Tubeimoside I Protects Dopaminergic Neurons Against Inflammation-Mediated Damage in Lipopolysaccharide (LPS)-Evoked Model of Parkinson’s Disease in Rats
Abstract
:1. Introduction
2. Results
2.1. TBMS1 Treatment Improves the Behavioral Dysfunction of LPS-Injected PD Rat Model
2.2. TBMS1 Treatment Protects Dopaminergic Neurons in the SN
2.3. TBMS1 Inhibits LPS-Induced Over-Activation of Microglia in the SN
2.4. Effect of TBMS1 on the Viability of BV-2 Cells
2.5. TBMS1 Inhibits the Production of Pro-Inflammatory Cytokines in LPS-Exposed BV-2 Cells
2.6. TBMS1 Inhibits the Production of Pro-Inflammatory Enzymes in LPS-Exposed BV-2 Cells
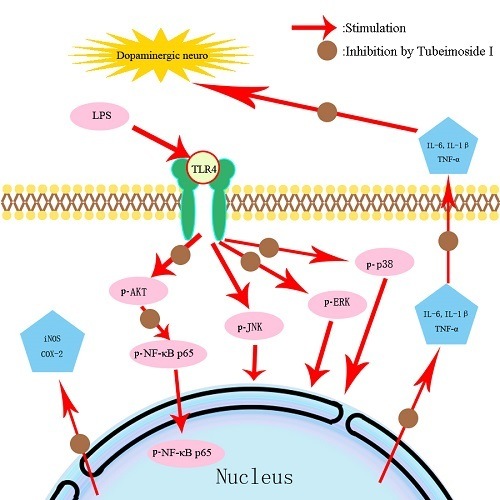
2.7. TBMS1 Inhibits the Phosphorylation of AKT, NF-κB p65, ERK1/2 and p38 in LPS-Exposed BV-2 Cells
3. Discussion
4. Materials and Methods
4.1. Animals and Treatment
4.2. Rotational Behavior Experiment
4.3. TH and IBA-1 Immunohistological Analysis
4.4. Murine Microglial BV-2 Cell Cultures and Treatments
4.5. Cell Viability
4.6. RNA Extraction and Quantitative Real-Time PCR
4.7. Western Blotting Analysis
4.8. Enzyme-Linked Immunosorbent Assay (ELISA)
4.9. Statistical Analysis
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| TBMS1 | Tubeimoside I |
| PD | Parkinson’s disease |
| LPS | Lipopolysaccharide |
| SNpc | Substantia nigra pars compacta |
| TH | Tyrosine hydroxylase |
| IBA-1 | Ionized calcium-binding adaptor molecule-1 |
References
- Obeso, J.A.; Rodriguez-Oroz, M.C.; Goetz, C.G.; Marin, C.; Kordower, J.H.; Rodriguez, M.; Hirsch, E.C.; Farrer, M.; Schapira, A.H.; Halliday, G. Missing pieces in the Parkinson′s disease puzzle. Nat. Med. 2010, 1, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Schapira, A.H. Neurobiology and treatment of Parkinson’s disease. Tr. Pharm. Sci. 2009, 30, 41–47. [Google Scholar] [CrossRef] [PubMed]
- McGeer, P.L.; Itagaki, S.; Akiyama, H.; McGeer, E.G. Rate of cell death in parkinsonism indicates active neuropathological process. Ann. Neurol. 1988, 24, 574–576. [Google Scholar] [CrossRef] [PubMed]
- Block, M.L.; Zecca, L.; Hong, J.S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef] [PubMed]
- McGeer, P.L.; McGeer, E.G. Inflammation and neurodegeneration in Parkinson’s disease. Parkinsonism Relat. Disord. 2004, 10, S3–CS7. [Google Scholar] [CrossRef] [PubMed]
- Graeber, M.B.; Streit, W.J. Microglia: Immune network in the CNS. Brain Pathol. 1990, 1, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Whitton, P.S. Inflammation as a causative factor in the aetiology of Parkinson′s disease. Br. J. Pharm. 2007, 150, 963–976. [Google Scholar] [CrossRef] [PubMed]
- Rojo, A.I.; McBean, G.; Cindric, M.; Egea, J.; Lopez, M.G.; Rada, P.; Zarkovic, N.; Cuadrado, A. Redox control of microglial function: Molecular mechanisms and functional significance. Antioxid. Redox Signal. 2014, 21, 1766–1801. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.; Herrera, A.J.; Venero, J.L.; Santiago, M.; de Pablos, R.M.; Villaran, R.F.; Espinosa-Oliva, A.M.; Arguelles, S.; Sarmiento, M.; Delgado-Cortes, M.J.; et al. Inflammatory animal model for Parkinson’s disease: The intranigral injection of LPS induced the inflammatory process along with the selective egeneration of nigrostriatal dopaminergic neurons. ISRN Neurol. 2011, 2011, 476158. [Google Scholar] [CrossRef] [PubMed]
