Involvement of Endocytosis in the Transdermal Penetration Mechanism of Ketoprofen Nanoparticles
Abstract
:1. Introduction
2. Results
2.1. Evaluation of the Release of Ketoprofen Nanoparticles from KET-NPs Formulation
2.2. Effect of Energy Dependent Endocytosis on the Transdermal Delivery of Ketoprofen Nanoparticles in the KET-NPs Formulation
2.3. Determination of the Endocytosis Pathway for Ketoprofen Nanoparticles Using Pharmacological Inhibitors
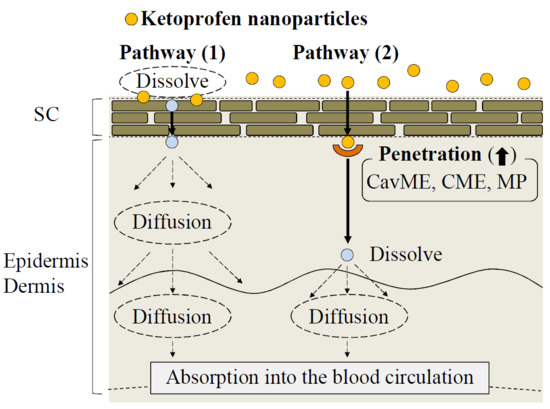
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Chemicals
4.3. Preparation of the Ketoprofen Transdermal Formulation
4.4. Measurement of Ketoprofen by an HPLC Method
4.5. Drug Release from KET-NPs Formulations
4.6. In Vitro Transdermal Penetration of KET-NPs Formulation
4.7. In Vivo Percutaneous Absorption of KET-NPs Formulation
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ANOVA | one-way analysis of variance |
| AUC | area under the ketoprofen concentration-time curve |
| carbopol | Carbopol® 934 |
| CavME | caveolae-mediated endocytosis |
| CME | clathrin-mediated endocytosis |
| COX | cyclooxygenase enzyme |
| D | penetration coefficient through the skin |
| Jc | penetration rate |
| KET-NPs formulation | transdermal formulation containing ketoprofen solid nanoparticles |
| KET-MPs formulation | transdermal formulation containing ketoprofen microparticles |
| Km | skin/preparation partition coefficient |
| NSAIDs | non-steroidal anti-inflammatory drug |
| MC | methylcellulose |
| MP | macropinocytosis |
| PGs | prostaglandins |
| SC | stratum corneum |
| SPM | scanning probe microscope |
| tlag | lag time |
References
- Malfait, A.M.; Schnitzer, T.J. Towards a mechanism-based approach to pain management in osteoarthritis. Nat. Rev. Rheumatol. 2013, 9, 654–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voilley, N.; de Weille, J.; Mamet, J.; Lazdunski, M. Nonsteroid anti-inflammatory drugs inhibit both the activity and the inflammation-induced expression of acid-sensing ion channels in nociceptors. J. Neurosci. 2001, 21, 8026–8033. [Google Scholar] [CrossRef] [PubMed]
- Fossgreen, J.; Ketoprofen. A survey of current publications. Scand. J. Rheumatol. Suppl. 1976, 14, 11–32. [Google Scholar]
- Chi, S.C.; Jun, H.W. Release rates of ketoprofen from poloxamer gels in a membraneless diffusion cell. J. Pharm. Sci. 1991, 80, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Irion, G.D.; Garrison, M.D.; Abraham, W. Effect of PGML excipient mixture in a transdermal system on the in vitro transport of estradiol across the skin. Pharm. Res. 1995, 12, 1618–1622. [Google Scholar] [CrossRef] [PubMed]
- Sinh, S.K.; Durrani, M.J.; Reddy, I.R.; Khan, M.A. Effect of permeation enhancers on the release of ketoprofen through transdermal drug delivery systems. Pharmazie 1996, 53, 741–744. [Google Scholar]
- Cho, Y.I.; Choi, H.K. Enhancement of percutaneous absorption of ketoprofen: Effect of vehicles and adhesive matrix. Int. J. Pharm. 1998, 169, 95–104. [Google Scholar] [CrossRef]
- Sridevi, S.; Diwan, P.V.R. Optimized transdermal delivery of ketoprofen using pH and hydroxypropyl-β-cyclodextrin as co-enhancers. Eur. J. Pharm. Biopharm. 2002, 54, 151–154. [Google Scholar] [CrossRef]
