Polyphenols Derived from Lychee Seed Suppress Aβ (1-42)-Induced Neuroinflammation
Abstract
:1. Introduction
2. Results
2.1. Isolation, Purification, Identification, and Elucidation of Catechin and Procyanidin A2 in LSF
2.2. Catechin and Procyanidin A2 Improve the Morphology of BV-2 Cells
2.3. Catechin and Procyanidin A2 Inhibit Pro-Inflammatory Cytokines in Aβ(1-42)-Induced BV-2 Cells
2.4. Catechin and Procyanidin A2 Inhibit the Activation of NF-κB Signaling Pathway
2.5. Catechin and Procyanidin A2 Inhibit Cell Apoptosis in Aβ(1-42)-Induced BV-2 Cells
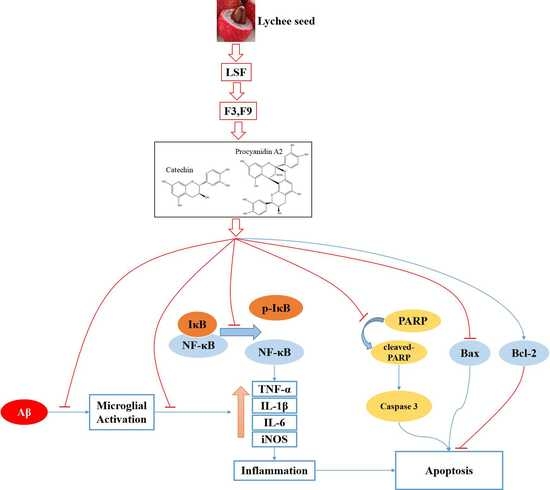
3. Discussion
4. Materials and Methods
4.1. Reagent and Instrument
4.2. Instrument and Chromatograph Condition for the Separation of the Components in LSF
4.3. Cell Culture
4.4. Cell Viability
4.5. Identification of the Components in LSF
4.6. Wright–Giemsa Staining
4.7. Cytokines ELISA
4.8. Quantitative Reverse Transcription PCR (qRT-PCR)
| TNF-α | Forward: 5′-GAGCACAGAAAGCATGATCC-3′ |
| Reverse: 5′-GAGAAGAGGCTGAGACATAG-3′ | |
| IL-1β | Forward: 5′-CTAGGGACTTAGGTGCTGTC-3′ |
| Reverse: 5′-CTCTGCCTTTGCTTCCAAGC-3′ | |
| iNOS | Forward: 5′-CGTTGGATTTGGAGCAGAAG-3′ |
| Reverse: 5′-CCTCTTTCAGGTCACTTTGG-3′ | |
| GAPDH | Forward: 5′-GACAGTCGGAAACTGGGAAG-3′ |
| Reverse: 5′-CATCACGTCCTCCATCATCC-3′ |
4.9. Western Blotting
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Aβ | Amyloid-β |
| AD | Alzheimer’s disease |
| LSF | Lychee seed fraction |
| pre-HPLC | Preparation of high performance liquid chromatography |
| NMR | Nuclear magnetic resonance |
| TNF-α | Tumor necrosis factor-α |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| NF-κB | Nuclear factor-κB |
| ELISA | Enzyme linked immunosorbent assay |
| RT-PCR | Real time-PCR |
| SPs | Senile plaques |
| NFTs | Neurofibrillary tangles |
| CNS | Central nervous system |
| PD | Parkinson’s disease |
| HD | Huntington’s disease |
| NO | Nitric oxide |
| ROS | Reactive oxygen species |
| TCMs | Traditional Chinese medicines |
| T2DM | Type 2 diabetes mellitus |
| COX-2 | Cyclooxygenase 2 |
| iNOS | Inducible nitric oxide synthase |
| IκBα | NF-κB inhibitor α |
| PARP | Poly ADP-ribose polymerase |
| IR | Insulin resistance |
| EGCG | Epigallocatechin gallate |
| IKK | IκB kinase |
| BBB | Blood–brain barrier |
References
- Wang, X.; Wang, W.; Li, L.; Perry, G.; Lee, H.; Zhu, X. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. BBA Mol. Basis Dis. 2014, 1842, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Scheff, S.W.; Price, D.A.; Schmitt, F.A.; Mufson, E.J. Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol. Aging 2006, 27, 1372–1384. [Google Scholar] [CrossRef] [PubMed]
- Varnum, M.M.; Ikezu, T. The Classification of Microglial Activation Phenotypes on Neurodegeneration and Regeneration in Alzheimer’s Disease Brain. Arch. Immunol. Ther. Exp. 2012, 60, 251–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heneka, M.T.; Carson, M.J.; Khoury, J.E.; Landreth, G.E. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef]
- Ferreira, S.T.; Clarke, J.R.; Bomfim, T.R.; de Felice, F.G. Inflammation, defective insulin signaling, and neuronal dysfunction in Alzheimer’s disease. Alzheimers Dement. 2014, 10, S76–S83. [Google Scholar] [CrossRef] [PubMed]
