2 haplotype 46/1 and JAK2 V617F allele burden in MPN: New evidence against the “hypermutability” hypothesis? Int. J. Lab. Hematol. 2018, 40, e8–e10. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.V.; Campbell, P.J.; Beer, P.A.; Schnittger, S.; Vannucchi, A.M.; Zoi, K.; Percy, M.J.; McMullin, M.F.; Scott, L.M.; Tapper, W.; et al. The JAK2 46/1 haplotype predisposes to MPL-mutated myeloproliferative neoplasms. Blood 2010, 115, 4517–4523. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, M.M.; Lasho, T.L.; Finke, C.M.; Gangat, N.; Caramazza, D.; Siragusa, S.; Hanson, C.A.; Pardanani, A.; Tefferi, A. MPL mutation effect on JAK2 46/1 haplotype frequency in JAK2V617F-negative myeloproliferative neoplasms. Leukemia 2010, 24, 859–860. [Google Scholar] [CrossRef] [PubMed]
- Trifa, A.P.; Cucuianu, A.; Petrov, L.; Urian, L.; Militaru, M.S.; Dima, D.; Pop, I.V.; Popp, R.A. The G allele of the JAK2 rs10974944 SNP, part of JAK2 46/1 haplotype, is strongly associated with JAK2 V617F-positive myeloproliferative neoplasms. Ann. Hematol. 2010, 89, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Pardanani, A.; Lasho, T.L.; Finke, C.M.; Gangat, N.; Wolanskyj, A.P.; Hanson, C.A.; Tefferi, A. The JAK2 46/1 haplotype confers susceptibility to essential thrombocythemia regardless of JAK2V617F mutational status-clinical correlates in a study of 226 consecutive patients. Leukemia 2010, 24, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Guglielmelli, P.; Biamonte, F.; Spolverini, A.; Pieri, L.; Isgrò, A.; Antonioli, E.; Pancrazzi, A.; Bosi, A.; Barosi, G.; Vannucchi, A.M. Frequency and clinical correlates of JAK2 46/1 (GGCC) haplotype in primary myelofibrosis. Leukemia 2010, 24, 1533–1537. [Google Scholar] [CrossRef] [PubMed]
- Gau, J.-P.; Chen, C.-C.; Chou, Y.-S.; Liu, C.-J.; Yu, Y.-B.; Hsiao, L.-T.; Liu, J.-H.; Hsu, H.-C.; Chiou, T.-J.; Chen, P.-M.; et al. No increase of JAK2 46/1 haplotype frequency in essential thrombocythemia with CALR mutations: Functional effect of the haplotype limited to allele with JAK2V617F mutation but not CALR mutation. Blood Cells Mol. Dis. 2015, 55, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Trifa, A.P.; Bănescu, C.; Tevet, M.; Bojan, A.; Dima, D.; Urian, L.; Török-Vistai, T.; Popov, V.M.; Zdrenghea, M.; Petrov, L.; et al. TERT rs2736100 A>C SNP and JAK2 46/1 haplotype significantly contribute to the occurrence of JAK2 V617F and CALR mutated myeloproliferative neoplasms—A multicentric study on 529 patients. Br. J. Haematol. 2016, 174, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Trifa, A.P.; Bănescu, C.; Bojan, A.S.; Voina, C.M.; Ștefana, P.; Vișan, S.; Ciubean, A.D.; Tripon, F.; Dima, D.; Popov, V.M.; et al. MECOM, HBS1L-MYB, THRB-RARB, JAK2, and TERT polymorphisms defining the genetic predisposition to myeloproliferative neoplasms: A study on 939 patients. Am. J. Hematol. 2018, 93, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Spolverini, A.; Jones, A.V.; Hochhaus, A.; Pieri, L.; Cross, N.C.P.; Vannucchi, A.M. The myeloproliferative neoplasm-associated JAK2 46/1 haplotype is not overrepresented in chronic myelogenous leukemia. Ann. Hematol. 2011, 90, 365–366. [Google Scholar] [CrossRef] [PubMed]
- Nahajevszky, S.; Andrikovics, H.; Batai, A.; Adam, E.; Bors, A.; Csomor, J.; Gopcsa, L.; Koszarska, M.; Kozma, A.; Lovas, N.; et al. The prognostic impact of germline 46/1 haplotype of Janus kinase 2 in cytogenetically normal acute myeloid leukemia. Haematologica 2011, 96, 1613–1618. [Google Scholar] [CrossRef] [PubMed]