- Mastroeni, D.; Grover, A.; Leonard, B.; Joyce, J.N.; Coleman, P.D.; Kozik, B.; Bellinger, D.L.; Rogers, J. Microglial responses to dopamine in a cell culture model of Parkinson’s disease. Neurobiol. Ag. 2009, 30, 1805–1817. [Google Scholar] [CrossRef] [PubMed]
- Iczkiewicz, J.; Broom, L.; Cooper, J.D.; Wong, A.M.; Rose, S.; Jenner, P. The RGD-containing peptide fragment of osteopontin protects tyrosine hydroxylase positive cells against toxic insult in primary ventral mesencephalic cultures and in the rat substantia nigra. J. Neurochem. 2010, 114, 1792–1804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, B.; Liu, J.; Ju, C.; Yang, D.; Chen, G.; Xu, S.; Zeng, Y.; Yan, X.; Wang, W.; Liu, D.; et al. Licochalcone A Prevents the Loss of Dopaminergic Neurons by Inhibiting Microglial Activation in Lipopolysaccharide (LPS)-Induced Parkinson’s Disease Models. Int. J. Mol. Sci. 2017, 18, 2043. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Liu, J.; Jiang, L.; Ran, X.; He, D.; Li, Y.; Huang, B.; Wang, W.; Fu, S. Galangin reduces the loss of dopaminergic neurons in an LPS-evoked model of Parkinson’s disease in rats. Int. J. Mol. Sci. 2017, 19, 12. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Liu, J.; Jiang, L.; Ran, X.; He, D.; Li, Y.; Huang, B.; Wang, W.; Liu, D.; Fu, S. Peiminine Protects Dopaminergic Neurons from Inflammation-Induced Cell Death by Inhibiting the ERK1/2 and NF-kappaB Signalling Pathways. Int. J. Mol. Sci. 2018, 19, 821. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, X.; Han, H.; Wang, L.; Bao, W.; Wang, S.; Hoffman, R.M.; Yang, M.; Qi, H.; An, C.; et al. Inhibition of growth and metastasis of triple-negative breast cancer targeted by Traditional Chinese Medicine Tubeimu in orthotopic mice models. Chin. J. Cancer Res. 2018, 30, 112. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Zhao, M.; An, C.; Yang, M.; Li, Q.; Zhang, Y.; Suetsugu, A.; Tome, Y.; Yano, S.; Fu, Y.; et al. Real-time imaging of apoptosis induction of human breast cancer cells by the traditional Chinese medicinal herb tubeimu. Anticancer Res. 2012, 32, 2509–2514. [Google Scholar] [PubMed]
- Xie, Z.; Liu, X.; Cao, L. Study on the pharmacognosy of traditional Chinese medicine tubeimu. J. Chin. Med. Mater. 1998, 21, 548–551. [Google Scholar]
- Zhang, J.B.; Zhang, L.; Li, S.Q.; Hou, A.H.; Liu, W.C.; Dai, L.L. Tubeimoside I attenuates inflammation and oxidative damage in a mice model of PM2.5-induced pulmonary injury. Exp. Ther. Med. 2018, 15, 1602–1607. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, L.; Ma, X.; Sun, S.; Qiu, H.; Li, H.; Xu, J.; Liu, M. Inhibitory effects of tubeimoside I on synoviocytes and collagen-induced arthritis in rats. J. Cell. Physiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Sun, G.; Yuan, X.; Soromou, L.W.; Chen, N.; Xiong, Y.; Feng, H. Tubeimoside-1 attenuates LPS-induced inflammation in RAW 264.7 macrophages and mouse models. Immunopharmacol. Immunotoxicol. 