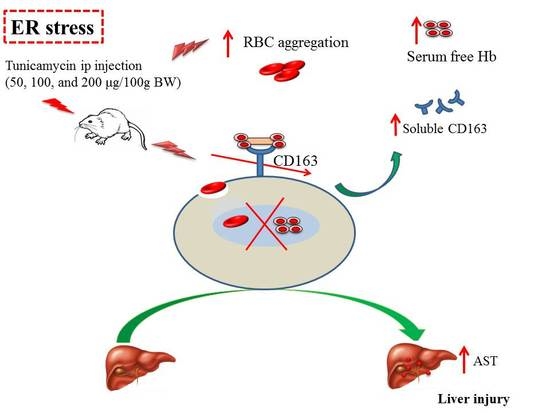
Effect of Endoplasmic Reticular Stress on Free Hemoglobin Metabolism and Liver Injury
Abstract
:1. Introduction
2. Results
2.1. ER Stress Attenuates RBC Aggregability and Free Hb Uptake
2.2. ER Stress Alters Hepatic Hepcidin-Ferroportin Expression
2.3. ER Stress Suppresses Globin Degradation
2.4. ER Stress Alters Heme Iron Degradation via Increasing Heme Oxygesnase-1 Trafficking
3. Discussion
4. Materials and Methods
4.1. Rat Experiment
4.2. Blood Biochemistry
4.3. Western Blot Analysis
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pagliassotti, M.J. Endoplasmic reticulum stress in nonalcoholic fatty liver disease. Annu. Rev. Nutr. 2012, 32, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Trak-Smayra, V.; Dargere, D.; Noun, R.; Albuquerque, M.; Yaghi, C.; Gannage-Yared, M.H.; Bedossa, P.; Paradis, V. Serum proteomic profiling of obese patients: Correlation with liver pathology and evolution after bariatric surgery. Gut 2009, 58, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, Y.; Senates, E.; Ayyildiz, T.; Colak, Y.; Tuncer, I.; Ovunc, A.O.; Dolar, E.; Kalayci, C. Characterization of nonalcoholic fatty liver disease unrelated to the metabolic syndrome. Eur. J. Clin. Investig. 2012, 42, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Xu, C.; Xu, L.; Yu, J.; Miao, M.; Li, Y. Serum proteomic analysis revealed diagnostic value of hemoglobin for nonalcoholic fatty liver disease. J. Hepatol. 2012, 56, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Fjeldborg, K.; Christiansen, T.; Bennetzen, M.; H, J.M.; Pedersen, S.B.; Richelsen, B. The macrophage-specific serum marker, soluble cd163, is increased in obesity and reduced after dietary-induced weight loss. Obesity 2013, 21, 2437–2443. [Google Scholar] [CrossRef] [PubMed]
- Kazankov, K.; Barrera, F.; Moller, H.J.; Rosso, C.; Bugianesi, E.; David, E.; Ibrahim Kamal Jouness, R.; Esmaili, S.; Eslam, M.; McLeod, D.; et al. The macrophage activation marker scd163 is associated with morphological disease stages in patients with non-alcoholic fatty liver disease. Liver Int. 2016, 36, 1549–1557. [Google Scholar] [CrossRef] [PubMed]
- Kazankov, K.; Moller, H.J.; Lange, A.; Birkebaek, N.H.; Holland-Fischer, P.; Solvig, J.; Horlyck, A.; Kristensen, K.; Rittig, S.; Handberg, A.; et al. The macrophage activation marker scd163 is associated with changes in nafld and metabolic profile during lifestyle intervention in obese children. Pediatr. Obes. 2015, 10, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Mueller, J.L.; Feeney, E.R.; Zheng, H.; Misdraji, J.; Kruger, A.J.; Alatrakchi, N.; King, L.Y.; Gelrud, L.; Corey, K.E.; Chung, R.T. Circulating soluble cd163 is associated with steatohepatitis and advanced fibrosis in nonalcoholic fatty liver disease. Clin. Transl. Gastroenterol. 2015, 6, e114. [Google Scholar] [CrossRef] [PubMed]
- Kazankov, K.; Tordjman, J.; Moller, H.J.; Vilstrup, H.; Poitou, C.; Bedossa, P.; Bouillot, J.L.; Clement, K.; Gronbaek, H. Macrophage activation marker soluble cd163 and non-alcoholic fatty liver disease in morbidly obese patients undergoing bariatric surgery. J. Gastroenterol. Hepatology. 2015, 30, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Rodgaard-Hansen, S.; St George, A.; Kazankov, K.; Bauman, A.; George, J.; Gronbaek, H.; Jon Moller, H. Effects of lifestyle intervention on soluble cd163, a macrophage activation marker, in patients with non-alcoholic fatty liver disease. Scand. J. Clin. Lab. Investig. 2017, 77, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Macrophages and systemic iron homeostasis. J. Innate Immun. 2012, 4, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, J.H.; Etzerodt, A.; Svendsen, P.; Moestrup, S.K. The haptoglobin-cd163-heme oxygenase-1 pathway for hemoglobin scavenging. Oxid. Med. Cell. Longev. 2013, 2013, 523652. [Google Scholar] [CrossRef] [PubMed]
