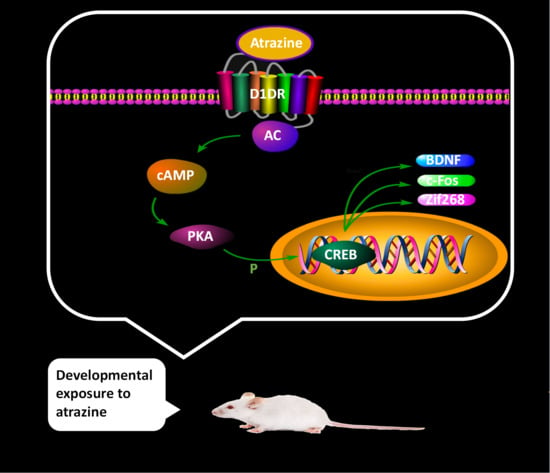
Developmental Exposure to Atrazine Impairs Spatial Memory and Downregulates the Hippocampal D1 Dopamine Receptor and cAMP-Dependent Signaling Pathway in Rats
Abstract
:1. Introduction
2. Results
2.1. Changes in Bodyweight (BW) and Physical Status
2.2. Effects of Atrazine (ATR) Exposure on Spatial Learning and Memory
2.3. Effects of ATR Exposure on the Ultrastructural Features of Hippocampal Neurons
2.4. Effects of ATR Exposure on Dopamine and cAMP Levels
2.5. Effects of ATR Exposure on D1 Dopamine Receptor (D1DR)-Positive Neurons in the Hippocampus
2.6. Effects of ATR Exposure on mRNA Expression Levels
2.7. Effects of ATR Exposure on Protein Expression Levels
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Animals and ATR Exposure
4.3. Morris Water Maze (MWM) Test
4.4. Transmission Electron Microscopy
4.5. Enzyme-Linked Immunosorbent Assay (ELISA) for Dopamine and cAMP
4.6. Immunohistochemical Analysis
4.7. RNA Collection and Reverse Transcription-Polymerase Chain Reaction (RT-PCR) Analysis
4.8. Western Blot Analysis
4.9. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ATR | Atrazine |
| PND | Postnatal day |
| CNS | Central nervous system |
| D1DR | D1 dopamine receptor |
| cAMP | Cyclic adenosine monophosphate |
| PKA | Protein kinase A |
| CREB | cAMP response element-binding protein |
| BDNF | Brain derived neurotrophic factor |
| IEGs | Immediate early genes |
| MWM | Morris water maze |
| Elisa | Enzyme-linked immunosorbent assay |
| RT-PCR | Reverse transcription-polymerase Chain reaction |
| PBS | Phosphate buffered solution |
| TBST | Tris-buffered saline with Tween 20 |
References
- Boffetta, P.; Adami, H.O.; Berry, S.C.; Mandel, J.S. Atrazine and cancer: A review of the epidemiologic evidence. Eur. J. Cancer Prev. 2013, 22, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Jowa, L.; Howd, R. Should atrazine and related chlorotriazines be considered carcinogenic for human health risk assessment. J. Environ. Sci. Health C 2011, 29, 91–144. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, B.G.; Chen, H.; Folmer, J.; Liu, J.; Papadopoulos, V.; Zirkin, B.R. Gestational exposure to atrazine: Effects on the postnatal development of male offspring. J. Androl. 2008, 29, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Stanko, J.P.; Enoch, R.R.; Rayner, J.L.; Davis, C.C.; Wolf, D.C.; Malarkey, D.E.; Fenton, S.E. Effects of prenatal exposure to a low dose atrazine metabolite mixture on pubertal timing and prostate development of male Long-Evans rats. Reprod. Toxicol. 2010, 30, 540–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abarikwu, S.O.; Adesiyan, A.C.; Oyeloja, T.O.; Oyeyemi, M.O.; Farombi, E.O. Changes in sperm characteristics and induction of oxidative stress in the testis and epididymis of experimental rats by a herbicide, atrazine. Arch. Environ. Contam. Toxicol. 2010, 58, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Narotsky, M.G.; Best, D.S.; Guidici, D.L.; Cooper, R.L. Strain comparisons of atrazine-induced pregnancy loss in the rat. Reprod. Toxicol. 2001, 15, 61–69. [Google Scholar] [CrossRef]
- Foradori, C.D.; Hinds, L.R.; Quihuis, A.M.; Lacagnina, A.F.; Breckenridge, C.B.; Handa, R.J. The differential effect of atrazine on luteinizing hormone release in adrenalectomized adult female Wistar rats. Biol. Reprod. 2011, 85, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Bardullas, U.; Giordano, M.; Rodríguez, V.M. Chronic atrazine exposure causes disruption of the spontaneous locomotor activity and alters the striatal dopaminergic system of the male Sprague-Dawley rat. Neurotoxicol. Teratol. 