- Olcaydu, D.; Rumi, E.; Harutyunyan, A.; Passamonti, F.; Pietra, D.; Pascutto, C.; Berg, T.; Jäger, R.; Hammond, E.; Cazzola, M.; et al. The role of the JAK2 GGCC haplotype and the TET2 gene in familial myeloproliferative neoplasms. Haematologica 2011, 96, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Jäger, R.; Harutyunyan, A.S.; Rumi, E.; Pietra, D.; Berg, T.; Olcaydu, D.; Houlston, R.S.; Cazzola, M.; Kralovics, R. Common germline variation at the TERT locus contributes to familial clustering of myeloproliferative neoplasms. Am. J. Hematol. 2014, 89, 1107–1110. [Google Scholar] [CrossRef] [PubMed]
- Rumi, E.; Passamonti, F.; della Porta, M.G.; Elena, C.; Arcaini, L.; Vanelli, L.; del Curto, C.; Pietra, D.; Boveri, E.; Pascutto, C.; et al. Familial chronic myeloproliferative disorders: Clinical phenotype and evidence of disease anticipation. J. Clin. Oncol. 2007, 25, 5630–5635. [Google Scholar] [CrossRef] [PubMed]
- Harutyunyan, A.S.; Kralovics, R. Role of germline genetic factors in MPN pathogenesis. Hematol. Oncol. Clin. N. Am. 2012, 26, 1037–1051. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.V.; Cross, N.C.P. Inherited predisposition to myeloproliferative neoplasms. Ther. Adv. Hematol. 2013, 4, 237–253. [Google Scholar] [CrossRef] [PubMed]
- Rumi, E.; Cazzola, M. Advances in understanding the pathogenesis of familial myeloproliferative neoplasms. Br. J. Haematol. 2017, 178, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Oddsson, A.; Kristinsson, S.Y.; Helgason, H.; Gudbjartsson, D.F.; Masson, G.; Sigurdsson, A.; Jonasdottir, A.; Jonasdottir, A.; Steingrimsdottir, H.; Vidarsson, B.; et al. The germline sequence variant rs2736100_C in TERT associates with myeloproliferative neoplasms. Leukemia 2014, 28, 1371–1374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bojesen, S.E. Telomeres and human health. J. Intern. Med. 2013, 274, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Alter, B.P.; Rosenberg, P.S.; Giri, N.; Baerlocher, G.M.; Lansdorp, P.M.; Savage, S.A. Telomere length is associated with disease severity and declines with age in dyskeratosis congenita. Haematologica 2012, 97, 353–359. [Google Scholar] [CrossRef] [PubMed]
- McKay, J.D.; Hung, R.J.; Gaborieau, V.; Boffetta, P.; Chabrier, A.; Byrnes, G.; Zaridze, D.; Mukeria, A.; Szeszenia-Dabrowska, N.; Lissowska, J.; et al. Lung cancer susceptibility locus at 5p15.33. Nat. Genet. 2008, 40, 1404–1406. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Broderick, P.; Webb, E.; Wu, X.; Vijayakrishnan, J.; Matakidou, A.; Qureshi, M.; Dong, Q.; Gu, X.; Chen, W.V.; et al. Common 5p15.33 and 6p21.33 variants influence lung cancer risk. Nat. Genet. 2008, 40, 1407–1409. [Google Scholar] [CrossRef] [PubMed]
- Gago-Dominguez, M.; Jiang, X.; Conti, D.V.; Castelao, J.E.; Stern, M.C.; Cortessis, V.K.; Pike, M.C.; Xiang, Y.-B.; Gao, Y.-T.; Yuan, J.-M.; et al. Genetic variations on chromosomes 5p15 and 15q25 and bladder cancer risk: Findings from the Los Angeles-Shanghai bladder case-control study. Carcinogenesis 2011, 32, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Shete, S.; Hosking, F.J.; Robertson, L.B.; Dobbins, S.E.; Sanson, M.; Malmer, B.; Simon, M.; Marie, Y.; Boisselier, B.; Delattre, J.-Y.; et al. Genome-wide association study identifies five susceptibility loci for glioma. Nat. Genet. 2009, 41, 899–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Xu, Y.; Mei, H.; Peng, L.; Li, X.; Tang, J. The TERT rs2736100 polymorphism increases cancer risk: A meta-analysis. Oncotarget 2017, 8, 38693–38705. [Google Scholar] [CrossRef] [PubMed]
