Ethanolic Extracts from Azadirachta indica Leaves Modulate Transcriptional Levels of Hormone Receptor Variant in Breast Cancer Cell Lines
Abstract
:1. Introduction
2. Results
2.1. Bioactive Compounds and Antiproliferative Effects of EENL
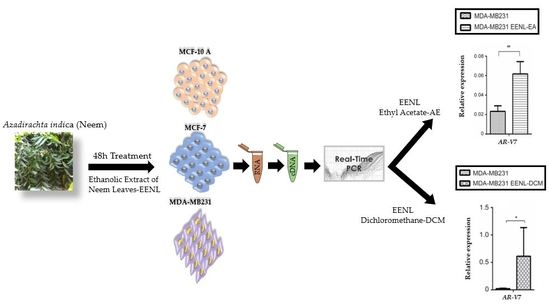
2.2. Transcriptional Profile of Hormone Receptors after Treatment with EENL
2.3. In Vivo Experiments with Drosophila Melanogaster
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Ethanolic Extraction and Determination of Phenolic Compounds
4.3. In Vitro Assays
4.3.1. Breast Cell Lines
4.3.2. Antiproliferative Assay
4.3.3. Quantitative Real-Time PCR
4.4. In Vivo Assays
4.4.1. Chemical Agents
4.4.2. Test for the Detection of Epithelial Tumor Clones (Warts)
4.4.3. Experimental Procedure
4.4.4. Analysis of the Flies
4.5. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BC | Breast cancer |
| neem | Azadirachta indica |
| DCM | Dichloromethane |
| EA | Ethyl acetate |
| EENL | Ethanolic extracts of neem leaves |
| DXR | Doxorubicin |
| ESR1 | Estrogen receptor alpha |
| ESR2 | Estrogen receptor beta |
| AR | Androgen receptor |
| wts | Test warts |
References
- World Health Organization (WHO). Breast Cancer: Prevention and Control. 2018. Available online: http://www.who.int/cancer/detection/breastcancer/en/ (accessed on 14 April 2018).
- World Health Organization (WHO). Media Centre-Cancer. 2018. Available online: http://www.who.int/mediacentre/factsheets/fs297/en/ (accessed on 18 April 2018).
- Araujo, T.G.; Paiva, C.E.; Rocha, R.M.; Maia, Y.C.; Sena, A.A.; Ueira-Vieira, C.; Carneiro, A.P.; Almeida, J.F.; de Faria, P.R.; Santos, D.W.; et al. A novel highly reactive Fab antibody for breast cancer tissue diagnostics and staging also discriminates a subset of good prognostic triple-negative breast cancers. Cancer Lett. 2014, 343, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Vogelstein, B.; Kinzler, K.W. Cancer genes and the pathways they control. Nat Med. 2004, 10, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Schulz, W.A. Molecular Biology of Human Cancers. An Advanced Student’s Textbook; Springer: Dordrecht, The Netherlands, 2005; pp. 12–14. ISBN 1-4020-3186-6. [Google Scholar]
- Suter, R.; Marcum, J.A. The molecular genetics of breast cancer and targeted therapy. Biologics 2007, 1, 241–258. [Google Scholar] [PubMed]
- Frei, A.; Macdonald, G.; Lund, I.; Gustafsson, J.-A.; Hynes, N.E.; Nalvarte, I. Memo interacts with c-Src to control Estrogen Receptor α sub-celular localization. Oncotarget 2016, 7, 56170–56182. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Dahlman-Wright, K.; Gustafsson, J.A. Estrogen receptor alpha and beta in health and disease. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Osborne, C.K.; Schiff, R. Mechanisms of endocrine resistance in breast cancer. Annu. Rev. Med. 2011, 62, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Mosselman, S.; Polman, J.; Dijkema, R. ERbeta: Identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996, 392, 49–53. [Google Scholar] [CrossRef]
- Yim, O.S.; Zhang, K.; Shalev, I.; Monakhov, M.; Zhong, S.; Hsu, M.; Chew, S.H.; Lai, P.S.; Ebstein, R.P. Delay discouting, genetic sensitivity, and leukocyte telomete length. Proc. Natl. Acad. Sci. USA 2016, 113, 2780–2785. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Liu, L.; Liu, W.; Zhang, P.; Huang, H.; Zang, L.; Hayashi, T.; Tashiro, S.; Onodera, S.; Xia, M.; et al. ERbeta up-regulation was involved in silibinin-induced growth inhibition of human breast cancer MCF-7 cells. Arch. Biochem. Biophys. 2016, 591, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.S.; Kokontis, J.; Liao, S.T. Molecular cloning of human and rat complementary DNA econding androgen receptors. Science 1988, 240, 324–326. [Google Scholar] [CrossRef] [PubMed]
- Trapman, J.; Klaassen, P.; Kuiper, G.G.; van der Korput, J.A.; Faber, P.W.; Van Rooij, H.C.; Geurts van Kessel, A.; Voorhorst, M.M.; Mulder, E.; Brinkmann, A.O. Cloning, structure and expression of cDNA econdiing the human androgen receptor. Biochem. Biophys. Res. Commun. 1988, 153, 241–248. [Google Scholar] [CrossRef]