2011, 33, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, V.M.; Limón-Pacheco, J.H.; Mendoza-Trejo, M.S.; González-Gallardo, A.; Hernández-Plata, I.; Giordano, M. Repeated exposure to the herbicide atrazine alters locomotor activity and the nigrostriatal dopaminergic system of the albino rat. Neurotoxicology 2013, 34, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Dodd, C.A.; Filipov, N.M. Short-term atrazine exposure causes behavioral deficits and disrupts monoaminergic systems in male C57BL/6 mice. Neurotoxicol. Teratol. 2013, 39, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Song, X.Y.; Li, J.N.; Wu, Y.P.; Zhang, B.; Li, B.X. Atrazine Causes Autophagy- and Apoptosis-Related Neurodegenerative Effects in Dopaminergic Neurons in the Rat Nigrostriatal Dopaminergic System. Int. J. Mol. Sci. 2015, 16, 13490–13506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wise, R.A. Dopamine, learning and motivation. Nat. Rev. Neurosci. 2004, 5, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.V.; Castner, S.A. Under the curve: Critical issues for elucidating D1 receptor function in working memory. Neuroscience 2006, 139, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Stoof, J.C.; Drukarch, B.; de Boer, P.; Westerink, B.H.; Groenewegen, H.J. Regulation of the activity of striatal cholinergic neurons by dopamine. Neuroscience 1992, 47, 755–770. [Google Scholar] [CrossRef]
- Walters, J.L.; Lansdell, T.A.; Lookingland, K.J.; Baker, L.E. The effects of gestational and chronic atrazine exposure on motor behaviors and striatal dopamine in male Sprague-Dawley rats. Toxicol. Appl. Pharmacol. 2015, 289, 185–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Z.; Dodd, C.A.; Xiao, S.; Krishna, S.; Ye, X.; Filipov, N.M. Gestational and lactational exposure to atrazine via the drinking water causes specific behavioral deficits and selectively alters monoaminergic systems in C57BL/6 mouse dams, juvenile and adult offspring. Toxicol. Sci. 2014, 141, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Kale, O.E.; Oyesola, T.O.; Raji, F.S. Celecoxib, a cyclooxygenase-2 inhibitor, offers chemoprevention against reproductive and neurobehavioural abnormalities induced by atrazine in male Wistar rats. Environ. Toxicol. Pharmacol. 2018, 58, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Hansen, N.; Manahan-Vaughan, D. Dopamine D1/D5 receptors mediate informational saliency that promotes persistent hippocampal long-term plasticity. Cereb. Cortex 2014, 24, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Lisman, J.; Grace, A.A.; Duzel, E. A neoHebbian framework for episodic memory; role of dopamine-dependent late LTP. Trends Neurosci. 2011, 34, 536–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Missale, C.; Nash, S.R.; Robinson, S.W.; Jaber, M.; Caron, M.G. Dopamine receptors: From structure to function. Physiol. Rev. 1998, 78, 189–225. [Google Scholar] [CrossRef] [PubMed]
- Rossato, J.I.; Bevilaqua, L.R.; Izquierdo, I.; Medina, J.H.; Cammarota, M. Dopamine controls persistence of long-term memory storage. Science 2009, 325, 1017–1020. [Google Scholar] [CrossRef] [PubMed]
- Xing, B.; Kong, H.; Meng, X.; Wei, S.G.; Xu, M.; Li, S.B. Dopamine D1 but not D3 receptor is critical for spatial learning and related signaling in the hippocampus. Neuroscience 2010, 169, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Stuchlik, A.; Rehakova, L.; Telensky, P.; Vales, K. Morris water maze learning in Long-Evans rats is differentially affected by blockade of D1-like and D2-like dopamine receptors. Neurosci. Lett. 2007, 422, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Neve, K.A.; Seamans, J.K.; Trantham-Davidson, H. Dopamine receptor signaling. J. Recept. Signal Transduct. Res. 2004, 24, 165–205. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Martínez, S. A new perspective on the role of the CREB family of transcription factors in memory consolidation via adult hippocampal neurogenesis. Front. Mol. Neurosci. 2015, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, C.; Kessels, H.W. The developmental stages of synaptic plasticity. J. Physiol. 2014, 592, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Semple, B.D.; Blomgren, K.; Gimlin, K.; Ferriero, D.M.; Noble-Haeusslein, L.J. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 2013, 106–107, 1–16. [Google Scholar] [CrossRef] [PubMed]