- Hung, A.S.M.; Liang, Y.; Chow, T.C.H.; Tang, H.C.; Wu, S.L.Y.; Wai, M.S.M.; Yew, D.T. Mutated tau, amyloid and neuroinflammation in Alzheimer disease—A brief review. Prog. Histochem. Cytochem. 2016, 51, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Streit, W.J.; Mrak, R.E.; Griffin, W.S. Microglia and neuroinflammation: A pathological perspective. J. Neuroinflamm. 2004, 1, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, L.; Wu, Y.; Ren, X.; Liu, Q.; Wang, J.; Liu, X. Isoorientin attenuates lipopolysaccharide-induced pro-inflammatory responses through down-regulation of ROS-related MAPK/NF-κB signaling pathway in BV-2 microglia. Mol. Cell. Biochem. 2014, 386, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Jung, Y.Y.; Hwang, C.J.; Lee, H.P.; Sok, C.H.; Kim, J.H.; Lee, S.M.; Seo, H.O.; Hyun, B.K.; Choi, D.Y.; et al. Inhibitory effect of ent-Sauchinone on amyloidogenesis via inhibition of STAT3-mediated NF-κB activation in cultured astrocytes and microglial BV-2 cells. J. Neuroinflamm. 2014, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, K.; Maphis, N.; Xu, G.; Varvel, N.H.; Kokiko-Cochran, O.N.; Weick, J.P.; Staugaitis, S.M.; Cardona, A.; Ransohoff, R.M.; Herrup, K.; et al. Microglial derived tumor necrosis factor-α drives Alzheimer’s disease-related neuronal cell cycle events. Neurobiol. Dis. 2014, 62, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Beauchet, O.; Launay, C.P.; Allali, G.; Annweiler, C. Changes in Gait Variability with Anti-dementia Drugs: A Systematic Review and Meta-analysis. CNS Drugs 2014, 28, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Wei, S. Potential therapeutic action of natural products from traditional Chinese medicine on Alzheimer’s disease animal models targeting neurotrophic factors. Fund. Clin. Pharmacol. 2016, 30, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Wang, C.; Shi, J.; Lin, Z. Effects of Ginkgo biloba on dementia: An overview of systematic reviews. J. Ethnopharmacol. 2017, 195, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, W.; Xu, L.; Chen, L. In Salvia miltiorrhiza, phenolic acids possess protective properties against amyloid beta-induced cytotoxicity, and tanshi-nones act as acetylcholinesterase inhibitors. Environ. Toxicol. Pharmacol. 2011, 31, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Zeng, W.; Wong, V.K.; Zhu, Y.; Lo, A.C.Y.; Liu, L.; Law, B.Y. Hederagenin and α-hederin promote degradation of proteins in neurodegenerative diseases and improve motor deficits in MPTP-mice. Pharmacol. Res. 2017, 115, 25–44. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Kam-Wai Wong, V.; Zeng, W.; Liu, L.; Yuen-Kwan Law, B. Identification of novel autophagic Radix Polygalae fraction by cell membrane chromatography and UHPLC-(Q)TOF-MS for degradation of neurodegenerative disease proteins. Sci. Rep. 2015, 5, 17199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, A.; Wong, V.K.; Xu, S.; Chan, W.; Ng, C.; Liu, L.; Law, B.Y. Onjisaponin B Derived from Radix Polygalae Enhances Autophagy and Accelerates the Degradation of Mutant α-Synuclein and Huntingtin in PC-12 Cells. Int. J. Mol. Sci. 2013, 14, 22618–22641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, V.K.; Wu, A.G.; Wang, J.R.; Liu, L.; Law, B.Y. Neferine Attenuates the Protein Level and Toxicity of Mutant Huntingtin in PC-12 Cells via Induction of Autophagy. Molecules 2015, 20, 3496–3514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Wu, J.; Yu, C.; Tang, Y.; Liu, J.; Chen, H.; Jin, B.; Mei, Q.; Cao, S.; Qin, D. Lychee Seed Saponins Improve Cognitive Function and Prevent Neuronal Injury via Inhibiting Neuronal Apoptosis in a Rat Model of Alzheimer’s Disease. Nutrients 2017, 9, 105. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, H.; Liu, J.; Chen, R.; Tang, Y.; Chen, H.; Gu, L.; Li, M.; Cao, S.; Qin, D.; et al. Inhibitory Effect of Lychee Seed Saponins on Apoptosis Induced by Aβ25-35 through Regulation of the Apoptotic and NF-κB Pathways in PC12 Cells. Nutrients 2017, 9, 337. [Google Scholar] [CrossRef] [PubMed]