- Krahling, T.; Balassa, K.; Kiss, K.P.; Bors, A.; Batai, A.; Halm, G.; Egyed, M.; Fekete, S.; Remenyi, P.; Masszi, T.; et al. Co-occurrence of myeloproliferative neoplasms and solid tumors is attributed to a synergism between cytoreductive therapy and the common TERT polymorphism rs2736100. Cancer Epidemiol. Biomark. Prev. 2016, 25, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Tapper, W.; Jones, A.V.; Kralovics, R.; Harutyunyan, A.S.; Zoi, K.; Leung, W.; Godfrey, A.L.; Guglielmelli, P.; Callaway, A.; Ward, D.; et al. Genetic variation at MECOM, TERT, JAK2 and HBS1L-MYB predisposes to myeloproliferative neoplasms. Nat. Commun. 2015, 6, 6691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinds, D.A.; Barnholt, K.E.; Mesa, R.A.; Kiefer, A.K.; Do, C.B.; Eriksson, N.; Mountain, J.L.; Francke, U.; Tung, J.Y.; Nguyen, H.M.; et al. Germ line variants predispose to both JAK2 V617F clonal hematopoiesis and myeloproliferative neoplasms. Blood 2016, 128, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Larrán, A.; Angona, A.; Martínez-Avilés, L.; Bellosillo, B.; Besses, C. Influence of JAK2 46/1 haplotype in the natural evolution of JAK2V617F allele burden in patients with myeloproliferative neoplasms. Leuk. Res. 2012, 36, 324–326. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, C.; Birgens, H.S.; Nordestgaard, B.G.; Bojesen, S.E. Diagnostic value of JAK2 V617F somatic mutation for myeloproliferative cancer in 49,488 individuals from the general population. Br. J. Haematol. 2013, 160, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Gieger, C.; Radhakrishnan, A.; Cvejic, A.; Tang, W.; Porcu, E.; Pistis, G.; Serbanovic-Canic, J.; Elling, U.; Goodall, A.H.; Labrune, Y.; et al. New gene functions in megakaryopoiesis and platelet formation. Nature 2011, 480, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Newton-Cheh, C.; Johnson, T.; Gateva, V.; Tobin, M.D.; Bochud, M.; Coin, L.; Najjar, S.S.; Zhao, J.H.; Heath, S.C.; Eyheramendy, S.; et al. Genome-wide association study identifies eight loci associated with blood pressure. Nat. Genet. 2009, 41, 666–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Harst, P.; Zhang, W.; Mateo Leach, I.; Rendon, A.; Verweij, N.; Sehmi, J.; Paul, D.S.; Elling, U.; Allayee, H.; Li, X.; et al. Seventy-five genetic loci influencing the human red blood cell. Nature 2012, 492, 369–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Trillos, A.; Maffioli, M.; Colomer, D.; Alvarez-Larrán, A.; Pereira, A.; Angona, A.; Bellosillo, B.; Cervantes, F. Relationship between the 46/1 haplotype of the JAK2 gene and the JAK2 mutational status and allele burden, the initial findings, and the survival of patients with myelofibrosis. Ann. Hematol. 2014, 93, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Colaizzo, D.; Tiscia, G.L.; Bafunno, V.; Amitrano, L.; Vergura, P.; Grandone, E.; Guardascione, M.A.; Margaglione, M. The JAK2 rs12343867 CC genotype frequently occurs in patients with splanchnic venous thrombosis without the JAK2V617F mutation: A retrospective study. J. Thromb. Haemost. 2010, 8, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Smalberg, J.H.; Koehler, E.; Darwish Murad, S.; Plessier, A.; Seijo, S.; Trebicka, J.; Primignani, M.; de Maat, M.P.M.; Garcia-Pagan, J.-C.; Valla, D.C.; et al. The JAK2 46/1 haplotype in Budd-Chiari syndrome and portal vein thrombosis. Blood 2011, 117, 3968–3973. [Google Scholar] [CrossRef] [PubMed]