- Lu, C.; Luo, J. Decoding the androgen receptor splice variants. Transl. Adrol. Urol. 2013, 2, 178–186. [Google Scholar] [CrossRef]
- Lu, J.; van der Steen, T.; Tindall, D.J. Are androgen receptor variants a substitute for the full-length receptor? Nat. Rev. Urol. 2015, 12, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Coram, M.A.; Nolley, R.; Reese, S.W.; Young, S.R.; Peehl, D.M. Transcript levels of androgen receptor variant AR-V1 or AR-V7 do not predict recurrence in patients with prostate cancer at indeterminate risk for progression. J. Urol. 2012, 188, 2158–2164. [Google Scholar] [CrossRef] [PubMed]
- Wadosky, K.M.; Koochekpour, S. Androgen receptor splice variants and prostate cancer: From bench to bedside. Oncotarget 2017, 14, 18550–18576. [Google Scholar] [CrossRef] [PubMed]
- Hickey, T.E.; Robinson, J.L.; Carroll, J.S.; Tilley, W.D. Minireview: The androgen receptor in breast tissues: Growth inhibitor, tumor suppressor, oncogene? Mol. Endocrinol. 2012, 26, 1252–1267. [Google Scholar] [CrossRef] [PubMed]
- Agoff, S.N.; Swanson, P.E.; Linden, H.; Hawes, S.E.; Lawton, T.J. Androgen receptor expression in estrogen receptor-negative breast cancer. Immunohistochemical, clinical, and prognostic associations. Am. J. Clin. Pathol. 2003, 120, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Koo, J.; Park, H.S.; Kim, J.H.; Choi, S.Y.; Lee, J.H.; Park, B.W.; Lee, K.S. Expression of androgen receptors in primary breast cancer. Ann. Oncol. 2010, 21, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Asano, Y.; Kashiwagi, S.; Onoda, N.; Kurata, K.; Morisaki, T.; Noda, S.; Takashima, T.; Ohsawa, M.; Kitagawa, S.; Hirakawa, K. Clinical verification of sensitivity to preoperative chemotherapy in cases of androgen receptor-expressing positive breast cancer. Br. J. Cancer 2016, 114, 14–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moinfar, F.; Okcu, M.; Tsybrovskyy, O.; Regitning, P.; Lax, S.F.; Weybora, W.; Ratscheck, M.; Tavassoli, F.A.; Denk, H. Androgen receptors frequently are expressed in breast carcinomas: Potential relevance to new therapeutic strategies. Cancer 2003, 98, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Leite, J.P.V. Fitoterapia: Bases Científicas e Tecnológicas, 1st ed.; Atheneu: São Paulo, Brazil, 2009; ISBN 9788573792379. [Google Scholar]
- Fan, S.; Zhang, J.; Nie, W.; Zhou, W.; Jin, L.; Chen, X.; Lu, J. Antitumor effects of polysaccharide from Sargassum fusiforme against human hepatocellular carcinoma HepG2 cells. Food Chem. Toxicol. 2017, 102, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.H.; Liu, X.; Shen, M.Y.; Nie, S.P.; Zhang, H.; Li, C.; Gong, D.M.; Xie, M.Y. Purification: Physicochemical characterisation and anticancer activity of a polysaccharide from Cyclocarya paliurus leaves. Food Chem. 2013, 136, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Nie, S.P.; Wang, J.Q.; Huang, D.F.; Li, W.J.; Xie, M.Y. Toll like receptor 4 mediates the antitumor host response induced by Ganoderma atrum polysaccharide. J. Agric. Food Chem. 2015, 63, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Zong, A.; Cao, H.; Wang, F. Anticancer polysaccharides from natural resources: A review of recent research. Carb. Pol. 2012, 90, 1395–1410. [Google Scholar] [CrossRef] [PubMed]
- Locke, J.C. Fungi. In The Neem Tree: Source of Unique Natural Products for Integrated Pest Management, Medicine, Industry and Other Purposes; Schmutterer, H., Ed.; VCH: Weinheim, Germany, 1995; pp. 118–125. [Google Scholar]
- Verkerk, R.H.J.; Wright, D.J. Biological activity of neem seed kernel extracts and synthetic azadirachtin against larvae of Plutella xylostella L. Pest Manag. Sci. 1993, 37, 83–91. [Google Scholar] [CrossRef]
- Santos, L.U.; Andrade, C.F.S. Azadiractha Indica—A Árvore do Nim e o Controle de Piolhos. Department of Zoology of the State University of Campinas-UNICAMP. Marc, 2000. Available online: http://www.piolho.org.br/artigos/arvoredonim.pdf (accessed on 18 April 2018).