2013, 35, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Ma, R.; Wang, Y.; Nishino, H. Potent anti-tumor activity and low toxicity of tubeimoside 1 isolated from Bolbostemma paniculatum. Planta Med. 1994, 60, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.J.; Zhang, W.D.; Zhang, C.; Liu, R.H.; Shen, Y.H.; Li, H.L.; Wang, X.L.; Wang, X.W.; Zhu, J.B.; Chen, C.L. Quantitative determination of the anticancer agent tubeimoside I in rat plasma by liquid chromatography coupled with mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 845, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Vivekanantham, S.; Shah, S.; Dewji, R.; Dewji, A.; Khatri, C.; Ologunde, R. Neuroinflammation in Parkinson’s disease: Role in neurodegeneration and tissue repair. Int. J. Neurosci. 2015, 125, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Le, W.; Wu, J.; Tang, Y. Protective microglia and their regulation in Parkinson’s disease. Front. Mol. Neurosci. 2016, 9, 89. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, Y.; Zhou, J. Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Transl. Neurodegener. 2015, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.W.; Lee, W.S.; Shin, S.C.; Kim, G.Y.; Choi, B.T.; Choi, Y.H. Anthocyanins downregulate lipopolysaccharide-induced inflammatory responses in BV2 microglial cells by suppressing the NF-kappaB and Akt/MAPKs signaling pathways. Int. J. Mol. Sci. 2013, 14, 1502–1515. [Google Scholar] [CrossRef] [PubMed]
- Syed Hussein, S.S.; Kamarudin, M.N.; Kadir, H.A. (+)-Catechin Attenuates NF-kappaB activation through regulation of akt, MAPK, and AMPK signaling pathways in LPS-induced BV-2 microglial cells. Am. J. Chin. Med. 2015, 43, 927–952. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, C.; Wang, J.; Zhao, S.; Zhang, K.; Wang, J.; Zhang, W.; Wu, C.; Yang, J. Pseudoginsenoside-F11 (PF11) exerts anti-neuroinflammatory effects on LPS-activated microglial cells by inhibiting TLR4-mediated TAK1/IKK/NF-kappaB, MAPKs and Akt signaling pathways. Neuropharmacology 2014, 79, 642–656. [Google Scholar] [CrossRef] [PubMed]
- Olanow, C.W.; Tatton, W.G. Etiology and pathogenesis of Parkinson’s disease. Annu. Rev. Neurosci. 1999, 22, 123–144. [Google Scholar] [CrossRef] [PubMed]
- Cebrian, C.; Loike, J.D.; Sulzer, D. Neuroinflammation in Parkinson’s disease animal models: A cell stress response or a step in neurodegeneration? Curr. Top. Behav. Neurosci. 2015, 22, 237–270. [Google Scholar] [PubMed]
- Shimoji, M.; Pagan, F.; Healton, E.B.; Mocchetti, I. CXCR4 and CXCL12 expression is increased in the nigro-striatal system of Parkinson’s disease. Neurotox. Res. 2009, 16, 318–328. [Google Scholar] [CrossRef] [PubMed]
- McGeer, P.L.; McGeer, E.G. The inflammatory response system of brain: Implications for therapy of Alzheimer and other neurodegenerative diseases. Brain Res. Brain Res. Rev. 1995, 21, 195–218. [Google Scholar] [CrossRef]
- Herrera, A.J.; Castano, A.; Venero, J.L.; Cano, J.; Machado, A. The single intranigral injection of LPS as a new model for studying the selective effects of inflammatory reactions on dopaminergic system. Neurobiol. Dis. 2000, 7, 429–447. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.A.; Das, A.; Ray, S.K.; Banik, N.L. Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res. Bull. 2012, 87, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Vijitruth, R.; Liu, M.; Choi, D.Y.; Nguyen, X.V.; Hunter, R.L.; Bing, G. Cyclooxygenase-2 mediates microglial activation and secondary dopaminergic cell death in the mouse MPTP model of Parkinson’s disease. J. Neuroinflamm. 