- Podlogar, F.; Bester Rogac, M.; Gasperlin, M. The effect of internal structure of selected water–Tween 40®–Imwitor 308®–IPM microemulsions on ketoprofene release. Int. J. Pharm. 2005, 302, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, L.; Primorac, M.; Stupar, M. In vitro release of diclofenac diethylamine from caprylocaproyl macrogolglycerides based microemulsions. Int. J. Pharm. 2005, 296, 73–79. [Google Scholar] [CrossRef] [PubMed]
- So, J.W.; Park, H.H.; Lee, S.S.; Kim, D.C.; Shin, S.C.; Cho, C.W. Effect of microneedle on the pharmacokinetics of ketoprofen from its transdermal formulations. Drug Deliv. 2009, 16, 52–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shinkai, N.; Korenaga, K.; Mizu, H.; Yamauchi, H. Intra-articular penetration of ketoprofen and analgesic effects after topical patch application in rats. J. Control. Release 2008, 131, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Albanese, A.; Tang, P.S.; Chan, W.C. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lian, Y.; Zhang, L.T.; Aldousari, S.M.; Hedia, H.S.; Asiri, S.A.; Liu, W.K. Cell and nanoparticle transport in tumour microvasculature: The role of size, shape and surface functionality of nanoparticles. Interface Focus 2016, 6, 20150086. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Nieh, M.P.; Li, Y. Decorating Nanoparticle Surface for Targeted Drug Delivery: Opportunities and Challenges. Polymers 2016, 8, 83. [Google Scholar] [CrossRef]
- Ying, L.; Wylie, S.; Tae-Rin, L.; Sung, K.; Han, M.; Dean, H.; Paolo, D.; Wing, K.L. Multiscale modeling and uncertainty quantification in nanoparticle-mediated drug/gene delivery. Comput. Mech. 2014, 53, 511–537. [Google Scholar]
- Chithrani, B.D.; Chan, W.C. Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Lett. 2007, 7, 1542–1550. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gao, H.; Bao, G. Physical Principles of Nanoparticle Cellular Endocytosis. ACS Nano 2015, 9, 8655–8671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, H.; Shi, W.; Freund, L.B. Mechanics of receptor-mediated endocytosis. Proc. Natl. Acad. Sci. USA 2005, 102, 9469–9474. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Iwamae, A.; Tanimoto, S.; Yoshioka, C.; Ito, Y. Pharmacokinetics and Antiinflammatory Effect of a Novel Gel System Containing Ketoprofen Solid Nanoparticles. Biol. Pharm. Bull. 2015, 38, 1918–1924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gratton, S.E.; Ropp, P.A.; Pohlhaus, P.D.; Luft, J.C.; Madden, V.J.; Napier, M.E.; DeSimone, J.M. The effect of particle design on cellular internalization pathways. Proc. Natl. Acad. Sci. USA 2008, 105, 11613–11618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youm, I.; Bazzil, J.D.; Otto, J.W.; Caruso, A.N.; Murowchick, J.B.; Youan, B.B. Influence of surface chemistry on cytotoxicity and cellular uptake of nanocapsules in breast cancer and phagocytic cells. AAPS J. 2014, 16, 550–567. [Google Scholar] [CrossRef] [PubMed]