- Mossini, S.A.G.; Kemmelmeir, C. A árvore nim (Azadirachta indica. A. Juss.): Múltiplos usos. Acta Farm. Bonaerense 2005, 24, 139–148. [Google Scholar]
- Lee, S.M.; Olsen, J.I.; Schewizer, M.P.; Klocke, J.A. 7-Deacetyl-17β-hydroxyazadiradione, a new limonoid insect growth inhibitor from Azadirachta indica. Phytochemistry 1988, 27, 2773–2775. [Google Scholar] [CrossRef]
- Martinez, S.S. O Nim–Azadirachta indica Natureza: Usos Múltiplos, Produção; IAPAR: Londrina, Brazil, 2002; ISBN 85-88184-05-2. [Google Scholar]
- Weng, C.J.; Yen, G.C. Chemopreventive effects of dietary phytochemicals against cancer invasion and metastasis: Phenolic acids, monophenol, polyphenol, and their derivatives. Cancer Treat. Rev. 2012, 38, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.J.; Wang, B.F.; Lu, W.F.; Wang, Z.D.; Ma, X.B.; Min, W.L.; Kang, H.F.; Wang, X.J.; Wu, W.Y. Total Flavonoids of Scutellaria barbata Inhibit Invasion of Hepatocarcinoma via MMP/TIMP in vitro. Molecules 2013, 18, 934–950. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.J.; Yen, G.C. Flavonoids, a ubiquitous dietary phenolic subclass, exert extensive in vitro anti-invasive and in vivo anti-metastatic activities. Cancer Metastasis Rev. 2012, 31, 323–351. [Google Scholar] [CrossRef] [PubMed]
- Chien, S.C.; Wu, Y.C.; Chen, Z.W.; Yang, W.C. Naturally Occurring Anthraquinones: Chemistry and Therapeutic Potential in Autoimmune Diabetes. Evid. Based Complement. Alternat. Med. 2015, 2015, 357357. [Google Scholar] [CrossRef] [PubMed]
- Abdelhady, M.I.S.; Bader, A.; Shaheen, U.; El-Malah, Y.; Abourehab, M.A.S.; Barghash, M. Azadirachta indica as source for Antioxidant and Cytotoxic Polyphenolic Compounds. Biosci. Biotechnol. Res. Asia 2015, 12, 1209–1222. [Google Scholar] [CrossRef]
- Balasenthil, S.; Arivazhagan, S.; Ramachandran, C.R.; Ramachandran, V.; Nagini, S. Chemopreventive potential of neem (Azadirachta indica) on 7,12-dimethylbenz[a]anthracene (DMBA) induced hamster buccal pouch carcinogenesis. J. Ethnopharmacol. 1999, 67, 189–195. [Google Scholar] [CrossRef]
- Baral, R.; Chattopadhyay, U. Neem (Azadirachta indica) leaf mediated immune activation causes prophylactic growth inhibition of murine Ehrlich carcinoma and B16 melanoma. Int. Immunopharmacol. 2004, 4, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, K.; Roy, M.K.; Ono, H.; Maeda, I.; Kameyama, M.O.; Yoshida, M.; Trakoontivakorn, G. Prenylated flavanones isolated from flowers of Azadirachta indica (the neem tree) as antimutagenic constituents against heterocyclic amines. J. Agric. Food Chem. 2003, 51, 6456–6460. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Teves, S.S.; Kemp, C.J.; Henikoff, S. Doxorubicin, DNA torsion, and chromatin dynamics. Biochim. Biophys. Acta 2014, 1845, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, A.; Mavi, A.; Kara, A.A. Determination of antioxidant and antimicrobial activities of Rumex cripus L. extracts. J. Agric. Food Chem. 2001, 49, 4083–4089. [Google Scholar] [CrossRef] [PubMed]
- Bendini, A.; Cerretani, L.; Pizzolante, L.; Gallina-Toschi, T.; Guzzo, F.; Ceoldo, S.; Marconi, A.M.; Andreetta, F.; Levi, M. Phenol Content Related to Antioxidant and An-timicrobial Activity of Passiflora spp. Extracts. Eur. Food Res. Technol. 2006, 223, 102–109. [Google Scholar] [CrossRef]
