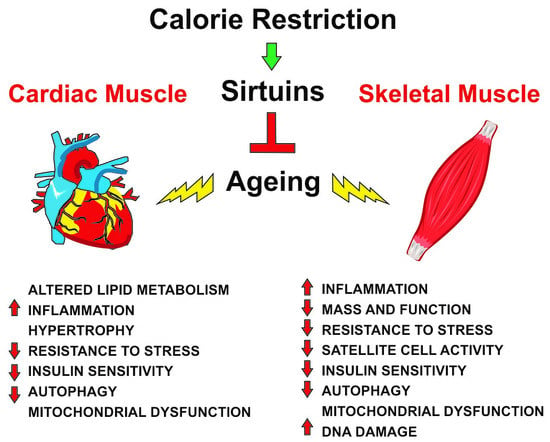
Sirtuins as Mediator of the Anti-Ageing Effects of Calorie Restriction in Skeletal and Cardiac Muscle
Abstract
:1. Introduction
2. Calorie Restriction, Sirtuins, and Ageing
3. CR and Sirtuins in Skeletal Muscle
4. CR and Sirtuins in Cardiac Muscle
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 4E-BP1 | eIF4E-binding protein1 |
| AMPK | AMP-activated protein kinase |
| CR | Calorie restriction |
| eNOS | Endothelial nitric oxide synthase |
| FOXOs | Forkhead box O |
| GLUT-4 | Glucose transporter type 4 |
| HSC | Hematopoietic stem cell |
| I/R | Ischemia/reperfusion |
| IGF-1 | Insulin growth factor type 1 |
| KO | Knockout |
| MEF2 | Myocyte enhancer factor-2 |
| Mn-SOD | Manganese-dependent superoxide dismutase |
| mTOR | Mammalian target of rapamycin |
| MYOD | Myogenic differentiation factor |
| NAMPT | Nicotinamide phosphoribosyltransferase |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| PDK | Pyruvate dehydrogenase kinase |
| PEPCK1 | Phosphoenolpyruvate carboxykinase 1 |
| PGC-1α | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| PPAR | Peroxisome proliferator-activated receptor |
| ROS | Reactive oxygen species |
| S6K1 | P70S6 kinase 1 |
| SIRT | Sirtuin |
| SREBP-1C | Sterol regulatory element-binding protein 1 |
References
- De Nigris, F.; Balestrieri, M.L.; Napoli, C. Targeting C-Myc, Ras and IGF Cascade to treat cancer and Vascular Disorders. Cell Cycle 2006, 5, 1621–1628. [Google Scholar] [CrossRef] [PubMed]
- Keys, A. Coronary Heart Disease in Seven Countries. Circulation 1970, 41, 1–211. [Google Scholar] [CrossRef]
- Mancini, M.; Stamler, J. Diet for preventing cardiovascular diseases: Light from Ancel Keys, Distinguished Centenarian Scientist. Nutr. Metab. Cardiovasc. Dis. 2004, 14, 52–57. [Google Scholar] [CrossRef]
- World Health Organization. Available online: http://www.Who.Int/Mediacentre/Factsheets/Fs317/En/ (accessed on 2 February 2018).
- Keys, A. Mediterranean Diet and Public Health: Personal Reflections. Am. J. Clin. Nutr. 1995, 61 (Suppl. 6), 1321s–1323s. [Google Scholar] [CrossRef] [PubMed]
- Rubba, P.; Mancini, F.P.; Gentile, M.; Mancini, M. The Mediterranean Diet in Italy: An Update. World Rev. Nutr. Diet. 2007, 97, 85–113. [Google Scholar] [PubMed]
- Balestrieri, M.L.; Fiorito, C.; Crimi, E.; Felice, F.; Schiano, C.; Milone, L.; Casamassimi, A.; Giovane, A.; Grimaldi, V.; Del Giudice, V.; et al. Effect of Red Wine Antioxidants and Minor Polyphenolic Constituents on Endothelial Progenitor Cells after Physical Training in Mice. Int. J. Cardiol. 2008, 126, 295–297. [Google Scholar] [CrossRef] [PubMed]
- Sacks, F.M.; Obarzanek, E.; Windhauser, M.M.; Svetkey, L.P.; Vollmer, W.M.; Mccullough, M.; Karanja, N.; Lin, P.H.; Steele, P.; Proschan, M.A. Rationale and Design of the Dietary Approaches to Stop Hypertension Trial (Dash). A Multicenter Controlled-Feeding Study of Dietary Patterns to Lower Blood Pressure. Ann. Epidemiol. 1995, 5, 108–118. [Google Scholar] [CrossRef]
- Fung, T.T.; Hu, F.B.; Wu, K.; Chiuve, S.E.; Fuchs, C.S.; Giovannucci, E. The Mediterranean and Dietary Approaches to Stop Hypertension (Dash) Diets and Colorectal Cancer. Am. J. Clin. Nutr. 2010, 92, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- Salehi-Abargouei, A.; Maghsoudi, Z.; Shirani, F.; Azadbakht, L. Effects of Dietary Approaches to Stop Hypertension (Dash)-Style Diet on Fatal or Nonfatal Cardiovascular Diseases—Incidence: A Systematic Review and Meta-Analysis on Observational Prospective Studies. Nutrition 2013, 29, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.A.; Kendall, C.W.C.; Marchie, A.; Faulkner, D.A.; Wong, J.M.W.; De Souza, R.; Emam, A.; Parker, T.L.; Vidgen, E.; Lapsley, K.G.; et al. Effects of a Dietary Portfolio of Cholesterol-Lowering Foods vs. Lovastatin on Serum Lipids and C-Reactive Protein. JAMA 2003, 290, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Kendall, C.W.C.; Jenkins, D.J.A. A Dietary Portfolio: Maximal Reduction of Low-Density Lipoprotein Cholesterol with Diet. Curr. Atheroscler. Rep. 2004, 6, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, D.A. Toward a Unified Theory of Caloric Restriction and Longevity Regulation. Mech. Ageing Dev. 2005, 126, 987–1002. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Partridge, L. Promoting Health and Longevity Through Diet: From Model Organisms to Humans. Cell 2015, 161, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Mattison, J.A.; Roth, G.S.; Beasley, T.M.; Tilmont, E.M.; Handy, A.M.; Herbert, R.L.; Longo, D.L.; Allison, D.B.; Young, J.E.; Bryant, M.; et al. Impact of Caloric Restriction on Health and Survival in Rhesus Monkeys from the Nia Study. Nature 2012, 489, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Colman, R.J.; Beasley, T.M.; Kemnitz, J.W.; Johnson, S.C.; Weindruch, R.; Anderson, R.M. Caloric Restriction Reduces Age-Related and All-Cause Mortality in Rhesus Monkeys. Nat. Commun. 2014, 5, 3557. [Google Scholar] [CrossRef] [PubMed]
- Trepanowski, J.F.; Canale, R.E.; Marshall, K.E.; Kabir, M.M.; Bloomer, R.J. Impact of Caloric and Dietary Restriction Regimens on Markers of Health and Longevity in Humans and Animals: A Summary of Available Findings. Nutr. J. 2011, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Romey-Glüsing, R.; Li, Y.; Hoffmann, J.; Von Frieling, J.; Knop, M.; Pfefferkorn, R.; Bruchhaus, I.; Fink, C.; Roeder, T. Nutritional Regimens with Periodically Recurring Phases of Dietary Restriction Extend Lifespan in Drosophila. FASEB J. 2018. [Google Scholar] [CrossRef] [PubMed]