- D’Hooge, R.; De Deyn, P.P. Applications of the Morris water maze in the study of learning and memory. Brain Res. Brain Res. Rev. 2001, 36, 60–90. [Google Scholar] [CrossRef]
- Vorhees, C.V.; Williams, M.T. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006, 1, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Cory-Slechta, D.A.; Merchant-Borna, K.; Allen, J.L.; Liu, S.; Weston, D.; Conrad, K. Variations in the nature of behavioral experience can differentially alter the consequences of developmental exposures to lead, prenatal stress, and the combination. Toxicol. Sci. 2013, 131, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Eichenbaum, H. A cortical-hippocampal system for declarative memory. Nat. Rev. Neurosci. 2000, 1, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Giusi, G.; Facciolo, R.M.; Canonaco, M.; Alleva, E.; Belloni, V.; Dessi’-Fulgheri, F.; Santucci, D. The endocrine disruptor atrazine accounts for a dimorphic somatostatinergic neuronal expression pattern in mice. Toxicol. Sci. 2006, 89, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Niu, Y.; Zhang, R.; Guo, H.; Gao, Y.; Li, Y.; Liu, R. The toxic influence of paraquat on hippocampus of mice: Involvement of oxidative stress. Neurotoxicology 2010, 31, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Canales-Aguirre, A.A.; Gomez-Pinedo, U.A.; Luquin, S.; Ramírez-Herrera, M.A.; Mendoza-Magaña, M.L.; Feria-Velasco, A. Curcumin protects against the oxidative damage induced by the pesticide parathion in the hippocampus of the rat brain. Nutr. Neurosci. 2012, 15, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Rubin, T.G.; Gray, J.D.; McEwen, B.S. Experience and the ever-changing brain: What the transcriptome can reveal. BioEssays 2014, 36, 1072–1081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chien, E.Y.; Liu, W.; Zhao, Q.; Katritch, V.; Han, G.W.; Hanson, M.A.; Shi, L.; Newman, A.H.; Javitch, J.A.; Cherezov, V.; et al. Structure of the human dopamine D3 receptor in complex with a D2/D3 selective antagonist. Science 2010, 330, 1091–1095. [Google Scholar] [CrossRef] [PubMed]
- El-Ghundi, M.; Fletcher, P.J.; Drago, J.; Sibley, D.R.; O’Dowd, B.F.; George, S.R. Spatial learning deficit in dopamine D(1) receptor knockout mice. Eur. J. Pharmacol. 1999, 383, 95–106. [Google Scholar] [CrossRef]
- Karasinska, J.M.; George, S.R.; El-Ghundi, M.; Fletcher, P.J.; O’Dowd, B.F. Modification of dopamine D(1) receptor knockout phenotype in mice lacking both dopamine D(1) and D(3) receptors. Eur. J. Pharmacol. 2000, 399, 171–181. [Google Scholar] [CrossRef]
- South, T.; Huang, X.F. High-fat diet exposure increases dopamine D2 receptor and decreases dopamine transporter receptor binding density in the nucleus accumbens and caudate putamen of mice. Neurochem. Res. 2008, 33, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Frey, J.U. Long-lasting hippocampal plasticity: Cellular model for memory consolidation. Res. Probl. Cell Differ. 2001, 34, 27–40. [Google Scholar] [CrossRef]