- Kouroupi, E.; Kiladjian, J.-J.; Chomienne, C.; Dosquet, C.; Bellucci, S.; Valla, D.; Cassinat, B. The JAK2 46/1 haplotype in splanchnic vein thrombosis. Blood 2011, 117, 5777–5778. [Google Scholar] [CrossRef] [PubMed]
- Villani, L.; Bergamaschi, G.; Primignani, M.; Rosti, V.; Carolei, A.; Poletto, V.; Catarsi, P.; Spolverini, A.; Vannucchi, A.M.; Barosi, G. JAK2 46/1 haplotype predisposes to splanchnic vein thrombosis-associated BCR-ABL negative classic myeloproliferative neoplasms. Leuk. Res. 2012, 36, e7–e9. [Google Scholar] [CrossRef] [PubMed]
- Westbrook, R.H.; Lea, N.C.; Mohamedali, A.M.; Smith, A.E.; Orr, D.W.; Roberts, L.N.; Heaton, N.D.; Wendon, J.A.; O’Grady, J.G.; Heneghan, M.A.; et al. Prevalence and clinical outcomes of the 46/1 haplotype, Janus kinase 2 mutations, and ten-eleven translocation 2 mutations in Budd-Chiari syndrome and their impact on thrombotic complications post liver transplantation. Liver Transplant. 2012, 18, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-L.; Zhang, P.-J.; Sun, G.-X.; Lu, Z.-J. The JAK2 46/1 haplotype (GGCC) in myeloproliferative neoplasms and splanchnic vein thrombosis: A pooled analysis of 26 observational studies. Ann. Hematol. 2014, 93, 1845–1852. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, L.R.; Han, D.Y.; Fraser, A.G.; Huebner, C.; Lam, W.J.; Morgan, A.R.; Duan, H.; Karunasinghe, N. Genetic factors in chronic inflammation: Single nucleotide polymorphisms in the STAT-JAK pathway, susceptibility to DNA damage and Crohn’s disease in a New Zealand population. Mutat. Res. 2010, 690, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-X.; Song, J.; Wang, J.; Dong, W.-G. JAK2 rs10758669 polymorphisms and susceptibility to ulcerative colitis and Crohn’s disease: A meta-analysis. Inflammation 2014, 37, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Can, G.; Tezel, A.; Gürkan, H.; Tozkır, H.; Ünsal, G.; Soylu, A.R.; Ümit, H.C. Investigation of IL23R, JAK2, and STAT3 gene polymorphisms and gene-gene interactions in Crohn’s disease and ulcerative colitis in a Turkish population. Turk. J. Gastroenterol. 2016, 27, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.C.; Hansoul, S.; Nicolae, D.L.; Cho, J.H.; Duerr, R.H.; Rioux, J.D.; Brant, S.R.; Silverberg, M.S.; Taylor, K.D.; Barmada, M.M.; et al. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn’s disease. Nat. Genet. 2008, 40, 955–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandzar, S.; Gupta, S.; Platt, M.O. Crohn’s disease: A review of treatment options and current research. Cell. Immunol. 2013, 286, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Hasselbalch, H.C. Perspectives on chronic inflammation in essential thrombocythemia, polycythemia vera, and myelofibrosis: Is chronic inflammation a trigger and driver of clonal evolution and development of accelerated atherosclerosis and second cancer? Blood 2012, 119, 3219–3225. [Google Scholar] [CrossRef] [PubMed]