- Min, K.; Flatt, T.; Kulaots, I.; Tatar, M. Counting Calories in Drosophila Diet Restriction. Exp. Gerontol. 2007, 42, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Lawler, D.F.; Larson, B.T.; Ballam, J.M.; Smith, G.K.; Biery, D.N.; Evans, R.H.; Greeley, E.H.; Segre, M.; Stowe, H.D.; Kealy, R.D. Diet Restriction and Ageing in the Dog: Major Observations over Two Decades. Br. J. Nutr. 2008, 99, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Comfort, A. Effect of Delayed and Resumed Growth on the Longevity of a Fish (Lebistes reticulatus, Peters) in Captivity. Gerontologia 1963, 49, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Mair, W.; Piper, M.D.W.; Partridge, L. Calories Do Not Explain Extension of Life Span by Dietary Restriction in Drosophila. PLoS Biol. 2005, 3, E223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willcox, D.C.; Willcox, B.J.; Todoriki, H.; Curb, J.D.; Suzuki, M. Caloric Restriction and Human Longevity: What Can We Learn from the Okinawans? Biogerontology 2006, 7, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Kagawa, Y. Impact of Westernization on the Nutrition of Japanese: Changes in Physique, Cancer, Longevity and Centenarians. Prev. Med. (Baltim) 1978, 7, 205–217. [Google Scholar] [CrossRef]
- Feldman, J.L.; Dittenhafer-Reed, K.E.; Denu, J.M. Sirtuin Catalysis and Regulation. J. Biol. Chem. 2012, 287, 42419–42427. [Google Scholar] [CrossRef] [PubMed]
- Bales, C.W.; Kraus, W.E. Caloric Restriction: Implications for Human Cardiometabolic Health. J. Cardiopulm. Rehabil. Prev. 2013, 33, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Mccay, C.M.; Crowell, M.F.; Maynard, L.A. The Effect of Retarded Growth upon the Length of Life Span and upon the Ultimate Body Size. Nutrition 1935, 5, 155–171. [Google Scholar] [CrossRef]
- Ross, M.H. Length of Life and Nutrition in the Rat. J. Nutr. 1961, 75, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.P.; Masoro, E.J.; Murata, I.; Bertrand, H.A.; Lynd, F.T. Life Span Study of SPF Fischer 344 Male Rats Fed ad Libitum or Restricted Diets: Longevity, Growth, Lean Body Mass and Disease. J. Gerontol. 1982, 37, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Weindruch, R.; Walford, R.L.; Fligiel, S.; Guthrie, D. The Retardation of Aging in Mice by Dietary Restriction: Longevity, Cancer, Immunity and Lifetime Energy Intake. J. Nutr. 1986, 116, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Kapahi, P.; Kaeberlein, M.; Hansen, M. Dietary Restriction and Lifespan: Lessons from Invertebrate Models. Ageing Res. Rev. 2017, 39, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Colman, R.J.; Anderson, R.M. Nonhuman Primate Calorie Restriction. Antioxid. Redox Signal. 2011, 14, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Bodkin, N.L.; Alexander, T.M.; Ortmeyer, H.K.; Johnson, E.; Hansen, B.C. Mortality and Morbidity in Laboratory-Maintained Rhesus Monkeys and Effects of Long-Term Dietary Restriction. J. Gerontol. A Biol. Sci. Med. Sci. 2003, 58, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Mattison, J.A.; Colman, R.J.; Beasley, T.M.; Allison, D.B.; Kemnitz, J.W.; Roth, G.S.; Ingram, D.K.; Weindruch, R.; De Cabo, R.; Anderson, R.M. Caloric Restriction Improves Health and Survival of Rhesus Monkeys. Nat. Commun. 2017, 8, 14063. [Google Scholar] [CrossRef] [PubMed]
- Strom, A.; Jensen, R.A. Mortality from Circulatory Diseases in Norway 1940–1945. Lancet (Lond. Engl.) 1951, 1, 126–129. [Google Scholar] [CrossRef]
- Malmros, H. The Relation of Nutrition to Health; A Statistical Study of the Effect of the War-Time on Arteriosclerosis, Cardiosclerosis, Tuberculosis and Diabetes. Acta Med. Scand. Suppl. 1950, 246, 137–153. [Google Scholar] [PubMed]
- Suzuki, M.; Wilcox, B.J.; Wilcox, C.D. Implications from and for Food Cultures for Cardiovascular Disease: Longevity. Asia Pac. J. Clin. Nutr. 2001, 10, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Klein, S. Aging, Adiposity, and Calorie Restriction. JAMA 2007, 297, 986. [Google Scholar] [CrossRef] [PubMed]
- Masoro, E.J.; Yu, B.P.; Bertrand, H.A. Action of Food Restriction in Delaying the Aging Process. Proc. Natl. Acad. Sci. USA 1982, 79, 4239–4241. [Google Scholar] [CrossRef] [PubMed]
- Barger, J.L.; Walford, R.L.; Weindruch, R. The Retardation of Aging By Caloric Restriction: Its Significance in the Transgenic Era. Exp. Gerontol. 2003, 38, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Selman, C.; Phillips, T.; Staib, J.L.; Duncan, J.S.; Leeuwenburgh, C.; Speakman, J.R. Energy Expenditure of Calorically Restricted Rats Is Higher Than Predicted from Their Altered Body Composition. Mech. Ageing Dev. 2005, 126, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Most, J.; Tosti, V.; Redman, L.M.; Fontana, L. Calorie Restriction in Humans: An Update. Ageing Res. Rev. 2017, 39, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Vassilopoulos, A.; Fritz, K.S.; Petersen, D.R.; Gius, D. The Human Sirtuin Family: Evolutionary Divergences and Functions. Hum. Genomics 2011, 5, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Ota, H.; Eto, M.; Kano, M.R.; Kahyo, T.; Setou, M.; Ogawa, S.; Iijima, K.; Akishita, M.; Ouchi, Y. Induction of Endothelial Nitric Oxide Synthase, SIRT1, and Catalase By Statins Inhibits Endothelial Senescence through the Akt Pathway. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2205–2211. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, Y.; Kosuge, Y.; Awaji, H.; Tamura, E.; Takai, A.; Yanai, T.; Yamamoto, R.; Kokame, K.; Miyata, T.; Nakata, R.; et al. Up-Regulation of Endothelial Nitric Oxide Synthase (Enos), Silent Mating Type Information Regulation 2 Homologue 1 (SIRT1) and Autophagy-Related Genes By Repeated Treatments with Resveratrol in Human Umbilical Vein Endothelial Cells. Br. J. Nutr. 2013, 110, 2150–2155. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Strand, S.; Schlufter, F.; Siuda, D.; Reifenberg, G.; Kleinert, H.; Förstermann, U.; Li, H. Role of SIRT1 and Foxo Factors in Enos Transcriptional Activation By Resveratrol. Nitric Oxide 2013, 32, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Vaitheesvaran, B.; Hartil, K.; Robinson, A.J.; Hoopmann, M.R.; Eng, J.K.; Kurland, I.J.; Bruce, J.E. The Fasted/Fed Mouse Metabolic Acetylome: N6-Acetylation Differences Suggest Acetylation Coordinates Organ-Specific Fuel Switching. J. Proteome Res. 2011, 10, 4134–4149. [Google Scholar] [CrossRef] [PubMed]