- Proulx, S.T.; Luciani, P.; Dieterich, L.C.; Karaman, S.; Leroux, J.C.; Detmar, M. Expansion of the lymphatic vasculature in cancer and inflammation: New opportunities for in vivo imaging and drug delivery. J. Control. Release 2013, 172, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Zhang, F.; Lu, X.; Wei, X.; Wang, J.; Fang, X.; Si, D.; Wang, Y.; Zhang, C.; Yang, R.; et al. Polymeric micelles for enhanced lymphatic drug delivery to treat metastatic tumors. J. Control. Release 2013, 171, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hu, W.; Li, J.; Tao, L.; Wei, Y. A comparative study of cellular uptake and cytotoxicity of multi-walled carbon nanotubes, graphene oxide, and nanodiamond. Toxicol. Res. 2012, 1, 62–68. [Google Scholar] [CrossRef]
- Wang, J.; Byrne, J.D.; Napier, M.E.; DeSimone, J.M. More effective nanomedicines through particle design. Small 2011, 7, 1919–1931. [Google Scholar] [CrossRef] [PubMed]
- Rappoport, J. Focusing on clathrin-mediated endocytosis. Biochem. J. 2008, 412, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Benmerah, A.; Lamaze, C. Clathrin-coated Pits: Vive la différence? Traffic 2007, 8, 970–982. [Google Scholar] [CrossRef] [PubMed]
- Medina-Kauwe, L.K. “Alternative” endocytic mechanisms exploited by pathogens: New avenues for therapeutic delivery? Adv. Drug Del. Rev. 2007, 59, 798–809. [Google Scholar] [CrossRef] [PubMed]
- Swanson, J.A. Shaping Cups into Phagosomes and Macropinosomes. Nat. Rev. Mol. Cell Biol. 2008, 9, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Swanson, J.A.; Watts, C. Macropinocytosis. Trends Cell Biol. 1995, 5, 424–428. [Google Scholar] [CrossRef]
- Tamaru, M.; Akita, H.; Fujiwara, T.; Kajimoto, K.; Harashima, H. Leptinderived peptide; a targeting ligand for mouse brain-derived endothelial cells via macropinocytosis. Biochem. Biophys. Res. Commun. 2010, 394, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Roxbury, D.; Gong, X.; Mukhopadhyay, D.; Jagota, A. DNA conjugated SWCNTs enter endothelial cells via Rac1 mediated macropinocytosis. Nano Lett. 2012, 12, 1826–1830. [Google Scholar] [CrossRef] [PubMed]
- Longfa, K.; Jin, S.; Yinglei, Z.; Zhonggui, H. The endocytosis and intracellular fate of nanomedicines: Implication for rational design. Asian J. Pharm. Sci. 2013, 8, 1–10. [Google Scholar]
- Aderem, A.; Underhill, D.M. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 1999, 17, 593–623. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Liu, K.; Manaloto, E.; Casey, A.; Cribaro, G.P.; Byrne, H.J.; Tian, F.; Barcia, C.; Conway, G.E.; Cullen, P.J.; et al. Cold Atmospheric Plasma Induces ATP-Dependent Endocytosis of Nanoparticles and Synergistic U373MG Cancer Cell Death. Sci. Rep. 2018, 8, 5298. [Google Scholar] [CrossRef] [PubMed]
- Mäger, I.; Langel, K.; Lehto, T.; Eiríksdóttir, E.; Langel, U. The role of endocytosis on the uptake kinetics of luciferin-conjugated cell-penetrating peptides. Biochim. Biophys. Acta 2012, 1818, 502–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malomouzh, A.I.; Mukhitov, A.R.; Proskurina, S.E.; Vyskocil, F.; Nikolsky, E.E. The effect of dynasore, a blocker of dynamin-dependent endocytosis, on spontaneous quantal and non-quantal release of acetylcholine in murine neuromuscular junctions. Dokl. Biol. Sci. 2014, 459, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Hufnagel, H.; Hakim, P.; Lima, A.; Hollfelder, F. Fluid phase endocytosis contributes to transfection of DNA by PEI-25. Mol. Ther. 2009, 17, 1411–1417. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, J.; Lykotrafitis, G.; Bao, G.; Suresh, S. Size-dependent endocytosis of nanoparticles. Adv. Mater. 