- Miketova, P.; Schram, K.H.; Whitney, J.; Li, M.; Huang, R.; Kerns, E.; Valcic, S.; Timmermann, B.N.; Rourick, R.; Klohr, S. Tandem mass spectrometry studies of green tea catechins. Identification of three minor components in the polyphenolic extract of green tea. J. Mass Spectrom. 2000, 35, 860–869. [Google Scholar] [CrossRef]
- Porter, L.J.; Newman, R.H.; Foo, L.Y.; Wong, H.; Hemingway, R.W. Polymeric Proanthocyanidins. I3C Nmr Studies of Procyanidins. J. Chem. Soc. Perkin Trans. 1982, 1, 1217–1221. [Google Scholar] [CrossRef]
- Koerner, J.L.; Hsu, V.L.; Lee, J.; Kennedy, J.A. Determination of proanthocyanidin A2 content in phenolic polymer isolates by reversed-phase high-performance liquid chromatography. J. Chromatogr. A 2009, 1216, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Latta, C.H.; Sudduth, T.L.; Weekman, E.M.; Brothers, H.M.; Abner, E.L.; Popa, G.J.; Mendenhall, M.D.; Gonzalez-Oregon, F.; Braun, K.; Wilcock, D.M. Determining the role of IL-4 induced neuroinflammation in microglial activity and amyloid-β using BV2 microglial cells and APP/PS1 transgenic mice. J. Neuroinflamm. 2015, 12, 41. [Google Scholar] [CrossRef] [PubMed]
- Godyń, J.; Jończyk, J.; Panek, D.; Malawska, B. Therapeutic strategies for Alzheimer’s disease in clinical trials. Pharmacol. Rep. 2016, 68, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Sevigny, J.; Chiao, P.; Bussière, T.; Weinreb, P.H.; Williams, L.; Maier, M.; Dunstan, R.; Salloway, S.; Chen, T.; Ling, Y.; et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 2016, 537, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Alarcón, R.; Fuenzalida, C.; Santibáñez, M.; Bernhardi, R. Expression of Scavenger Receptors in Glial Cells: Comparing the adhesion of astrocytes and microglia from neonatal rats to surface-bound β-amyloid. J. Biol. Chem. 2005, 280, 30406–30415. [Google Scholar] [CrossRef] [PubMed]
- Grathwohl, S.A.; Kälin, R.E.; Bolmont, T.; Prokop, S.; Winkelmann, G.; Kaeser, S.A.; Odenthal, J.; Radde, R.; Eldh, T.; Gandy, S.; et al. Formation and maintenance of Alzheimer’s disease β-amyloid plaques in the absence of microglia. Nat. Neurosci. 2009, 12, 1361–1363. [Google Scholar] [CrossRef] [PubMed]
- Jantzen, P.T.; Connor, K.E.; DiCarlo, G.; Wenk, G.L.; Wallace, J.L.; Rojiani, A.M.; Coppola, D.; Morgan, D.; Gordon, M.N. Microglial Activation and β-Amyloid Deposit Reduction Caused by a Nitric Oxide-Releasing Nonsteroidal Anti-Inflammatory Drug in Amyloid Precursor Protein Plus Presenilin-1 Transgenic Mice. J. Neurosci. 2002, 22, 2246–2254. [Google Scholar] [CrossRef] [PubMed]