- López-Lluch, G.; Navas, P. Calorie Restriction as an Intervention in Ageing. J. Physiol. 2016, 594, 2043–2060. [Google Scholar] [CrossRef] [PubMed]
- Selman, C.; Kerrison, N.D.; Cooray, A.; Piper, M.D.W.; Lingard, S.J.; Barton, R.H.; Schuster, E.F.; Blanc, E.; Gems, D.; Nicholson, J.K.; et al. Coordinated Multitissue Transcriptional and Plasma Metabonomic Profiles Following Acute Caloric Restriction in Mice. Physiol. Genomics 2006, 27, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Jové, M.; Naudí, A.; Ramírez-Núñez, O.; Portero-Otín, M.; Selman, C.; Withers, D.J.; Pamplona, R. Caloric Restriction Reveals a Metabolomic and Lipidomic Signature in Liver of Male Mice. Aging Cell 2014, 13, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Vera, E.; Bernardes De Jesus, B.; Foronda, M.; Flores, J.M.; Blasco, M.A. Telomerase Reverse Transcriptase Synergizes with Calorie Restriction to Increase Health Span and Extend Mouse Longevity. PLoS ONE 2013, 8, E53760. [Google Scholar] [CrossRef] [PubMed]
- Makino, N.; Oyama, J.; Maeda, T.; Koyanagi, M.; Higuchi, Y.; Tsuchida, K. Calorie Restriction Increases Telomerase Activity, Enhances Autophagy, and Improves Diastolic Dysfunction in Diabetic Rat Hearts. Mol. Cell. Biochem. 2015, 403, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Makino, N.; Oyama, J.; Maeda, T.; Koyanagi, M.; Higuchi, Y.; Shimokawa, I.; Mori, N.; Furuyama, T. Foxo1 Signaling Plays a Pivotal Role in the Cardiac Telomere Biology Responses to Calorie Restriction. Mol. Cell. Biochem. 2016, 412, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Tomiyama, A.J.; Milush, J.M.; Lin, J.; Flynn, J.M.; Kapahi, P.; Verdin, E.; Sinclair, E.; Melov, S.; Epel, E.S. Long-Term Calorie Restriction in Humans Is Not Associated with Indices of Delayed Immunologic Aging: A Descriptive Study. Nutr. Healthy Aging 2017, 4, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Cuthbertson, D.; Smith, K.; Babraj, J.; Leese, G.; Waddell, T.; Atherton, P.; Wackerhage, H.; Taylor, P.M.; Rennie, M.J. Anabolic Signaling Deficits Underlie Amino Acid Resistance of Wasting, Aging Muscle. FASEB J. 2005, 19, 422–424. [Google Scholar] [CrossRef] [PubMed]
- Weraarchakul, N.; Strong, R.; Wood, W.G.; Richardson, A. The Effect of Aging and Dietary Restriction on DNA Repair. Exp. Cell Res. 1989, 181, 197–204. [Google Scholar] [CrossRef]
- Matt, K.; Burger, K.; Gebhard, D.; Bergemann, J. Influence of Calorie Reduction on DNA Repair Capacity of Human Peripheral Blood Mononuclear Cells. Mech. Ageing Dev. 2016, 154, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Langie, S.A.S.; Knaapen, A.M.; Houben, J.M.J.; Van Kempen, F.C.; De Hoon, J.P.J.; Gottschalk, R.W.H.; Godschalk, R.W.L.; Van Schooten, F.J. The Role of Glutathione in the Regulation of Nucleotide Excision Repair during Oxidative Stress. Toxicol. Lett. 2007, 168, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Heydari, A.R.; Unnikrishnan, A.; Lucente, L.V.; Richardson, A. Caloric Restriction and Genomic Stability. Nucleic Acids Res. 2007, 35, 7485–7496. [Google Scholar] [CrossRef] [PubMed]
- Vermeij, W.P.; Dollé, M.E.T.; Reiling, E.; Jaarsma, D.; Payan-Gomez, C.; Bombardieri, C.R.; Wu, H.; Roks, A.J.M.; Botter, S.M.; Van Der Eerden, B.C.; et al. Restricted Diet Delays Accelerated Ageing and Genomic Stress in DNA-Repair-Deficient Mice. Nature 2016, 537, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Yun, M.H. Changes in Regenerative Capacity through Lifespan. Int. J. Mol. Sci. 2015, 16, 25392–25432. [Google Scholar] [CrossRef] [PubMed]
- Sommese, L.; Zullo, A.; Schiano, C.; Mancini, F.P.; Napoli, C. Possible Muscle Repair in the Human Cardiovascular System. Stem Cell Rev. Rep. 2017, 13, 170–191. [Google Scholar] [CrossRef] [PubMed]
- Nödl, M.-T.; Fossati, S.M.; Domingues, P.; Sánchez, F.J.; Zullo, L. The Making of an Octopus Arm. Evodevo 2015, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Fossati, S.M.; Candiani, S.; Nödl, M.-T.; Maragliano, L.; Pennuto, M.; Domingues, P.; Benfenati, F.; Pestarino, M.; Zullo, L. Identification and Expression of Acetylcholinesterase in Octopus Vulgaris Arm Development and Regeneration: A Conserved Role for Ache? Mol. Neurobiol. 2015, 52, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, V.; Schiano, C.; Casamassimi, A.; Zullo, A.; Soricelli, A.; Mancini, F.P.; Napoli, C. Imaging Techniques to Evaluate Cell Therapy in Peripheral Artery Disease: State of the Art and Clinical Trials. Clin. Physiol. Funct. Imaging 2016, 36, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Brandhorst, S.; Choi, I.Y.; Wei, M.; Cheng, C.W.; Sedrakyan, S.; Navarrete, G.; Dubeau, L.; Yap, L.P.; Park, R.; Vinciguerra, M.; et al. A Periodic Diet that Mimics Fasting Promotes Multi-System Regeneration, Enhanced Cognitive Performance, and Healthspan. Cell Metab. 2015, 22, 86–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, I.Y.; Piccio, L.; Childress, P.; Bollman, B.; Ghosh, A.; Brandhorst, S.; Suarez, J.; Michalsen, A.; Cross, A.H.; Morgan, T.E.; et al. A Diet Mimicking Fasting Promotes Regeneration and Reduces Autoimmunity and Multiple Sclerosis Symptoms. Cell Rep. 2016, 15, 2136–2146. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.; Thuret, S. The Systemic Milieu as a mediator of dietary influence on stem cell function during ageing. Ageing Res. Rev. 2015, 19, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Tao, S.; Chen, Z.; Koliesnik, I.O.; Calmes, P.G.; Hoerr, V.; Han, B.; Gebert, N.; Zörnig, M.; Löffler, B.; et al. Dietary Restriction Improves Repopulation But Impairs Lymphoid Differentiation Capacity of Hematopoietic Stem Cells in Early Aging. J. Exp. Med. 2016, 213, 535–553. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Glass, Z.; Sayed, K.; Michurina, T.V.; Lazutkin, A.; Mineyeva, O.; Velmeshev, D.; Ward, W.F.; Richardson, A.; Enikolopov, G. Calorie Restriction Alleviates the Age-Related Decrease in Neural Progenitor Cell Division in the Aging Brain. Eur. J. Neurosci. 2013, 37, 1987–1993. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, Ö.H.; Katajisto, P.; Lamming, D.W.; Gültekin, Y.; Bauer-Rowe, K.E.; Sengupta, S.; Birsoy, K.; Dursun, A.; Yilmaz, V.O.; Selig, M.; et al. mTORC1 in the Paneth Cell Niche Couples Intestinal Stem-Cell Function to Calorie Intake. Nature 2012, 486, 490–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerletti, M.; Jang, Y.C.; Finley, L.W.S.; Haigis, M.C.; Wagers, A.J. Short-Term Calorie Restriction Enhances Skeletal Muscle Stem Cell Function. Cell Stem Cell 2012, 10, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Mazzoccoli, G.; Tevy, M.F.; Borghesan, M.; Vergini, M.R.D.; Vinciguerra, M. Caloric Restriction and Aging Stem Cells: The Stick and the Carrot? Exp. Gerontol. 2014, 50, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Klimova, B.; Novotny, M.; Kuca, K. Anti-Aging Drugs–Prospect of Longer Life? Curr. Med. Chem. 2017, 25. [Google Scholar] [CrossRef] [PubMed]
- Anisimov, V.N.; Berstein, L.M.; Popovich, I.G.; Zabezhinski, M.A.; Egormin, P.A.; Piskunova, T.S.; Semenchenko, A.V.; Tyndyk, M.L.; Yurova, M.N.; Kovalenko, I.G.; et al. If Started Early in Life, Metformin Treatment Increases Life Span and Postpones Tumors in Female SHR Mice. Aging 2011, 3, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Cabreiro, F.; Au, C.; Leung, K.-Y.; Vergara-Irigaray, N.; Cochemé, H.M.; Noori, T.; Weinkove, D.; Schuster, E.; Greene, N.D.E.; Gems, D. Metformin Retards Aging in C. elegans By Altering Microbial Folate and Methionine Metabolism. Cell 2013, 153, 228–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Haes, W.; Frooninckx, L.; Van Assche, R.; Smolders, A.; Depuydt, G.; Billen, J.; Braeckman, B.P.; Schoofs, L.; Temmerman, L. Metformin Promotes Lifespan through Mitohormesis via the Peroxiredoxin PRDX-2. Proc. Natl. Acad. Sci. USA 2014, 111, E2501-9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allard, J.S.; Perez, E.J.; Fukui, K.; Carpenter, P.; Ingram, D.K.; De Cabo, R. Prolonged Metformin Treatment Leads to Reduced Transcription of Nrf2 and Neurotrophic Factors without Cognitive Impairment in Older C57BL/6J Mice. Behav. Brain Res. 2016, 301, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Onken, B.; Driscoll, M. Metformin Induces a Dietary Restriction–Like State and the Oxidative Stress Response to Extend C. elegans Healthspan via AMPK, LKB1, and SKN-1. PLoS ONE 2010, 5, E8758. [Google Scholar] [CrossRef] [PubMed]
- Martin-Montalvo, A.; Mercken, E.M.; Mitchell, S.J.; Palacios, H.H.; Mote, P.L.; Scheibye-Knudsen, M.; Gomes, A.P.; Ward, T.M.; Minor, R.K.; Blouin, M.-J.; et al. Metformin Improves Healthspan and Lifespan in Mice. Nat. Commun. 2013, 4, 2192. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-R.; Li, S.; Lin, C.-C. Effect of Resveratrol and Pterostilbene on Aging and Longevity. Biofactors 2018, 44, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Tung, B.T.; Rodriguez-Bies, E.; Thanh, H.N.; Le-Thi-Thu, H.; Navas, P.; Sanchez, V.M.; López-Lluch, G. Organ and Tissue-Dependent Effect of Resveratrol and Exercise on Antioxidant Defenses of Old Mice. Aging Clin. Exp. Res. 2015, 27, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Marchal, J.; Blanc, S.; Epelbaum, J.; Aujard, F.; Pifferi, F. Effects of Chronic Calorie Restriction or Dietary Resveratrol Supplementation on Insulin Sensitivity Markers in a Primate, Microcebus murinus. PLoS ONE 2012, 7, E34289. [Google Scholar] [CrossRef] [PubMed]
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.H.; Van De Weijer, T.; Goossens, G.H.; Hoeks, J.; Van Der Krieken, S.; Ryu, D.; Kersten, S.; et al. Calorie Restriction-Like Effects of 30 Days of Resveratrol Supplementation on Energy Metabolism and Metabolic Profile in Obese Humans. Cell Metab. 2011, 14, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Demontis, F.; Piccirillo, R.; Goldberg, A.L.; Perrimon, N. Mechanisms of Skeletal Muscle Aging: Insights from Drosophila and Mammalian Models. Dis. Model. Mech. 2013, 6, 1339–1352. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.-M.; Yu, M.-X.; Shen, Z.-Y.; Guo, C.-Y.; Zhuang, S.-Q.; Qiu, X.-S. Leucine Promotes Proliferation and Differentiation of Primary Preterm Rat Satellite Cells in Part through mTORC1 Signaling Pathway. Nutrients 2015, 7, 3387–3400. [Google Scholar] [CrossRef] [PubMed]
- Yue, T.; Yin, J.; Li, F.; Li, D.; Du, M. High Glucose Induces Differentiation and Adipogenesis in Porcine Muscle Satellite Cells via mTOR. BMB Rep. 2010, 43, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Aspnes, L.E.; Lee, C.M.; Weindruch, R.; Chung, S.S.; Roecker, E.B.; Aiken, J.M. Caloric Restriction Reduces Fiber Loss and Mitochondrial Abnormalities in Aged Rat Muscle. FASEB J. 1997, 11, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Phillips, T.; Leeuwenburgh, C. Muscle Fiber Specific Apoptosis and TNF-Alpha Signaling in Sarcopenia Are Attenuated By Life-Long Calorie Restriction. FASEB J. 2005, 19, 668–670. [Google Scholar] [CrossRef] [PubMed]
- Hepple, R.T.; Qin, M.; Nakamoto, H.; Goto, S. Caloric Restriction Optimizes the Proteasome Pathway with Aging in Rat Plantaris Muscle: Implications for Sarcopenia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R1231–R1237. [Google Scholar] [CrossRef] [PubMed]
- Mckiernan, S.H.; Colman, R.J.; Lopez, M.; Beasley, T.M.; Aiken, J.M.; Anderson, R.M.; Weindruch, R. Caloric Restriction Delays Aging-Induced Cellular Phenotypes in Rhesus Monkey Skeletal Muscle. Exp. Gerontol. 2011, 46, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Inoki, K.; Zhu, T.; Guan, K.-L. TSC2 Mediates Cellular Energy Response to Control Cell Growth and Survival. Cell 2003, 115, 577–590. [Google Scholar] [CrossRef]