- Schaer, C.A.; Schoedon, G.; Imhof, A.; Kurrer, M.O.; Schaer, D.J. Constitutive endocytosis of cd163 mediates hemoglobin-heme uptake and determines the noninflammatory and protective transcriptional response of macrophages to hemoglobin. Circ. Res. 2006, 99, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Gomes, I.; Dale, C.S.; Casten, K.; Geigner, M.A.; Gozzo, F.C.; Ferro, E.S.; Heimann, A.S.; Devi, L.A. Hemoglobin-derived peptides as novel type of bioactive signaling molecules. AAPS J. 2010, 12, 658–669. [Google Scholar] [CrossRef] [PubMed]
- Fruitier, I.; Garreau, I.; Lacroix, A.; Cupo, A.; Piot, J.M. Proteolytic degradation of hemoglobin by endogenous lysosomal proteases gives rise to bioactive peptides: Hemorphins. FEBS Lett. 1999, 447, 81–86. [Google Scholar] [CrossRef]
- Nielsen, M.J.; Moestrup, S.K. Receptor targeting of hemoglobin mediated by the haptoglobins: Roles beyond heme scavenging. Blood 2009, 114, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.L.; Chen, P.Y.; Wang, C.M.; Chen, W.Y.; Chen, C.W.; Owaga, E.; Chang, J.S. Dose-related effects of ferric citrate supplementation on endoplasmic reticular stress responses and insulin signalling pathways in streptozotocin-nicotinamide-induced diabetes. Food Funct. 2016, 7, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-M.; Chen, Y.-H.; Lee, Y.-C.; Chang, J.-S. Endoplasmic reticulum stress contributes to ferritin molecules-mediated macrophage migration via P-selectin glycoprotein ligand-1. Mol. Nutr. Food Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.M.; Li, S.J.; Wu, C.H.; Hu, C.M.; Cheng, H.W.; Chang, J.S. Transient knock down of grp78 reveals roles in serum ferritin mediated pro-inflammatory cytokine secretion in rat primary activated hepatic stellate cells. Asian Pac. J. Cancer Prev. 2014, 15, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Vecchi, C.; Montosi, G.; Zhang, K.; Lamberti, I.; Duncan, S.A.; Kaufman, R.J.; Pietrangelo, A. Er stress controls iron metabolism through induction of hepcidin. Science 2009, 325, 877–880. [Google Scholar] [CrossRef] [PubMed]
- Etzerodt, A.; Maniecki, M.B.; Moller, K.; Moller, H.J.; Moestrup, S.K. Tumor necrosis factor alpha-converting enzyme (tace/adam17) mediates ectodomain shedding of the scavenger receptor cd163. J. Leukoc. Biol. 2010, 88, 1201–1205. [Google Scholar] [CrossRef] [PubMed]
- Etzerodt, A.; Rasmussen, M.R.; Svendsen, P.; Chalaris, A.; Schwarz, J.; Galea, I.; Moller, H.J.; Moestrup, S.K. Structural basis for inflammation-driven shedding of cd163 ectodomain and tumor necrosis factor-α in macrophages. J. Biol. Chem. 2014, 289, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Etzerodt, A.; Kjolby, M.; Nielsen, M.J.; Maniecki, M.; Svendsen, P.; Moestrup, S.K. Plasma clearance of hemoglobin and haptoglobin in mice and effect of cd163 gene targeting disruption. Antioxid. Redox Signal. 2013, 18, 2254–2263. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, F.; Sanderson, K.; Glamsta, E.L. The hemorphins: A new class of opioid peptides derived from the blood protein hemoglobin. Biopolymers 1997, 43, 147–156. [Google Scholar] [CrossRef]
- Fruitier-Arnaudin, I.; Cohen, M.; Bordenave, S.; Sannier, F.; Piot, J.M. Comparative effects of angiotensin iv and two hemorphins on angiotensin-converting enzyme activity. Peptides 2002, 23, 1465–1470. [Google Scholar] [CrossRef]
- Lee, J.; Albiston, A.L.; Allen, A.M.; Mendelsohn, F.A.; Ping, S.E.; Barrett, G.L.; Murphy, M.; Morris, M.J.; McDowall, S.G.; Chai, S.Y. Effect of i.C.V. Injection of at4 receptor ligands, nle1-angiotensin iv and lvv-hemorphin 7, on spatial learning in rats. Neuroscience 2004, 124, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Fruiter, A., II; Cohen, M.M.; Nervi, S.S.; Bordenave, S.S.; Sannier, F.F.; Piot, J.M. Reduced level of opioid peptides, hemorphin-7 peptides, in serum of diabetic patients. Diabetes Care 2003, 26, 2480. [Google Scholar] [CrossRef]