- Lull, M.E.; Block, M.L. Microglial Activation and Chronic Neurodegeneration. Neurotherapeutics 2010, 7, 354–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dheen, S.T.; Kaur, C.; Ling, E.A. Microglial Activation and its Implications in the Brain Diseases. Curr. Med. Chem. 2007, 14, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Condello, C.; Yuan, P.; Schain, A.; Grutzendler, J. Microglia constitute a barrier that prevents neurotoxic protofibrillar Aβ42 hotspots around plaques. Nat. Commun. 2015, 6, 6176. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lin, J.; Wang, C.; Chen, H.; Yang, D. Antioxidant properties of various solvent extracts from lychee (Litchi chinenesis Sonn.) flowers. Food Chem. 2009, 114, 577–581. [Google Scholar] [CrossRef]
- Sakurai, T.; Nishioka, H.; Fujii, H.; Nakano, N.; Kizaki, T.; Radak, Z.; Iizawa, T.; Haga, S.; Ohno, H. Antioxidative Effects of a New Lychee Fruit-Derived Polyphenol Mixture, Oligonol, Converted into a Low-Molecular Form in Adipocytes. Biosci. Biotechnol. Biochem. 2008, 72, 463–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Xie, H.; Wang, Y.; Wei, X. A-Type Proanthocyanidins from Lychee Seeds and Their Antioxidant and Antiviral Activities. J. Agric. Food Chem. 2010, 58, 11667–11672. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Huang, H.; Huang, J.; Wang, Q.; Wei, Q. Lychee (Litchi chinensis Sonn.) seed water extract as potential antioxidant and antiobese natural additive in meat products. Food Control. 2015, 50, 195–201. [Google Scholar] [CrossRef]
- Heneka, M.T.; Golenbock, D.T.; Latz, E. Innate immunity in Alzheimer’s disease. Nat. Immunol. 2015, 16, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Tarasoff-Conway, J.M.; Carare, R.O.; Osorio, R.S.; Glodzik, L.; Butler, T.; Fieremans, E.; Axel, L.; Rusinek, H.; Nicholson, C.; Zlokovic, B.V.; et al. Clearance systems in the brain-implications for Alzheimer disease. Nat. Rev. Neurol. 2015, 11, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Rajmohan, R.; Reddy, P.H. Amyloid-Beta and Phosphorylated Tau Accumulations Cause Abnormalities at Synapses of Alzheimer’s disease Neurons. J. Alzheimers Dis. 2017, 57, 975–999. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.T.; Lourenco, M.V.; Oliveira, M.M.; De Felice, F.G. Soluble amyloid-β oligomers as synaptotoxins leading to cognitive impairment in Alzheimer’s disease. Front. Cell. Neurosci. 2015, 9, 191. [Google Scholar] [CrossRef] [PubMed]
- Couturier, J.; Paccalin, M.; Morel, M.; Terro, F.; Milin, S.; Pontcharraud, R.; Fauconneau, B.; Page, G. Prevention of the b-amyloid peptide-induced inflammatory process by inhibition of double-stranded RNA-dependent protein kinase in primary murine mixed co-cultures. J. Neuroinflamm. 2011, 8, 72–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zujovic, V.; Benavides, J.; Vige, X.; Carter, C.; Taupin, V. Fractalkine Modulates TNF-α Secretion and Neurotoxicity Induced by Microglial Activation. Glia 2000, 29, 305–315. [Google Scholar] [CrossRef]
- Faro, M.L.L.; Fox, B.; Whatmore, J.L.; Winyard, P.G.; Whiteman, M. Hydrogen sulfide and nitric oxide interactions in inflammation. Nitric Oxide 2014, 41, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Deng, Y.Y.; Hou, D.R.; Li, W.; Feng, X.L.; Yu, Z.L. Association of IL-1, IL-18, and IL-33 gene polymorphisms with late-onset Alzheimer’s disease in a Hunan Han Chinese population. Brain Res. 2015, 1596, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Wang, N.; Liu, X. Resveratrol and Amyloid-Beta: Mechanistic Insights. Nutrients 2017, 9, 1122. [Google Scholar] [CrossRef] [PubMed]