- Dlugosz, A.; Lembas-Bogaczyk, J.; Lamer-Zaraw-Ska. Antoxid Increases Ferric Reducing Antioxidant Po-wer (FRAP) even Stronger than Vitamin C. Acta Pol. Pharm. 2006, 63, 446–448. [Google Scholar] [PubMed]
- Wojdylo, A.; Oszmianski, J.; Czemerys, R. Antioxidant Activity and Phenolic Compounds in 32 Selected Herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Mohamed, H.; Masmoudi, O.; Yosra, E.-T.; Rayda, S.; Gharsallah, N.; Moncef, N. Chemical composition and antioxidant and radical scavenging activities of Periploca laevigata rook bark extracts. J. Sci. Food Agric. 2008, 89, 897–905. [Google Scholar] [CrossRef]
- Singh, U.P.; Maurya, S.; Singh, D.P. Phenolic acids in neem (Azadirachta indica): A major preexisting secondary metabolite. J. Herb. Pharm. 2005, 5, 35–43. [Google Scholar]
- Paul, R.; Prasad, M.; Sah, N.K. Anticancer biology of Azadirachta indica L (neem): A mini review. Cancer Biol. Ther. 2011, 12, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Phan, D.D.K.; Zhang, J.; Ong, P.-S.; Thuya, W.L.; Soo, R.; Wong, A.L.-A.; Yong, W.P.; Lee, S.C.; Ho, P.C.-L.; et al. Anticancer properties of nimbolide and pharmacokinetic considerations to accelerate its development. Oncotarget 2016, 7, 44790–44802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmieri, G.; Capone, M.; Ascierto, M.L.; Gentilcore, G.; Stroncek, D.F.; Casula, M.; Sini, M.C.; Palla, M.; Mozzilli, N.; Ascierto, P.A. Main roads to melanoma. J. Transl. Med. 2009, 7, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, M.K.; Kobori, M.; Takenaka, M.; Nakahara, K.; Shinmoto, H.; Tsushida, T. Inhibition of colon cancer (HT-29) cell proliferation by a triterpenoid isolated from Azadirachta indica is accompanied by cell cycle arrest and up-regulation of p21. Planta Med. 2006, 72, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Kohli, M.; Bergen, H.R.; Cheville, J.C.; Karnes, R.J.; Cao, H.; Young, C.Y.; Tindall, D.J.; Mcniven, M.A.; Donkena, K.V. Preclinical evaluation of the supercritical extract of Azadirachta indica (neem) leaves in vitro and in vivo on inhibition of prostate cancer tumor growth. Mol. Cancer Ther. 2014, 13, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Aleskandarany, M.A.; Abduljabbar, R.; Ashankyty, I.; Elmouna, A.; Jerjees, D.; Ali, S.; Buluwela, L.; Diez-Rodriguez, M.; Caldas, C.; Green, A.R.; et al. Prognostic significance of androgen receptor expression in invasive breast cancer: Transcriptonic and protein expression analysis. Breast Cancer Res. Treat. 2016, 159, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Hickey, T.E.; Irvine, C.M.; Dvinge, H.; Tarulli, G.A.; Hanson, A.R.; Ryan, N.K.; Pickering, M.A.; Birrell, S.N.; Hu, D.G.; Mackenzie, P.I.; et al. Expression of androgen receptor splice variants in clinical breast Cancers. Oncotarget 2015, 6, 44728–44744. [Google Scholar] [CrossRef] [PubMed]
- Rampurwala, M.; Wisinski, K.B.; O’Regan, R. Role of the androgen receptor in triple-negative breast cancer. Clin. Adv. Hematol. Oncol. 2016, 14, 186–193. [Google Scholar] [PubMed]