- Lee, C.K.; Klopp, R.G.; Weindruch, R.; Prolla, T.A. Gene Expression Profile of Aging and Its Retardation by Caloric Restriction. Science 1999, 285, 1390–1393. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Bies, E.; Navas, P.; López-Lluch, G. Age-Dependent Effect of Every-Other-Day Feeding and Aerobic Exercise in Ubiquinone Levels and Related Antioxidant Activities in Mice Muscle. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Boldrin, L.; Ross, J.A.; Whitmore, C.; Doreste, B.; Beaver, C.; Eddaoudi, A.; Pearce, D.J.; Morgan, J.E. The Effect of Calorie Restriction on Mouse Skeletal Muscle Is Sex, Strain and Time-Dependent. Sci. Rep. 2017, 7, 5160. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.Y.; Lee, E.K.; Choi, Y.J.; Kim, J.M.; Kim, D.H.; Zou, Y.; Kim, C.H.; Lee, J.; Kim, H.S.; Kim, N.D.; et al. Molecular Inflammation as an Underlying Mechanism of the Aging Process and Age-Related Diseases. J. Dent. Res. 2011, 90, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Horrillo, D.; Sierra, J.; Arribas, C.; García-San Frutos, M.; Carrascosa, J.M.; Lauzurica, N.; Fernández-Agulló, T.; Ros, M. Age-Associated Development of Inflammation in Wistar Rats: Effects of Caloric Restriction. Arch. Physiol. Biochem. 2011, 117, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Wåhlin-Larsson, B.; Carnac, G.; Kadi, F. The Influence of Systemic Inflammation on Skeletal Muscle in Physically Active Elderly Women. Age (Dordr.) 2014, 36, 9718. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Villareal, D.T.; Das, S.K.; Smith, S.R.; Meydani, S.N.; Pittas, A.G.; Klein, S.; Bhapkar, M.; Rochon, J.; Ravussin, E.; et al. Effects of 2-Year Calorie Restriction on Circulating Levels of IGF-1, IGF-Binding Proteins and Cortisol in Nonobese Men and Women: A Randomized Clinical Trial. Aging Cell 2016, 15, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-J.; Gavrilova, O.; Brown, A.L.; Soto, J.E.; Bremner, S.; Kim, J.; Xu, X.; Yang, S.; Um, J.-H.; Koch, L.G.; et al. DNA-PK Promotes The Mitochondrial, Metabolic, and Physical Decline that Occurs during Aging. Cell Metab. 2017, 25, 1135–1146.E7. [Google Scholar] [CrossRef] [PubMed]
- Hord, J.M.; Botchlett, R.; Lawler, J.M. Age-Related Alterations in the Sarcolemmal Environment Are Attenuated by Lifelong Caloric Restriction and Voluntary Exercise. Exp. Gerontol. 2016, 83, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sharma, N.; Arias, E.B.; Cartee, G.D. Insulin Signaling and Glucose Uptake in the Soleus Muscle of 30-Month-Old Rats after Calorie Restriction with or without Acute Exercise. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2016, 71, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Arias, E.B.; Yu, C.S.; Verkerke, A.R.P.; Cartee, G.D. Effects of Calorie Restriction and Fiber Type on Glucose Uptake and Abundance of Electron Transport Chain and Oxidative Phosphorylation Proteins in Single Fibers from Old Rats. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1638–1646. [Google Scholar] [CrossRef] [PubMed]
- Ohanna, M.; Sobering, A.K.; Lapointe, T.; Lorenzo, L.; Praud, C.; Petroulakis, E.; Sonenberg, N.; Kelly, P.A.; Sotiropoulos, A.; Pende, M. Atrophy of S6k1(-/-) Skeletal Muscle Cells Reveals Distinct mTOR Effectors for Cell Cycle and Size Control. Nat. Cell Biol. 2005, 7, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Risson, V.; Mazelin, L.; Roceri, M.; Sanchez, H.; Moncollin, V.; Corneloup, C.; Richard-Bulteau, H.; Vignaud, A.; Baas, D.; Defour, A.; et al. Muscle Inactivation of mTOR Causes Metabolic and Dystrophin Defects Leading to Severe Myopathy. J. Cell Biol. 2009, 187, 859–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mounier, R.; Lantier, L.; Leclerc, J.; Sotiropoulos, A.; Foretz, M.; Viollet, B. Antagonistic Control of Muscle Cell Size by AMPK and mTORC1. Cell Cycle 2011, 10, 2640–2646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terada, N.; Patel, H.R.; Takase, K.; Kohno, K.; Nairn, A.C.; Gelfand, E.W. Rapamycin Selectively Inhibits Translation of mRNAs Encoding Elongation Factors and Ribosomal Proteins. Proc. Natl. Acad. Sci. USA 1994, 91, 11477–11481. [Google Scholar] [CrossRef] [PubMed]
- Welsh, G.I.; Stokes, C.M.; Wang, X.; Sakaue, H.; Ogawa, W.; Kasuga, M.; Proud, C.G. Activation of Translation Initiation Factor eIF2B By Insulin Requires Phosphatidyl Inositol 3-Kinase. FEBS Lett. 1997, 410, 418–422. [Google Scholar] [CrossRef]
- Pallafacchina, G.; Calabria, E.; Serrano, A.L.; Kalhovde, J.M.; Schiaffino, S. A Protein Kinase B-Dependent and Rapamycin-Sensitive Pathway Controls Skeletal Muscle Growth But Not Fiber Type Specification. Proc. Natl. Acad. Sci. USA 2002, 99, 9213–9218. [Google Scholar] [CrossRef] [PubMed]
- Léger, B.; Derave, W.; De Bock, K.; Hespel, P.; Russell, A.P. Human Sarcopenia Reveals an Increase in SOCS-3 and Myostatin and a Reduced Efficiency of AKT Phosphorylation. Rejuvenation Res. 2008, 11, 163–175b. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharples, A.P.; Hughes, D.C.; Deane, C.S.; Saini, A.; Selman, C.; Stewart, C.E. Longevity and Skeletal Muscle Mass: The Role of IGF Signalling, the Sirtuins, Dietary Restriction and Protein Intake. Aging Cell 2015, 14, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Pardo, P.S.; Mohamed, J.S.; Lopez, M.A.; Boriek, A.M. Induction of SIRT1 by Mechanical Stretch of Skeletal Muscle through the Early Response Factor EGR1 Triggers an Antioxidative Response. J. Biol. Chem. 2011, 286, 2559–2566. [Google Scholar] [CrossRef] [PubMed]
- Schenk, S.; Mccurdy, C.E.; Philp, A.; Chen, M.Z.; Holliday, M.J.; Bandyopadhyay, G.K.; Osborn, O.; Baar, K.; Olefsky, J.M. SIRT1 Enhances Skeletal Muscle Insulin Sensitivity in Mice during Caloric Restriction. J. Clin. Investig. 2011, 121, 4281–4288. [Google Scholar] [CrossRef] [PubMed]
- Fulco, M.; Schiltz, R.L.; Iezzi, S.; King, M.T.; Zhao, P.; Kashiwaya, Y.; Hoffman, E.; Veech, R.L.; Sartorelli, V. SIR2 Regulates Skeletal Muscle Differentiation as a Potential Sensor of the Redox State. Mol. Cell 2003, 12, 51–62. [Google Scholar] [CrossRef]