2008, 21, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Liu, D.; Qin, M.; Chen, B.; Song, S.; Dai, W.; Zhang, H.; Wang, X.; Wang, Y.; He, B.; et al. Intestinal Mucin Induces More Endocytosis but Less Transcytosis of Nanoparticles across Enterocytes by Triggering Nanoclustering and Strengthening the Retrograde Pathway. ACS Appl. Mater. Interfaces 2018, 10, 11443–11456. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Tanino, T.; Ito, Y. Pharmacokinetic Studies of Gel System Containing Ibuprofen Solid Nanoparticles. J. Oleo Sci. 2016, 65, 1045–1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Parameter | Normal Skin | SC-Removed Skin | ||
|---|---|---|---|---|
| 4 °C | 37 °C | 4 °C | 37 °C | |
| Jc (nmol·cm−2·min−1) | 26.7 ± 13.5 ** | 208.7 ± 10.8 *,*** | 28.2 ± 4.6 ** | 261.4 ± 8.2 *,**,*** |
| Kp (×10−3·min−1) | 7.5 ± 2.8 ** | 59.0 ± 3.1 *,*** | 7.3 ± 0.2 ** | 74.3 ± 2.5 *,**,*** |
| Km (×10−3) | 5.5 ± 2.9 | 4.8 ± 0.8 | 5.5 ± 0.6 | 3.4 ± 0.9 |
| tlag (min) | 8.0 ± 2.2 **,*** | 1.0 ± 0.2 *,*** | 2.2 ± 0.4 *,** | 0.6 ± 0.1 *,*** |
| D (×10−3·cm2·min−1) | 0.2 ± 0.1 ** | 1.1 ± 0.3 *,*** | 0.4 ± 0.1 ** | 1.4 ± 0.5 *,*** |
| AUCPenetration (μmol·h/cm2) | 6.1 ± 1.0 ** | 49.7 ± 6.1 *,*** | 8.8 ± 1.1 ** | 53.2 ± 5.4 *,*** |
| Parameter | Control | Nystatine | Dynasore | Rottlerin | Cytochalasin D | Nys + Dyn + Rot |
|---|---|---|---|---|---|---|
| Jc (nmol·cm−2·min−1) | 187.9 ± 19.0 ** | 127.3 ± 0.9 *,** | 128.5 ± 1.5 *,** | 128.3 ± 29.5 *,** | 199.5 ± 26.2 ** | 27.1 ± 9.3 * |
| Kp (×10−3·min−1) | 53.1 ± 5.4 ** | 35.9 ± 5.5 *,** | 36.3 ± 9.3 *,** | 36.3 ± 4.2 *,** | 56.4 ± 7.3 ** | 7.2 ± 1.2 * |
| Km (×10−3) | 3.2 ± 0.9 | 3.7 ± 0.2 | 4.2 ± 0.2 | 2.8 ± 0.8 | 2.2 ± 0.8 | 4.3 ± 0.8 |
| tlag (min) | 0.8 ± 0.3 ** | 1.3 ± 0.2 *,** | 1.5 ± 0.2 *,** | 0.8 ± 0.2 ** | 0.5 ± 0.2 ** | 8.0 ± 1.0 * |
| D (×10−3·cm2·min−1) | 1.5 ± 0.3 ** | 0.7 ± 0.1 *,** | 0.6 ± 0.1 *,** | 1.4 ± 0.4 ** | 3.2 ± 1.7 ** | 0.2 ± 0.1 * |
| AUCPenetration (μmol·h/cm2) | 50.7 ± 8.7 ** | 32.9 ± 6.1 *,** | 35.2 ± 6.3 *,** | 36.8 ± 7.4 *,** | 52.6 ± 9.5 ** | 6.8 ± 2.7 * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagai, N.; Ogata, F.; Ishii, M.; Fukuoka, Y.; Otake, H.; Nakazawa, Y.; Kawasaki, N. Involvement of Endocytosis in the Transdermal Penetration Mechanism of Ketoprofen Nanoparticles. Int. J. Mol. Sci. 2018, 19, 2138. https://doi.org/10.3390/ijms19072138
Nagai N, Ogata F, Ishii M, Fukuoka Y, Otake H, Nakazawa Y, Kawasaki N. Involvement of Endocytosis in the Transdermal Penetration Mechanism of Ketoprofen Nanoparticles. International Journal of Molecular Sciences. 2018; 19(7):2138. https://doi.org/10.3390/ijms19072138
Chicago/Turabian StyleNagai, Noriaki, Fumihiko Ogata, Miyu Ishii, Yuya Fukuoka, Hiroko Otake, Yosuke Nakazawa, and Naohito Kawasaki. 2018. "Involvement of Endocytosis in the Transdermal Penetration Mechanism of Ketoprofen Nanoparticles" International Journal of Molecular Sciences 19, no. 7: 2138. https://doi.org/10.3390/ijms19072138
APA StyleNagai, N., Ogata, F., Ishii, M., Fukuoka, Y., Otake, H., Nakazawa, Y., & Kawasaki, N. (2018). Involvement of Endocytosis in the Transdermal Penetration Mechanism of Ketoprofen Nanoparticles. International Journal of Molecular Sciences, 19(7), 2138. https://doi.org/10.3390/ijms19072138