2006, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Stone, D.K.; Kiyota, T.; Mosley, R.L.; Gendelman, H.E. A model of nitric oxide induced alpha-synuclein misfolding in Parkinson’s disease. Neurosci. Lett. 2012, 523, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.; Francisco, V.; Lopes, M.C.; Cruz, M.T.; Batista, M.T. Intracellular signaling pathways modulated by phenolic compounds: Application for new anti-inflammatory drugs discovery. Curr. Med. Chem. 2012, 19, 2876–2900. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.P.; Wang, J.F.; Xue, W.J.; Liu, H.M.; Liu, B.R.; Zeng, Y.L.; Li, S.N.; Huang, B.X.; Lv, Q.K.; Wang, W.; et al. Anti-inflammatory effects of BHBA in both in vivo and in vitro Parkinson’s disease models are mediated by GPR109A-dependent mechanisms. J. Neuroinflamm. 2015, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.Q.; Kong, X.C.; Qian, C.; Zhang, D. FLZ protects dopaminergic neuron through activating protein kinase B/mammalian target of rapamycin pathway and inhibiting RTP801 expression in Parkinson’s disease models. Neuroscience 2012, 202, 396–404. [Google Scholar] [CrossRef] [PubMed]









| Gene | Sequences | Length (bp) |
|---|---|---|
| β-actin | (F) 5′-GTCAGGTCATCACTATCGGCAAT-3′ | 147 |
| (R) 5′-AGAGGTCTTTACGGATGTCAACGT-3′ | ||
| iNOS | (F) 5′-GAACTGTAGCACAGCACAGGAAAT-3′ | 158 |
| (R) 5′-CGTACCGGATGAGCTGTGAAT-3′ | ||
| COX-2 | (F) 5′-CAGTTTATGTTGTCTGTCCAGAGTTTC-3′ | 127 |
| (R) 5′-CCAGCACTTCACCCATCAGTT-3′ | ||
| TNF-α | (F) 5′-CCCCAAAGGGATGAGAAGTTC-3′ | 136 |
| (R) 5′-CCTCCACTTGGTGGTTTGCT-3′ | ||
| IL-1β | (F) 5′-GTTCCCATTAGACAACTGCACTACAG-3′ | 139 |
| (R) 5′-GTCGTTGCTTGGTTCTCCTTGTA-3′ | ||
| IL-6 | (F) 5′-CCAGAAACCGCTATGAAGTTCC-3′ | 138 |
| (R) 5′-GTTGGGAGTGGTATCCTCTGTGA-3′ |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, D.; Huang, B.; Fu, S.; Li, Y.; Ran, X.; Liu, Y.; Chen, G.; Liu, J.; Liu, D. Tubeimoside I Protects Dopaminergic Neurons Against Inflammation-Mediated Damage in Lipopolysaccharide (LPS)-Evoked Model of Parkinson’s Disease in Rats. Int. J. Mol. Sci. 2018, 19, 2242. https://doi.org/10.3390/ijms19082242
He D, Huang B, Fu S, Li Y, Ran X, Liu Y, Chen G, Liu J, Liu D. Tubeimoside I Protects Dopaminergic Neurons Against Inflammation-Mediated Damage in Lipopolysaccharide (LPS)-Evoked Model of Parkinson’s Disease in Rats. International Journal of Molecular Sciences. 2018; 19(8):2242. https://doi.org/10.3390/ijms19082242
Chicago/Turabian StyleHe, Dewei, Bingxu Huang, Shoupeng Fu, Yuhang Li, Xin Ran, Yandan Liu, Guangxin Chen, Juxiong Liu, and Dianfeng Liu. 2018. "Tubeimoside I Protects Dopaminergic Neurons Against Inflammation-Mediated Damage in Lipopolysaccharide (LPS)-Evoked Model of Parkinson’s Disease in Rats" International Journal of Molecular Sciences 19, no. 8: 2242. https://doi.org/10.3390/ijms19082242
APA StyleHe, D., Huang, B., Fu, S., Li, Y., Ran, X., Liu, Y., Chen, G., Liu, J., & Liu, D. (2018). Tubeimoside I Protects Dopaminergic Neurons Against Inflammation-Mediated Damage in Lipopolysaccharide (LPS)-Evoked Model of Parkinson’s Disease in Rats. International Journal of Molecular Sciences, 19(8), 2242. https://doi.org/10.3390/ijms19082242