- Fulco, M.; Cen, Y.; Zhao, P.; Hoffman, E.P.; Mcburney, M.W.; Sauve, A.A.; Sartorelli, V. Glucose Restriction Inhibits Skeletal Myoblast Differentiation by Activating SIRT1 Through AMPK-Mediated Regulation of Nampt. Dev. Cell 2008, 14, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Gerhart-Hines, Z.; Rodgers, J.T.; Bare, O.; Lerin, C.; Kim, S.-H.; Mostoslavsky, R.; Alt, F.W.; Wu, Z.; Puigserver, P. Metabolic Control of Muscle Mitochondrial Function and Fatty Acid Oxidation through SIRT1/PGC-1alpha. Embo J. 2007, 26, 1913–1923. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, S.; Fergusson, M.M.; Finkel, T. Nutrient Availability Regulates SIRT1 through a forkhead-Dependent Pathway. Science 2004, 306, 2105–2108. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, S.; Fergusson, M.M.; Finkel, T. SIRT1 Functionally Interacts with the Metabolic Regulator and Transcriptional Coactivator PGC-1{Alpha}. J. Biol. Chem. 2005, 280, 16456–16460. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, J.T.; Lerin, C.; Haas, W.; Gygi, S.P.; Spiegelman, B.M.; Puigserver, P. Nutrient Control of Glucose Homeostasis through a Complex of PGC-1α and SIRT1. Nature 2005, 434, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Kume, S.; Haneda, M.; Kanasaki, K.; Sugimoto, T.; Araki, S.; Isshiki, K.; Isono, M.; Uzu, T.; Guarente, L.; Kashiwagi, A.; et al. SIRT1 Inhibits Transforming Growth Factor Beta-Induced Apoptosis in Glomerular Mesangial Cells Via Smad7 Deacetylation. J. Biol. Chem. 2007, 282, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Defour, A.; Dessalle, K.; Castro Perez, A.; Poyot, T.; Castells, J.; Gallot, Y.S.; Durand, C.; Euthine, V.; Gu, Y.; Béchet, D.; et al. Sirtuin 1 Regulates SREBP-1c Expression in a LXR-Dependent Manner in Skeletal Muscle. PLoS ONE 2012, 7, E43490. [Google Scholar] [CrossRef] [PubMed]
- Glass, D.J. A Signaling Role for Dystrophin: Inhibiting Skeletal Muscle Atrophy Pathways. Cancer Cell 2005, 8, 351–352. [Google Scholar] [CrossRef] [PubMed]
- Crosio, C.; Cermakian, N.; Allis, C.D.; Sassone-Corsi, P. Light Induces Chromatin Modification in Cells of the Mammalian Circadian Clock. Nat. Neurosci. 2000, 3, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Lees, S.J.; Rathbone, C.R.; Booth, F.W. Age-Associated Decrease in Muscle Precursor Cell Differentiation. Am. J. Physiol. Physiol. 2006, 290, C609–C615. [Google Scholar] [CrossRef] [PubMed]
- Machida, S.; Booth, F.W. Increased Nuclear Proteins in Muscle Satellite Cells in Aged Animals As Compared to Young Growing Animals. Exp. Gerontol. 2004, 39, 1521–1525. [Google Scholar] [CrossRef] [PubMed]
- Pardo, P.S.; Boriek, A.M. The Physiological Roles of SIRT1 in Skeletal Muscle. Aging (Albany NY) 2011, 3, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Brunet, A.; Sweeney, L.B.; Sturgill, J.F.; Chua, K.F.; Greer, P.L.; Lin, Y.; Tran, H.; Ross, S.E.; Mostoslavsky, R.; Cohen, H.Y.; et al. Stress-Dependent Regulation of Foxo Transcription Factors By the SIRT1 Deacetylase. Science 2004, 303, 2011–2015. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, H.S.; Mcburney, M.; Robbins, P.D. SIRT1 Negatively Regulates the Mammalian Target of Rapamycin. PLoS ONE 2010, 5, E9199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samant, S.A.; Kanwal, A.; Pillai, V.B.; Bao, R.; Gupta, M.P. The Histone Deacetylase SIRT6 Blocks Myostatin Expression and Development of Muscle Atrophy. Sci. Rep. 2017, 7, 11877. [Google Scholar] [CrossRef] [PubMed]
- Yeung, F.; Hoberg, J.E.; Ramsey, C.S.; Keller, M.D.; Jones, D.R.; Frye, R.A.; Mayo, M.W. Modulation of NF-KappaB-Dependent Transcription and Cell Survival by the SIRT1 Deacetylase. EMBO J. 2004, 23, 2369–2380. [Google Scholar] [CrossRef] [PubMed]
- Kanfi, Y.; Naiman, S.; Amir, G.; Peshti, V.; Zinman, G.; Nahum, L.; Bar-Joseph, Z.; Cohen, H.Y. The Sirtuin SIRT6 Regulates Lifespan in Male Mice. Nature 2012, 483, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Tilstra, J.S.; Robinson, A.R.; Wang, J.; Gregg, S.Q.; Clauson, C.L.; Reay, D.P.; Nasto, L.A.; St Croix, C.M.; Usas, A.; Vo, N.; et al. NF-ΚB Inhibition Delays DNA Damage-Induced Senescence and Aging in Mice. J. Clin. Investig. 2012, 122, 2601–2612. [Google Scholar] [CrossRef] [PubMed]
- Belman, J.P.; Bian, R.R.; Habtemichael, E.N.; Li, D.T.; Jurczak, M.J.; Alcázar-Román, A.; Mcnally, L.J.; Shulman, G.I.; Bogan, J.S. Acetylation of Tug Protein Promotes the Accumulation of GLUT4 Glucose Transporters in an Insulin-Responsive Intracellular Compartment. J. Biol. Chem. 2015, 290, 4447–4463. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.; Dey, C.S. SIRT2 Negatively Regulates Insulin Resistance in C2C12 Skeletal Muscle Cells. Biochim. Biophys. Acta 2014, 1842, 1372–1378. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Lei, Q.-Y.; Zhao, S.; Guan, K.-L. Regulation of Glycolysis and Gluconeogenesis by Acetylation of PKM and PEPCK. Cold Spring Harb. Symp. Quant. Biol. 2011, 76, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Palacios, O.M.; Carmona, J.J.; Michan, S.; Chen, K.Y.; Manabe, Y.; Ward, J.L.; Goodyear, L.J.; Tong, Q. Diet and Exercise Signals Regulate SIRT3 and Activate AMPK and PGC-1alpha in Skeletal Muscle. Aging (Albany NY) 2009, 1, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Seufert, C.D.; Graf, M.; Janson, G.; Kuhn, A.; Söling, H.D. Formation of Free Acetate by Isolated Perfused Livers from Normal, Starved and Diabetic Rats. Biochem. Biophys. Res. Commun. 1974, 57, 901–909. [Google Scholar] [CrossRef]
- Hallows, W.C.; Lee, S.; Denu, J.M. Sirtuins Deacetylate and Activate Mammalian Acetyl-Coa Synthetases. Proc. Natl. Acad. Sci. USA 2006, 103, 10230–10235. [Google Scholar] [CrossRef] [PubMed]