- Ehrnhoefer, D.E.; Bieschke, J.; Boeddrich, A.; Herbst, M.; Masino, L.; Lurz, R.; Engemann, S.; Pastore, A.; Wanker, E.E. EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat. Struct. Mol. Biol. 2008, 15, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Lim, G.P.; Begum, A.N.; Ubeda, O.J.; Simmons, M.R.; Ambegaokar, S.S.; Chen, P.P.; Kayed, R.; Glabe, C.G.; Frautschy, S.A.; et al. Curcumin Inhibits Formation of Amyloid β Oligomers and Fibrils, Binds Plaques, and Reduces Amyloid In Vivo. J. Biol. Chem. 2005, 280, 5892–5901. [Google Scholar] [CrossRef] [PubMed]
- Kook, Y. The inhibitory effect of Scutellaria baicalensis on type 1 interferon production in Raw 264.7 cells. Herb. Formula Sci. 2008, 16, 219–228. [Google Scholar]
- Pervin, M.; Unno, K.; Ohishi, T.; Tanabe, H.; Miyoshi, N.; Nakamura, Y. Beneficial Effects of Green Tea Catechins on Neurodegenerative Diseases. Molecules 2018, 23, 1297. [Google Scholar] [CrossRef] [PubMed]
- Toda, T.; Sunagawa, T.; Kanda, T.; Tagashira, M.; Shirasawa, T.; Shimizu, T. Apple Procyanidins Suppress Amyloid β-Protein Aggregation. Biochem. Res. Int. 2011, 2011, 784698. [Google Scholar] [CrossRef] [PubMed]
- Heo, H.J.; Lee, C.Y. Epicatechin and Catechin in Cocoa Inhibit Amyloid β Protein Induced Apoptosis. J. Agric. Food Chem. 2005, 53, 1445–1448. [Google Scholar] [CrossRef] [PubMed]
- Laurenzi, M.A.; Arcuri, C.; Rossi, R.; Marconi, P.; Bocchini, V. Effects of Microenvironment on Morphology and Function of the Microglial Cell Line BV-2. Neurochem. Res. 2001, 26, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, J.Y.; Zhou, F.; Sun, X.L.; Yao, H.H.; Yang, Y.; Ding, J.H.; Hu, G. The regulation of rotenone-induced inflammatory factor production by ATP-sensitive potassium channel expressed in BV-2 cells. Neurosci. Lett. 2006, 394, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Mincheva-Tasheva, S.; Soler, R.M. NF-κB Signaling Pathways. Neuroscientist 2013, 19, 175–194. [Google Scholar] [CrossRef] [PubMed]
- Alawdi, S.H.; El-Denshary, E.S.; Safar, M.M.; Eidi, H.; David, M.; Abdel-Wahhab, M.A. Neuroprotective Effect of Nanodiamond in Alzheimer’s Disease Rat Model: A Pivotal Role for Modulating NF-κB and STAT3 Signaling. Mol. Neurobiol. 2017, 54, 1906–1918. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Umemura, A.; Sanchez-Lopez, E.; Liang, S.; Shalapour, S.; Wong, J.; He, F.; Boassa, D.; Perkins, G.; Ali, S.R.; et al. NF-κB Restricts Inflammasome Activation via Elimination of Damaged Mitochondria. Cell 2016, 164, 896–910. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Sun, L.; Liu, Q.; Gong, C.; Yao, Y.; Lv, X.; Lin, L.; Yao, H.; Su, F.; Li, D.; et al. A Cytoplasmic NF-κB Interacting Long Noncoding RNA Blocks IκB Phosphorylation and Suppresses Breast Cancer Metastasis. Cancer Cell 2015, 27, 370–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, E.; Fischer, N.; Efferth, T. Phytochemicals as inhibitors of NF-κB for treatment of Alzheimer’s disease. Pharmacol. Res. 2018, 129, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, M.; Kim, Y.J.; Lee, Y.; Bae, D.; Kim, S.; Na, Y.; Yoon, H. Selective PCAF inhibitor ameliorates cognitive and behavioral deficits by suppressing NF-κB-mediated neuroinflammation induced by Aβ in a model of Alzheimer’s disease. Int. J. Mol. Med. 2015, 35, 1109–1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Z.; Han, Y.; Han, X.; Zhang, K.; Chang, Y.; Hu, Z.; Qi, H.; Ting, C.; Zhen, Z.; Hong, W. Upstream regulators and downstream effectors of NF-κB in Alzheimer’s disease. J. Neurol. Sci. 