- Kandel, E.R. The molecular biology of memory storage: A dialogue between genes and synapses. Science 2001, 294, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Fijał, K.; Nowak, E.; Leśkiewicz, M.; Budziszewska, B.; Filip, M. Working memory deficits and alterations of ERK and CREB phosphorylation following withdrawal from cocaine self-administration. Pharmacol. Rep. 2015, 67, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Richardson, C.L.; Tate, W.P.; Mason, S.E.; Lawlor, P.A.; Dragunow, M.; Abraham, W.C. Correlation between the induction of an immediate early gene, zif/268, and long-term potentiation in the dentate gyrus. Brain Res. 1992, 580, 147–154. [Google Scholar] [CrossRef]
- Matsumoto, T.; Rauskolb, S.; Polack, M.; Klose, J.; Kolbeck, R.; Korte, M.; Barde, Y.A. Biosynthesis and processing of endogenous BDNF: CNS neurons store and secrete BDNF, not pro-BDNF. Nat. Neurosci. 2008, 11, 131–133. [Google Scholar] [CrossRef] [PubMed]
- Dieni, S.; Matsumoto, T.; Dekkers, M.; Rauskolb, S.; Ionescu, M.S.; Deogracias, R.; Gundelfinger, E.D.; Kojima, M.; Nestel, S.; Frotscher, M.; et al. BDNF and its pro-peptide are stored in presynaptic dense core vesicles in brain neurons. J. Cell Biol. 2012, 196, 775–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.S.; Stranahan, A.M.; Chadwick, W.; Zhou, Y.; Wang, L.; Martin, B.; Becker, K.G.; Maudsley, S. Cortical gene transcription response patterns to water maze training in aged mice. BMC Neurosci. 2011, 12, 63. [Google Scholar] [CrossRef] [PubMed]
- Pugazhenthi, S.; Wang, M.; Pham, S.; Sze, C.I.; Eckman, C.B. Downregulation of CREB expression in Alzheimer’s brain and in Aβ-treated rat hippocampal neurons. Mol. Neurodegener. 2011, 6, 60. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, K.; Yamakuni, T.; Hashimoto, M.; Haque, A.M.; Shido, O.; Mimaki, Y.; Sashida, Y.; Ohizumi, Y. Nobiletin restoring beta-amyloid-impaired CREB phosphorylation rescues memory deterioration in Alzheimer’s disease model rats. Neurosci. Lett. 2006, 400, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jin, C.; Lu, X.; Yang, J.; Wu, S.; Liu, Q.; Chen, R.; Bai, C.; Zhang, D.; Zheng, L.; et al. Aluminium chloride impairs long-term memory and downregulates cAMP-PKA-CREB signalling in rats. Toxicology 2014, 323, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Frühauf-Perez, P.K.; Temp, F.R.; Pillat, M.M.; Signor, C.; Wendel, A.L.; Ulrich, H.; Mello, C.F.; Rubin, M.A. Spermine protects from LPS-induced memory deficit via BDNF and TrkB activation. Neurobiol. Learn. Mem. 2018, 149, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; He, X.; Sun, Y.; Li, B. Developmental exposure to paraquat and maneb can impair cognition, learning and memory in Sprague-Dawley rats. Mol. Biosyst. 2016, 12, 3088–3097. [Google Scholar] [CrossRef] [PubMed]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Li, X.; Bi, H.; Ma, K.; Li, B. Developmental Exposure to Atrazine Impairs Spatial Memory and Downregulates the Hippocampal D1 Dopamine Receptor and cAMP-Dependent Signaling Pathway in Rats. Int. J. Mol. Sci. 2018, 19, 2241. https://doi.org/10.3390/ijms19082241
Li J, Li X, Bi H, Ma K, Li B. Developmental Exposure to Atrazine Impairs Spatial Memory and Downregulates the Hippocampal D1 Dopamine Receptor and cAMP-Dependent Signaling Pathway in Rats. International Journal of Molecular Sciences. 2018; 19(8):2241. https://doi.org/10.3390/ijms19082241
Chicago/Turabian StyleLi, Jianan, Xueting Li, Haoran Bi, Kun Ma, and Baixiang Li. 2018. "Developmental Exposure to Atrazine Impairs Spatial Memory and Downregulates the Hippocampal D1 Dopamine Receptor and cAMP-Dependent Signaling Pathway in Rats" International Journal of Molecular Sciences 19, no. 8: 2241. https://doi.org/10.3390/ijms19082241
APA StyleLi, J., Li, X., Bi, H., Ma, K., & Li, B. (2018). Developmental Exposure to Atrazine Impairs Spatial Memory and Downregulates the Hippocampal D1 Dopamine Receptor and cAMP-Dependent Signaling Pathway in Rats. International Journal of Molecular Sciences, 19(8), 2241. https://doi.org/10.3390/ijms19082241