- Schwer, B.; Bunkenborg, J.; Verdin, R.O.; Andersen, J.S.; Verdin, E. Reversible Lysine Acetylation Controls the Activity of the Mitochondrial Enzyme Acetyl-Coa Synthetase 2. Proc. Natl. Acad. Sci. USA 2006, 103, 10224–10229. [Google Scholar] [CrossRef] [PubMed]
- Falcón, A.A.; Chen, S.; Wood, M.S.; Aris, J.P. Acetyl-Coenzyme a Synthetase 2 Is a Nuclear Protein Required for Replicative Longevity In Saccharomyces Cerevisiae. Mol. Cell. Biochem. 2010, 333, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Dittenhafer-Reed, K.E.; Richards, A.L.; Fan, J.; Smallegan, M.J.; Fotuhi Siahpirani, A.; Kemmerer, Z.A.; Prolla, T.A.; Roy, S.; Coon, J.J.; Denu, J.M. SIRT3 Mediates Multi-Tissue Coupling for Metabolic Fuel Switching. Cell Metab. 2015, 21, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Cantó, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK Regulates Energy Expenditure By Modulating Nad+ Metabolism and SIRT1 Activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef] [PubMed]
- Wronska, A.; Lawniczak, A.; Wierzbicki, P.M.; Kmiec, Z. Age-Related Changes in Sirtuin 7 Expression in Calorie-Restricted and Refed Rats. Gerontology 2016, 62, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Margolis, L.M.; Rivas, D.A.; Berrone, M.; Ezzyat, Y.; Young, A.J.; Mcclung, J.P.; Fielding, R.A.; Pasiakos, S.M. Prolonged Calorie Restriction Downregulates Skeletal Muscle mTORC1 Signaling Independent of Dietary Protein Intake and Associated Microrna Expression. Front. Physiol. 2016, 7, 445. [Google Scholar] [CrossRef] [PubMed]
- Treviño-Saldaña, N.; García-Rivas, G. Regulation of Sirtuin-Mediated Protein Deacetylation by Cardioprotective Phytochemicals. Oxid. Med. Cell. Longev. 2017, 2017, 1750306. [Google Scholar] [CrossRef] [PubMed]
- Winnik, S.; Auwerx, J.; Sinclair, D.A.; Matter, C.M. Protective Effects of Sirtuins in Cardiovascular Diseases: From Bench to Bedside. Eur. Heart J. 2015, 36, 3404–3412. [Google Scholar] [CrossRef] [PubMed]
- Spadari, R.C.; Cavadas, C.; De Carvalho, A.E.T.S.; Ortolani, D.; De Moura, A.L.; Vassalo, P.F. Role of Beta-Adrenergic Receptors and Sirtuin Signaling in the Heart During Aging, Heart Failure, and Adaptation to Stress. Cell. Mol. Neurobiol. 2018, 38, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, S.; Sadoshima, J. The Role of Sirtuins in Cardiac Disease. Am. J. Physiol. Circ. Physiol. 2015, 309, H1375–H1389. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Zhou, H.-F.; Lin, R.-B.; Fu, Y.-C.; Wang, W. Short-Term Calorie Restriction Activates SIRT1-4 and -7 in Cardiomyocytes In Vivo and In Vitro. Mol. Med. Rep. 2014, 9, 1218–1224. [Google Scholar] [CrossRef] [PubMed]
- Matsui, Y.; Takagi, H.; Qu, X.; Abdellatif, M.; Sakoda, H.; Asano, T.; Levine, B.; Sadoshima, J. Distinct Roles of Autophagy in the Heart during Ischemia and Reperfusion: Roles of AMP-Activated Protein Kinase and Beclin 1 In Mediating Autophagy. Circ. Res. 2007, 100, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, N.; Zhai, P.; Sadoshima, J. Oxidative Stress Stimulates Autophagic Flux during Ischemia/Reperfusion. Antioxid. Redox Signal. 2011, 14, 2179–2190. [Google Scholar] [CrossRef] [PubMed]
- Dolinsky, V.W.; Dyck, J.R.B. Calorie Restriction and Resveratrol in Cardiovascular Health and Disease. Biochim. Biophys. Acta Mol. Basis Dis. 2011, 1812, 1477–1489. [Google Scholar] [CrossRef] [PubMed]
- Fujitsuka, N.; Asakawa, A.; Morinaga, A.; Amitani, M.S.; Amitani, H.; Katsuura, G.; Sawada, Y.; Sudo, Y.; Uezono, Y.; Mochiki, E.; et al. Increased Ghrelin Signaling Prolongs Survival in Mouse Models of Human Aging through Activation of Sirtuin1. Mol. Psychiatry 2016, 21, 1613–1623. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Tamaki, K.; Shirakawa, K.; Ito, K.; Yan, X.; Katsumata, Y.; Anzai, A.; Matsuhashi, T.; Endo, J.; Inaba, T.; et al. Cardiac SIRT1 Mediates the Cardioprotective Effect of Caloric Restriction By Suppressing Local Complement System Activation after Ischemia-Reperfusion. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H1003-14. [Google Scholar] [CrossRef] [PubMed]
- Shinmura, K.; Tamaki, K.; Bolli, R. Impact of 6-Mo Caloric Restriction on Myocardial Ischemic tolerance: Possible Involvement of Nitric Oxide-Dependent Increase in Nuclear SIRT1. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H2348–H2355. [Google Scholar] [CrossRef] [PubMed]
- De Cavanagh, E.M.V.; Inserra, F.; Ferder, L. Angiotensin Ii Blockade: How Its Molecular Targets May Signal to Mitochondria and Slow Aging. Coincidences with Calorie Restriction and mTOR Inhibition. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H15–H44. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Lv, S.; Huang, M.; Lv, Y.; Yu, J.; Liu, J.; Tang, T.; Qi, H.; Di, W.; Ding, G. Opposing Effects on Cardiac Function by Calorie Restriction in Different-Aged Mice. Aging Cell 2017, 16, 1155–1167. [Google Scholar] [CrossRef] [PubMed]
- Booth, S.A.; Charchar, F.J. Cardiac Telomere Length in Heart Development, Function, and Disease. Physiol. Genomics 2017, 49, 368–384. [Google Scholar] [CrossRef] [PubMed]
- Zannolli, R.; Mohn, A.; Buoni, S.; Pietrobelli, A.; Messina, M.; Chiarelli, F.; Miracco, C. Telomere Length and Obesity. Acta Paediatr. 2008, 97, 952–954. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Fu, W.; Chen, J.; Olashaw, N.; Zhang, X.; Nicosia, S.V.; Bhalla, K.; Bai, W. SIRT1 Sumoylation Regulates Its Deacetylase Activity and Cellular Response to Genotoxic Stress. Nat. Cell Biol. 2007, 9, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Conrad, E.; Polonio-Vallon, T.; Meister, M.; Matt, S.; Bitomsky, N.; Herbel, C.; Liebl, M.; Greiner, V.; Kriznik, B.; Schumacher, S.; et al. HIPK2 Restricts SIRT1 Activity Upon Severe DNA Damage By A Phosphorylation-Controlled Mechanism. Cell Death Differ. 2016, 23, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Alcendor, R.R.; Gao, S.; Zhai, P.; Zablocki, D.; Holle, E.; Yu, X.; Tian, B.; Wagner, T.; Vatner, S.F.; Sadoshima, J. SIRT1 Regulates Aging and Resistance to Oxidative Stress in the Heart. Circ. Res. 2007, 100, 1512–1521. [Google Scholar] [CrossRef] [PubMed]