- Hu, D.G.; Hickey, T.E.; Irvine, C.; Wijayakumara, D.D.; Lu, L.; Tilley, W.D.; Selth, L.A.; Mackenzie, P.I. Identification of androgen receptor splice variant transcripts in breast cancer cell lines and human tissues. Horm. Cancer 2014, 5, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Kono, M.; Fujii, T.; Lyons, G.R.; Huo, L.; Bassett, R.; Gong, Y.; Karuturi, M.S.; Tripathy, D.; Ueno, N.T. Impact of androgen receptor expression. In fluoxymesterone-treated estrogen receptor-positive metastatic breast cancer refractory to contemporary hormonal therapy. Breast Cancer Res. Treat. 2016, 160, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Pietri, E.; Conteduca, V.; Andreis, D.; Masa, I.; Melegari, E.; Sarti, S.; Cecconetto, L.; Schirone, A.; Bravaccini, S.; Serra, P.; et al. Androgen receptor signaling pathways as a target for breast cancer treatment. Endocr. Relat. Cancer 2016, 23, R485–R498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chia, K.; O’brien, M.; Brown, M.; Lim, E. Targeting the androgen receptor in breast cancer. Curr. Oncol. Rep. 2015, 17, 4. [Google Scholar] [CrossRef] [PubMed]
- Fioretti, F.M.; Sita-Lumsden, A.; Bevan, C.L.; Brooke, G.N. Revising the role of the androgen receptor in breast cancer. J. Mol. Endocrinol. 2014, 52, R257–R265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birrell, S.N.; Bentel, J.M.; Hickey, T.E.; Ricciardelli, C.; Weger, M.A.; Horsfall, D.J.; Tilley, W.D. Androgens induce divergent proliferative responses in human breast cancer cell lines. J. Steroid Biochem. Mol. Biol. 1995, 52, 459–467. [Google Scholar] [CrossRef]
- Cochrane, D.R.; Bernales, S.; Jacobsen, B.M.; Cittelly, D.M.; Howe, E.N.; D’Amato, N.C.; Spoelstra, N.S.; Edgerton, S.M.; Jean, A.; Guerrero, J.; et al. Role of the androgen receptor in breast cancer and preclinical analysis of enzalutamide. Breast Cancer Res. 2014, 16, R7. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.; Ni, M.; Hazra, A.; Tamimi, R.; Brown, M. Elucidating the role of androgen receptor in breast cancer. J. Clin. Investig. 2012, 2, 1003–1011. [Google Scholar] [CrossRef]
- Lanzino, M.; Sisci, D.; Morelli, C.; Garofalo, C.; Catalano, S.; Casaburi, I.; Capparelli, C.; Giordano, C.; Giordano, F.; Maggiolini, M.; et al. Inhibition of cyclin D1 expression by androgen receptor in breast cancer cells—Identification of novel androgen response elemento. Nucleic Acids Res. 2010, 38, 5351–5365. [Google Scholar] [CrossRef] [PubMed]
- Cechinel-Filho, V.; Yunes, R.A. Estratégias para a obtenção de compostos farmacologicamente ativos a partir de plantas medicinais: Conceitos sobre modificação estrutural para otimização da atividade. Quím. Nova 1998, 21, 99–105. [Google Scholar] [CrossRef]
- Silva, V.C.L. Avaliação da Toxicidade Reprodutiva de Ratas Wistar Submetidas à Ingestão do Extrato Etanólico das Folhas de Nim (Azadirachta indica A. Jus). Master’s Thesis, Rural Federal University of Pernambuco, Recife, Brazil, 2010. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–158. [Google Scholar] [CrossRef]