- Maraninchi, M.; Feron, D.; Fruitier-Arnaudin, I.; Begu-Le Corroller, A.; Nogueira, J.P.; Mancini, J.; Valero, R.; Piot, J.M.; Vialettes, B. Serum hemorphin-7 levels are decreased in obesity. Obesity 2013, 21, 378–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gottlieb, Y.; Truman, M.; Cohen, L.A.; Leichtmann-Bardoogo, Y.; Meyron-Holtz, E.G. Endoplasmic reticulum anchored heme-oxygenase 1 faces the cytosol. Haematologica 2012, 97, 1489–1493. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Weis, S.; Yang, G.; Weng, Y.H.; Helston, R.; Rish, K.; Smith, A.; Bordner, J.; Polte, T.; Gaunitz, F.; et al. Heme oxygenase-1 protein localizes to the nucleus and activates transcription factors important in oxidative stress. J. Biol. Chem. 2007, 282, 20621–20633. [Google Scholar] [CrossRef] [PubMed]
- Biswas, C.; Shah, N.; Muthu, M.; La, P.; Fernando, A.P.; Sengupta, S.; Yang, G.; Dennery, P.A. Nuclear heme oxygenase-1 (ho-1) modulates subcellular distribution and activation of nrf2, impacting metabolic and anti-oxidant defenses. J. Biol. Chem. 2014, 289, 26882–26894. [Google Scholar] [CrossRef] [PubMed]
- Oslowski, C.M.; Urano, F. Measuring er stress and the unfolded protein response using mammalian tissue culture system. Methods Enzymol. 2011, 490, 71–92. [Google Scholar] [PubMed]
- Zhao, N.; Enns, C.A. N-linked glycosylation is required for transferrin-induced stabilization of transferrin receptor 2, but not for transferrin binding or trafficking to the cell surface. Biochemistry 2013, 52, 3310–3319. [Google Scholar] [CrossRef] [PubMed]
- Ashton, L.; Brewster, V.L.; Correa, E.; Goodacre, R. Detection of glycosylation and iron-binding protein modifications using raman spectroscopy. Analyst 2017, 142, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Oyadomari, S.; Mori, M. Roles of chop/gadd153 in endoplasmic reticulum stress. Cell Death Differ. 2004, 11, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Iurlaro, R.; Munoz-Pinedo, C. Cell death induced by endoplasmic reticulum stress. FEBS J. 2016, 283, 2640–2652. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Jian, J.; Katz, S.; Abramson, S.B.; Huang, X. 17beta-estradiol inhibits iron hormone hepcidin through an estrogen responsive element half-site. Endocrinology 2012, 153, 3170–3178. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Nam, J.H.; Hou, J.X.; Suh, J.S. A transient, microfluidic approach to the investigation of erythrocyte aggregation: The threshold shear-stress for erythrocyte disaggregation. Clin. Hemorheol. Microcirc. 2009, 42, 117–125. [Google Scholar] [PubMed]
- Alexy, T.; Baskurt, O.K.; Nemeth, N.; Uyuklu, M.; Wenby, R.B.; Meiselman, H.J. Effect of lanthanides on red blood cell deformability and response to mechanical stress: Role of lanthanide ionic radius. Biorheology 2011, 48, 173–183. [Google Scholar] [PubMed]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseng, S.-H.; Chang, T.-Y.; Shih, C.-K.; Hsieh, R.-H.; Chen, C.-W.; Chen, Y.-C.; Lin, M.-H.; Chang, J.-S. Effect of Endoplasmic Reticular Stress on Free Hemoglobin Metabolism and Liver Injury. Int. J. Mol. Sci. 2018, 19, 1977. https://doi.org/10.3390/ijms19071977
Tseng S-H, Chang T-Y, Shih C-K, Hsieh R-H, Chen C-W, Chen Y-C, Lin M-H, Chang J-S. Effect of Endoplasmic Reticular Stress on Free Hemoglobin Metabolism and Liver Injury. International Journal of Molecular Sciences. 2018; 19(7):1977. https://doi.org/10.3390/ijms19071977
Chicago/Turabian StyleTseng, Sung-Hui, Ting-Yun Chang, Chun-Kuang Shih, Rong-Hong Hsieh, Chia-Wen Chen, Yi-Chun Chen, Mei-Hsiang Lin, and Jung-Su Chang. 2018. "Effect of Endoplasmic Reticular Stress on Free Hemoglobin Metabolism and Liver Injury" International Journal of Molecular Sciences 19, no. 7: 1977. https://doi.org/10.3390/ijms19071977
APA StyleTseng, S. -H., Chang, T. -Y., Shih, C. -K., Hsieh, R. -H., Chen, C. -W., Chen, Y. -C., Lin, M. -H., & Chang, J. -S. (2018). Effect of Endoplasmic Reticular Stress on Free Hemoglobin Metabolism and Liver Injury. International Journal of Molecular Sciences, 19(7), 1977. https://doi.org/10.3390/ijms19071977