- Balassa, K.; Krahling, T.; Remenyi, P.; Batai, A.; Bors, A.; Kiss, K.P.; Torbagyi, E.; Gopcsa, L.; Lengyel, L.; Barta, A.; et al. Recipient and donor JAK2 46/1 haplotypes are associated with acute graft-versus-host disease following allogeneic hematopoietic stem cell transplantation. Leuk. Lymphoma 2017, 58, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.J. Somatic and germline genetics at the JAK2 locus. Nat. Genet. 2009, 41, 385–386. [Google Scholar] [CrossRef] [PubMed]
- Spasovski, V.; Tosic, N.; Nikcevic, G.; Stojiljkovic, M.; Zukic, B.; Radmilovic, M.; Karan-Djurasevic, T.; Srzentic, S.; Colovic, M.; Pavlovic, S. The influence of novel transcriptional regulatory element in intron 14 on the expression of Janus kinase 2 gene in myeloproliferative neoplasms. J. Appl. Genet. 2013, 54, 21–26. [Google Scholar] [CrossRef] [PubMed]

| Reference | Main Findings | Association between the GGCC Haplotype and Somatic Mutations | Association between the GGCC Haplotype and JAK2 V617F Neg MPN | Tagging SNPs | Series of Analyzed Patients | Clinical Findings |
|---|---|---|---|---|---|---|
| [6] | First identification of JAK2 SNPs significantly associated with PV or ET | - | - | rs7046736, rs10815148, rs12342421 | 84 PV, 58 PMF, and 37 ET | Association between JAK2 SNPs and leucocytosis, higher hemoglobin level, lower platelet count |
| [7] | Association between JAK2 46/1 haplotype and JAK2 V617F positive MPN | JAK2 V617F (48–56%) | Weak association | rs12340895 | 88 PMF | Hematologically normal individuals that carried at least one 46/1 allele grew significantly fewer CFU-GM |
| [19] | The incidence of the 46/1-linked C allele was significantly higher in ET than in population controls | JAK2 V617F (44%) | Significant association | rs12343867 | 226 ET | The clinical features of 46/1 positive and negative ET were indistinguishable, including blood counts, rate of thrombosis/disease transformation and survival |
| [12] | JAK2 germline genetic variation affects disease susceptibility in PMF regardless of VF mutational status | JAK2 V617F (50%) | Significant association | rs12343867 | 130 MF | Association between nullizygosity for the JAK2 46/1 haplotype SNP allele and shortened survival |
| [55] | The SNP rs10758669_C allele increase the risk of having Crohn’s disease | - | - | rs10758669 | 302 Crohn’s disease | This JAK2 variant strongly enhanced the risk of ileocolonic disease, with stricturing or ileal/stricturing behavior, requiring a bowel resection |
| [16] | The frequency of 46/1 was higher in MPL mutated cases compared with controls | MPL W515K/L mutations (36%) | The 46/1 haplotype was also overrepresented in cases without V617F mutation | rs12340895 | 176 MPL pos/V617F neg ET, and 212 V617F pos ET | No association between 46/1 and clinical or laboratory features |
| [20] | The frequency of the 46/1 haplotype, was significantly higher in PMF patients showing the highest V617F allele burden | JAK2 V617F (38.6%) | No statistical significant association | rs12343867 | 202 PMF | No statistically significant correlations between any of the possible rs12343867 genotypes and hematological or clinical variables |
| [13] | The 46/1 haplotype is a predisposition factor for JAK2 V617F positive MPN, and is also significantly associated with AML patients with normal karyotype | JAK2 V617F (85%) | Significant association | rs12343867 | 312 MPN, 339 AML | The 46/1 haplotype is not associated with MPN manifestations, like disease type, splenomegaly, signs of increased erythropoiesis or myelopoiesis and vascular complication, except the increased risk of the development of myelofibrosis in homozygous cases |