- Swain, T.; Hills, W.E. The phenolic constituents of Punnus domestica. Iquantitative analysis of phenolic constituents. J. Sci. Food Agric. 1959, 10, 63–68. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Araujo, T.G.; Marangoni, K.; Rocha, R.M.; Maia, Y.C.P.; Araujo, G.R.; Alcântara, T.M.; Alves, P.T.; Calábria, L.; Neves, A.F.; Soares, F.A.; et al. Dynamic dialog between cytokeratin 18 and annexin A1 in breast cancer: A transcriptional disequilibrium. Acta Histochem. 2014, 116, 1178–1184. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Yang, X.; Sun, F.; Jiang, R.; Linn, D.E.; Chen, H.; Chen, H.; Kong, X.; Melamed, J.; Tepper, C.G.; et al. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res. 2009, 69, 2305–2313. [Google Scholar] [CrossRef] [PubMed]
- Nepomuceno, J.C. Using the Drosophila melanogaster to assessment carcinogenic agents through the test for detection of epithelial tumor clones. Adv. Tech. Biol. Med. 2015, 3, 149. [Google Scholar] [CrossRef]
- Justice, R.W.; Zilian, O.; Woods, D.F.; Noll, M.; Bryant, P.J. The Drosophila tumor suppressor gene warts encodes a homolog o-f human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 1995, 9, 534–546. [Google Scholar] [CrossRef] [PubMed]
- Orsolin, P.C.; Silva-Oliveira, R.G.; Nepomuceno, J.C. Modulating effect of Modulating effect of synthetic statins against damage induced by doxorubicin in somatic cells of Drosophila melanogaster. Food Chem. Toxicol. 2015, 81, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Orsolin, P.C.; Silva-Oliveira, R.G.; Nepomuceno, J.C. Modulating effect of simvastatin on the DNA damage induced by doxorubicin in somatic cells of Drosophila melanogaster. Food Chem. Toxicol. 2016, 90, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, M.A.; Orsolin, P.C.; Silva-Oliveira, R.G.; Nepomuceno, J.C.; Spanó, M.A. Assessment of the carcinogenic potential of high intense-sweeteners through the test for detection of epithelial tumor clones (warts) in Drosophila melanogaster. Food Chem. Toxicol. 2017, 101, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.C.; Constante, S.A.R.; Orsolin, P.C.; Nepomuceno, J.C.; Rezende, A.A.A.; Spanó, M.A. Modulatory effects of metformin on mutagenicity and epithelial tumor incidence in doxorubicin-treated Drosophila melanogaster. Food Chem. Toxocol. 2017, 106, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Lima, P.M.A.P.; Orsoli, P.C.; Araújo, T.G.; Brandão, D. Effects of a Carbonated Soft Drink on Epitheial Tumor Incidence in Drosophila melanogaster. J. Pharm. Pharmacol. 2018, 6, 240–247. [Google Scholar] [CrossRef]
- Eeken, J.C.J.; Klink, I.; Veen, B.L.V.; Pastink, A.; Ferro, W. Induction of epithelial tumors in Drosophila melanogaster heterozygous for the tumor suppressor gene wts. Environ. Mol. Mutagen. 2002, 40, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Costa, W.F.; Oliveira, A.B.; Nepomuceno, J.C. Lapachol as an epithelial tumor inhibitor agent in Drosophila melanogaster heterozygote for tumor suppressor gene wts. Genet. Mol. Res. 2012, 10, 3236–3245. [Google Scholar] [CrossRef] [PubMed]





| Treatments | Indiv. (N) | Tumors | ||||||
|---|---|---|---|---|---|---|---|---|
| Eyes | Head | Wings | Body | Legs | Halteres | Total Tumors | ||
| Negative Control | 203 | 0.00 (00) | 0.00 (01) | 0.00 (00) | 0.00 (01) | 0.01 (02) | 0.00 (01) | 0.02 (05) |
| Positive Control | 176 | 0.01 (02) | 0.04 (07) | 0.20 (36) | 0.15 (26) | 0.05 (09) | 0.01 (01) | 0.46 (81) + |
| EENL 0.03125% | 200 | 0.00 (00) | 0.01 (02) | 0.10 (20) | 0.04 (08) | 0.03 (06) | 0.03 (05) | 0.21 (41) + |