2016, 366, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Lian, H.; Yang, L.; Cole, A.; Sun, L.; Chiang, A.C.A.; Fowler, S.W.; Shim, D.J.; Rodriguez-Rivera, J.; Taglialatela, G.; Jankowsky, J.L.; et al. NF-κB-Activated Astroglial Release of Complement C3 Compromises Neuronal Morphology and Function Associated with Alzheimer’s Disease. Neuron 2015, 85, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Martire, S.; Mosca, L.; d’Erme, M. PARP-1 involvement in neurodegeneration: A focus on Alzheimer’s and Parkinson’s diseases. Mech. Ageing Dev. 2015, 146, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Libien, J.; Shaik, F.; Wolk, J.; Hernández, A.I. Nucleolar PARP-1 Expression Is Decreased in Alzheimer’s Disease: Consequences for Epigenetic Regulation of rDNA and Cognition. Neural Plast. 2016, 2016, 8987928. [Google Scholar] [CrossRef] [PubMed]
- Figueira, I.; Garcia, G.; Pimpão, R.C.; Terrasso, A.P.; Costa, I.; Almeida, A.F.; Tavares, L.; Pais, T.F.; Pinto, P.; Ventura, M.R.; et al. Polyphenols journey through blood-brain barrier towards neuronal protection. Sci. Rep. 2017, 7, 11456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaffer, S.; Halliwell, B. Do polyphenols enter the brain and does it matter? Some theoretical and practical considerations. Genes Nutr. 2012, 7, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, Q.L.; Zhang, X.Y.; Lv, C.; Li, J.; Yuan, Y.; Yin, F.X. Pharmacokinetics and Blood-Brain Barrier Penetration of (+)-Catechin and (−)-Epicatechin in Rats by Microdialysis Sampling Coupled to High-Performance Liquid Chromatography with Chemiluminescence Detection. J. Agric. Food Chem. 2012, 60, 9377–9383. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.R.; Queiroz, J.F.; Reis, S. Brain-targeted delivery of resveratrol using solid lipid nanoparticles functionalized with apolipoprotein E. J. Nanobiotechnol. 2016, 14, 27. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Rani, V. Mode of treatment governs curcumin response on doxorubicin-induced toxicity in cardiomyoblasts. Mol. Cell. Biochem. 2018, 442, 81–96. [Google Scholar] [CrossRef] [PubMed]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; Xiong, R.; Wu, A.-G.; Yu, C.-L.; Zhao, Y.; Qiu, W.-Q.; Wang, X.-L.; Teng, J.-F.; Liu, J.; Chen, H.-X.; et al. Polyphenols Derived from Lychee Seed Suppress Aβ (1-42)-Induced Neuroinflammation. Int. J. Mol. Sci. 2018, 19, 2109. https://doi.org/10.3390/ijms19072109
Tang Y, Xiong R, Wu A-G, Yu C-L, Zhao Y, Qiu W-Q, Wang X-L, Teng J-F, Liu J, Chen H-X, et al. Polyphenols Derived from Lychee Seed Suppress Aβ (1-42)-Induced Neuroinflammation. International Journal of Molecular Sciences. 2018; 19(7):2109. https://doi.org/10.3390/ijms19072109
Chicago/Turabian StyleTang, Yong, Rui Xiong, An-Guo Wu, Chong-Lin Yu, Ya Zhao, Wen-Qiao Qiu, Xiu-Ling Wang, Jin-Feng Teng, Jian Liu, Hai-Xia Chen, and et al. 2018. "Polyphenols Derived from Lychee Seed Suppress Aβ (1-42)-Induced Neuroinflammation" International Journal of Molecular Sciences 19, no. 7: 2109. https://doi.org/10.3390/ijms19072109
APA StyleTang, Y., Xiong, R., Wu, A. -G., Yu, C. -L., Zhao, Y., Qiu, W. -Q., Wang, X. -L., Teng, J. -F., Liu, J., Chen, H. -X., Wu, J. -M., & Qin, D. -L. (2018). Polyphenols Derived from Lychee Seed Suppress Aβ (1-42)-Induced Neuroinflammation. International Journal of Molecular Sciences, 19(7), 2109. https://doi.org/10.3390/ijms19072109