- Bindu, S.; Pillai, V.B.; Gupta, M.P. Role of Sirtuins in Regulating Pathophysiology of the Heart. Trends Endocrinol. Metab. 2016, 27, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-P.; Zhai, P.; Yamamoto, T.; Maejima, Y.; Matsushima, S.; Hariharan, N.; Shao, D.; Takagi, H.; Oka, S.; Sadoshima, J. Silent Information Regulator 1 Protects the Heart from Ischemia/Reperfusion. Circulation 2010, 122, 2170–2182. [Google Scholar] [CrossRef] [PubMed]
- Sundaresan, N.R.; Pillai, V.B.; Wolfgeher, D.; Samant, S.; Vasudevan, P.; Parekh, V.; Raghuraman, H.; Cunningham, J.M.; Gupta, M.P.M.; Gupta, M.P.M. The Deacetylase SIRT1 Promotes Membrane Localization and Activation of AKT and PDK1 during Tumorigenesis and Cardiac Hypertrophy. Sci. Signal. 2011, 4, Ra46. [Google Scholar] [CrossRef] [PubMed]
- Oka, S.; Alcendor, R.; Zhai, P.; Park, J.Y.; Shao, D.; Cho, J.; Yamamoto, T.; Tian, B.; Sadoshima, J. PPARα-SIRT1 Complex Mediates Cardiac Hypertrophy and Failure through Suppression of the ERR Transcriptional Pathway. Cell Metab. 2011, 14, 598–611. [Google Scholar] [CrossRef] [PubMed]
- Sundaresan, N.R.; Gupta, M.; Kim, G.; Rajamohan, S.B.; Isbatan, A.; Gupta, M.P. SIRT3 Blocks the Cardiac Hypertrophic Response by Augmenting Foxo3a-Dependent Antioxidant Defense Mechanisms in Mice. J. Clin. Investig. 2009, 119, 2758–2771. [Google Scholar] [CrossRef] [PubMed]
- Sadoshima, J. SIRT3 Targets mPTP and Prevents Aging in the Heart. Aging (Albany NY) 2011, 3, 12–13. [Google Scholar] [CrossRef] [PubMed]
- Pillai, V.B.; Sundaresan, N.R.; Kim, G.; Gupta, M.; Rajamohan, S.B.; Pillai, J.B.; Samant, S.; Ravindra, P.V.; Isbatan, A.; Gupta, M.P. Exogenous Nad Blocks Cardiac Hypertrophic Response via Activation of the SIRT3-LKB1-AMP-Activated Kinase Pathway. J. Biol. Chem. 2010, 285, 3133–3144. [Google Scholar] [CrossRef] [PubMed]
- Sundaresan, N.R.; Bindu, S.; Pillai, V.B.; Samant, S.; Pan, Y.; Huang, J.-Y.; Gupta, M.; Nagalingam, R.S.; Wolfgeher, D.; Verdin, E.; et al. Sirt3 Blocks Aging-Associated Tissue Fibrosis in Mice by Deacetylating and Activating Glycogen Synthase Kinase 3β. Mol. Cell. Biol. 2015, 36, 678–692. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Che, W.; Xue, J.; Zheng, C.; Tang, K.; Zhang, J.; Wen, J.; Xu, Y. SIRT4 Prevents Hypoxia-Induced Apoptosis in H9C2 Cardiomyoblast Cells. Cell. Physiol. Biochem. 2013, 32, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Che, W.; Zheng, C.; Liu, W.; Wen, J.; Fu, H.; Tang, K.; Zhang, J.; Xu, Y. SIRT5: A Safeguard against Oxidative Stress-Induced Apoptosis in Cardiomyocytes. Cell. Physiol. Biochem. 2013, 32, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Rardin, M.J.; He, W.; Nishida, Y.; Newman, J.C.; Carrico, C.; Danielson, S.R.; Guo, A.; Gut, P.; Sahu, A.K.; Li, B.; et al. SIRT5 Regulates the Mitochondrial Lysine Succinylome and Metabolic Networks. Cell Metab. 2013, 18, 920–933. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Chen, X.-F.; Chen, H.-Z.; Liu, D.-P. Mitochondrial Sirtuins in Cardiometabolic Diseases. Clin. Sci. 2017, 131, 2063–2078. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, M.; Zullo, A.; Servillo, L.; Mancini, F.P.; Borriello, A.; Giovane, A.; Della Ragione, F.; D’onofrio, N.; Balestrieri, M.L. Multiple Pathways of SIRT6 at the Crossroads in the Control of Longevity, Cancer, and Cardiovascular Diseases. Ageing Res. Rev. 2017, 35, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Khan, D.; Sarikhani, M.; Maniyadath, B.; Pandit, A.S.; Mishra, S.; Ahamed, F.; Dubey, A.; Fathma, N.; Atreya, H.S.; Kolthur-Seetharam, U.; et al. SIRT6 Deacetylase Transcriptionally Regulates Glucose Metabolism in Heart. J. Cell. Physiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Vakhrusheva, O.; Smolka, C.; Gajawada, P.; Kostin, S.; Boettger, T.; Kubin, T.; Braun, T.; Bober, E. SIRT7 Increases Stress Resistance of Cardiomyocytes and Prevents Apoptosis and Inflammatory Cardiomyopathy in Mice. Circ. Res. 2008, 102, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Baird, M.F.; Grace, F.; Sculthorpe, N.; Graham, S.M.; Fleming, A.; Baker, J.S. Evidence of Direct Cardiac Damage Following High-Intensity Exercise in Chronic Energy Restriction: A Case Report and Literature Review. Medicine (Baltimore) 2017, 96, E7030. [Google Scholar] [CrossRef] [PubMed]

| Skeletal Muscle | Cardiac Muscle |
|---|---|
| ↑ Mass and function | Altered lipid metabolism |
| ↑ Satellite cell activity | Hypertrophy |
| ↓ Inflammation | ↓ Inflammation |
| ↑ Resistance to stress | ↑ Resistance to stress |
| ↑ Insulin sensitivity | ↑ Insulin sensitivity |
| ↑ Autophagy | ↑ Autophagy |
| Mitochondrial dysfunction | Mitochondrial dysfunction |
| ↓ DNA damage |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zullo, A.; Simone, E.; Grimaldi, M.; Musto, V.; Mancini, F.P. Sirtuins as Mediator of the Anti-Ageing Effects of Calorie Restriction in Skeletal and Cardiac Muscle. Int. J. Mol. Sci. 2018, 19, 928. https://doi.org/10.3390/ijms19040928
Zullo A, Simone E, Grimaldi M, Musto V, Mancini FP. Sirtuins as Mediator of the Anti-Ageing Effects of Calorie Restriction in Skeletal and Cardiac Muscle. International Journal of Molecular Sciences. 2018; 19(4):928. https://doi.org/10.3390/ijms19040928
Chicago/Turabian StyleZullo, Alberto, Emanuela Simone, Maddalena Grimaldi, Vincenzina Musto, and Francesco Paolo Mancini. 2018. "Sirtuins as Mediator of the Anti-Ageing Effects of Calorie Restriction in Skeletal and Cardiac Muscle" International Journal of Molecular Sciences 19, no. 4: 928. https://doi.org/10.3390/ijms19040928
APA StyleZullo, A., Simone, E., Grimaldi, M., Musto, V., & Mancini, F. P. (2018). Sirtuins as Mediator of the Anti-Ageing Effects of Calorie Restriction in Skeletal and Cardiac Muscle. International Journal of Molecular Sciences, 19(4), 928. https://doi.org/10.3390/ijms19040928