| [50] | The 46/1 haplotype was overrepresented in JAK2V617F positive SVT patients compared with controls | JAK2 V617F (43%) | JAK2V617F negative SVT patients with a proven MPN also exhibited an increased frequency of the 46/1 haplotype | rs12343867 | 199 SVT | The 46/1 haplotype was associated with increased erythropoiesis (higher hemoglobin levels, hematocrit, and red blood cell count) in JAK2 V617F negative SVT patients |
| [25] | Association of the JAK2 46/1 haplotype with disease characteristics and treatment outcome in AML patients | - | - | rs12343867 | 176 AML | The 46/1 haplotype was found to be a factor predisposing to the development of acute myelomonocytic leukemia. In NK-AML, the carriers of 46/1 haplotype are characterized by shorter disease-free survival and overall survival |
| [43] | Untreated PV patients with homozygous JAK2 46/1 haplotype experienced a progressive increase in the JAK2 V617F allele burden | JAK2 V617F (68%) | - | rs12340895, rs12343867 | 26 PV, 36 ET | The 46/1 JAK2 haplotype status was not statistically different according to age, gender, type of diagnosis (PV or ET) or baseline hematological values |
| [48] | Among JAK2 V617F positive patients, those who were homozygous for the 46/1 haplotype had a higher allele burden | JAK2 V617F (40%) | - | rs12340895 | 132 MF | Patients with homozygous 46/1 haplotype showed significantly higher hemoglobin values and leukocyte counts, but no association was seen with other clinic hematologic features |
| [21] | The frequency of 46/1 haplotype was significantly higher in JAK2 V617F positive PV/ET but not in ET patients with CALR mutations | JAK2 V617F (40–50%) | No statistical significant association | rs12340895 | 72 PV, 115 ET | The presence of 46/1 haplotype had a trend to have higher white blood cell count in JAK2 V617F mutated PV and ET patients but not in CALR mutated ET |
| [61] | Both, recipient and donor 46/1 haplotypes significantly affected aGvHD grades II–IV development | - | - | rs12343867 | 124 AML | The recipient haplotype remained independently related to aGvHD, while the donor not. Significantly less relapses were observed among haplotype carriers, but overall survival did not differ |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anelli, L.; Zagaria, A.; Specchia, G.; Albano, F. The JAK2 GGCC (46/1) Haplotype in Myeloproliferative Neoplasms: Causal or Random? Int. J. Mol. Sci. 2018, 19, 1152. https://doi.org/10.3390/ijms19041152
Anelli L, Zagaria A, Specchia G, Albano F. The JAK2 GGCC (46/1) Haplotype in Myeloproliferative Neoplasms: Causal or Random? International Journal of Molecular Sciences. 2018; 19(4):1152. https://doi.org/10.3390/ijms19041152
Chicago/Turabian StyleAnelli, Luisa, Antonella Zagaria, Giorgina Specchia, and Francesco Albano. 2018. "The JAK2 GGCC (46/1) Haplotype in Myeloproliferative Neoplasms: Causal or Random?" International Journal of Molecular Sciences 19, no. 4: 1152. https://doi.org/10.3390/ijms19041152
APA StyleAnelli, L., Zagaria, A., Specchia, G., & Albano, F. (2018). The JAK2 GGCC (46/1) Haplotype in Myeloproliferative Neoplasms: Causal or Random? International Journal of Molecular Sciences, 19(4), 1152. https://doi.org/10.3390/ijms19041152