| EENL 0.0625% | 200 | 0.02 (03) | 0.01 (02) | 0.07 (13) | 0.03 (06) | 0.04 (07) | 0.01 (01) | 0.16 (32) + |
| EENL 0.125% | 218 | 0.00 (00) | 0.01 (02) | 0.04 (08) | 0.13 (29) | 0.01 (03) | 0.01 (05) | 0.20 (44) + |
| EENL 0.03125% + DXR | 200 | 0.01 (01) | 0.26 (52) | 2.08 (416) | 0.98 (196) | 1.92 (384) | 0.19 (37) | 5.43 (1086) ** |
| EENL 0.0624% + DXR | 200 | 0.09 (18) | 0.59 (117) | 3.65 (730) | 1.82 (363) | 3.65 (729) | 0.45 (90) | 10.24 (2047) ** |
| EENL 0.125% + DXR | 165 | 0.03 (05) | 0.06 (10) | 0.24 (39) | 0.28 (46) | 0.06 (10) | 0.02 (04) | 0.69 (114) ** |
| GENE | Primers sequence (Forward/Reverse) (5′–3′) |
|---|---|
| β2M [73] | F: CCTGCCGTGTGAACCATGT/R: GCGGCATCTTCAAACCTCC |
| ESR1 | F: CTAACTTGCTCTTGGACAGGAAC/R: GATTTGAGGCACACAAACTCCTC |
| ESR2 | F: GGGAATGGTGAAGTGTGGCT/R: TCATGTGTACCAACTCCTTGTCGG |
| AR | F: CATGTGGAAGCTGCAAGGTCT/R: GTGTAAGTTGCGGAAGCCAGG |
| AR-V1 [74] | F: CTACTCCGGACCTTACGGGGACATGCG/R: GATTCTTTCAGAAACAACAACAGCTGCT |
| AR-V4 [74] | F: CTACTCCGGACCTTACGGGGACATGCG/R: CTTTTAATTTGTTCATTCTGAAAAATCCTC |
| AR-V7 [74] | F: CTACTCCGGACCTTACGGGGACATGCG/R: TGCCAACCCGGAATTTTTCTCCC |
| Treatments | Concentrations | Composition |
|---|---|---|
| Negative control (−) | - | MP + 5 mL ROW + 2.38% of ethanol P.A |
| Positive control (+) | - | MP + DXR 0.4 mM |
| T1 | 0.03% | MP + 5 mL of EENL 0.03125% |
| T2 | 0.06% | MP + 5 mL of EENL 0.0625% |
| T3 | 0.13% | MP + 5 mL of EENL 0.125% |
| T4 | 0.03125% + DXR | MP + 5 mL of EENL 0.03125% + DXR 0.4 mM |
| T5 | 0.0625% + DXR | MP + 5 mL of EENL 0.0625% + DXR 0.4 mM |
| T6 | 0.125% + DXR | MP + 5 mL of EENL 0.125% + DXR 0.4 mM |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braga, D.L.; Mota, S.T.S.; Zóia, M.A.P.; Lima, P.M.A.P.; Orsolin, P.C.; Vecchi, L.; Nepomuceno, J.C.; Fürstenau, C.R.; Maia, Y.C.P.; Goulart, L.R.; et al. Ethanolic Extracts from Azadirachta indica Leaves Modulate Transcriptional Levels of Hormone Receptor Variant in Breast Cancer Cell Lines. Int. J. Mol. Sci. 2018, 19, 1879. https://doi.org/10.3390/ijms19071879
Braga DL, Mota STS, Zóia MAP, Lima PMAP, Orsolin PC, Vecchi L, Nepomuceno JC, Fürstenau CR, Maia YCP, Goulart LR, et al. Ethanolic Extracts from Azadirachta indica Leaves Modulate Transcriptional Levels of Hormone Receptor Variant in Breast Cancer Cell Lines. International Journal of Molecular Sciences. 2018; 19(7):1879. https://doi.org/10.3390/ijms19071879
Chicago/Turabian StyleBraga, Deisi L., Sara T. S. Mota, Mariana A. P. Zóia, Paula M. A. P. Lima, Priscila C. Orsolin, Lara Vecchi, Júlio C. Nepomuceno, Cristina R. Fürstenau, Yara C. P. Maia, Luiz Ricardo Goulart, and et al. 2018. "Ethanolic Extracts from Azadirachta indica Leaves Modulate Transcriptional Levels of Hormone Receptor Variant in Breast Cancer Cell Lines" International Journal of Molecular Sciences 19, no. 7: 1879. https://doi.org/10.3390/ijms19071879
APA StyleBraga, D. L., Mota, S. T. S., Zóia, M. A. P., Lima, P. M. A. P., Orsolin, P. C., Vecchi, L., Nepomuceno, J. C., Fürstenau, C. R., Maia, Y. C. P., Goulart, L. R., & Araújo, T. G. (2018). Ethanolic Extracts from Azadirachta indica Leaves Modulate Transcriptional Levels of Hormone Receptor Variant in Breast Cancer Cell Lines. International Journal of Molecular Sciences, 19(7), 1879. https://doi.org/10.3390/ijms19071879