Magnolol: A Neolignan from the Magnolia Family for the Prevention and Treatment of Cancer
Abstract
:1. Introduction
2. Chemistry of Magnolol
3. Biological Activities of Magnolol
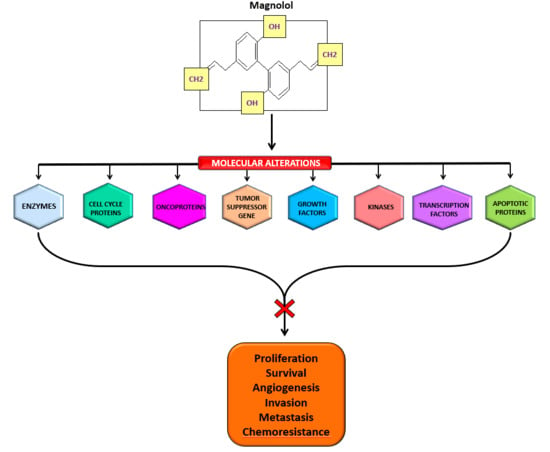
4. Molecular Targets of Magnolol
5. Cancer Chemopreventive and Therapeutic Properties of Magnolol
6. Effect of Magnolol in Different Cancers
6.1. Bladder Cancer
6.2. Brain Cancer
6.3. Breast Cancer
6.4. Colorectal Cancer
6.5. Leukemia
6.6. Liver Cancer
6.7. Lung Cancer
6.8. Ovarian Cancer
6.9. Prostate Cancer
6.10. Skin Cancer
6.11. Other Cancers
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AIF | apoptosis inducing factor |
| AMPK | AMP-activated protein kinase |
| Bak | Bcl-2 homologous antagonist/killer |
| Bax | Bcl-2-associated X protein |
| Bcl-2 | B-cell lymphoma 2 |
| Bcl-XL | B-cell lymphoma-extra large |
| Bid | BH3 interacting-domain death agonist |
| Ca (2+) | Calcium |
| CDC25A | cell division cycle 25 homolog A |
| CDK | cyclin-dependent kinase |
| Cip1 | CDK-interacting protein 1 |
| COX-2 | Cyclooxygenase-2 |
| cyt-c | cytochrome-c |
| DNA | Deoxyribo nucleic acid |
| DR5 | Death receptor 5 EGFR: epidermal growth factor receptor |
| ERK | extracellular phosphorylated signal-regulated kinase |
| FoxO3 | Forkhead box O3 |
| GAS5 | growth arrest-specific 5 HIF-1α:hypoxia-inducible factors-1α |
| IGF-1 | Insulin-like growth factor 1 |
| IGFBP-5 | Insulin-like growth factor binding Protein-5 |
| iNOS | inducible nitric oxide synthase |
| Kip1 | Kinase inhibitory protein |
| 5-LO | 5-lipoxygenase |
| LOX | Lysyl oxidase |
| LT | Leukotriene |
| MDR | Multidrug resistance |
| MMP | Matrix metalloproteinases |
| mTOR | mammalian target of rapamycin |
| NF-κB | Nuclear factor kappa B |
| NSCLC | Non-small cell lung cancer cell lines |
| PARP | Poly ADP ribose polymerase |
| PCNA | Proliferating cell nuclear antigen |
| P-gp | Phosphorylated-glycoprotein |
| PI3K | Phosphatidylinositol-4,5-bisphosphate 3-kinase |
| PKC | protein kinase C |
| PLA2 | phospholipase A2 |
| PLC | phospholipase C |
| PTEN | phosphatase and tensin homolog |
| SOC | Store-operated Ca (2+) channels |
| TNF-α | Tumor necrosis factor-alpha |
| TRAIL | TNF-related apoptosis-inducing ligand |
| uPA | urokinase plasminogen activator |
| VEGF | Vascular endothelial growth factor |
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Sailo, B.L.; Banik, K.; Padmavathi, G.; Javadi, M.; Bordoloi, D.; Kunnumakkara, A.B. Tocotrienols: The promising analogues of vitamin E for cancer therapeutics. Pharmacol. Res. 2018, 130, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Sailo, B.L.; Banik, K.; Harsha, C.; Prasad, S.; Gupta, S.C.; Bharti, A.C.; Aggarwal, B.B. Chronic diseases, inflammation, and spices: How are they linked? J. Transl. Med. 2018, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Khwairakpam, A.D.; Bordoloi, D.; Thakur, K.K.; Monisha, J.; Arfuso, F.; Sethi, G.; Mishra, S.; Kumar, A.P.; Kunnumakkara, A.B. Possible use of Punica granatum (Pomegranate) in cancer therapy. Pharmacol. Res. 2018, 133, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Padmavathi, G.; Roy, N.K.; Bordoloi, D.; Arfuso, F.; Mishra, S.; Sethi, G.; Bishayee, A.; Kunnumakkara, A.B. Butein in health and disease: A comprehensive review. Phytomedicine 2017, 25, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Bordoloi, D.; Padmavathi, G.; Monisha, J.; Roy, N.K.; Prasad, S.; Aggarwal, B.B. Curcumin, the golden nutraceutical: Multitargeting for multiple chronic diseases. Br. J. Pharmacol. 2017, 174, 1325–1348. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.K.; Deka, A.; Bordoloi, D.; Mishra, S.; Kumar, A.P.; Sethi, G.; Kunnumakkara, A.B. The potential role of boswellic acids in cancer prevention and treatment. Cancer Lett. 2016, 377, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Sethi, G. Targeting transcription factor NF-kappaB to overcome chemoresistance and radioresistance in cancer therapy. Biochim. Biophys. Acta 2010, 1805, 167–180. [Google Scholar] [PubMed]
- Manu, K.A.; Shanmugam, M.K.; Li, F.; Chen, L.; Siveen, K.S.; Ahn, K.S.; Kumar, A.P.; Sethi, G. Simvastatin sensitizes human gastric cancer xenograft in nude mice to capecitabine by suppressing nuclear factor-kappa B-regulated gene products. J. Mol. Med. 2014, 92, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Pan, Y.; Lai, R. New mechanism of magnolol and honokiol from Magnolia officinalis against Staphylococcus aureus. Nat. Prod. Commun. 2014, 9, 1307–1309. [Google Scholar] [PubMed]
- Siveen, K.S.; Mustafa, N.; Li, F.; Kannaiyan, R.; Ahn, K.S.; Kumar, A.P.; Chng, W.J.; Sethi, G. Thymoquinone overcomes chemoresistance and enhances the anticancer effects of bortezomib through abrogation of NF-kappaB regulated gene products in multiple myeloma xenograft mouse model. Oncotarget 2014, 5, 634–648. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Shanmugam, M.K.; Siveen, K.S.; Wang, F.; Ong, T.H.; Loo, S.Y.; Swamy, M.M.; Mandal, S.; Kumar, A.P.; Goh, B.C.; et al. Garcinol sensitizes human head and neck carcinoma to cisplatin in a xenograft mouse model despite downregulation of proliferative biomarkers. Oncotarget 2015, 6, 5147–5163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tahover, E.; Hubert, A.; Temper, M.; Salah, A.; Peretz, T.; Hamburger, T.; Uziely, B. An observational cohort study of bevacizumab and chemotherapy in metastatic colorectal cancer patients: Safety and efficacy with analysis by age group. Target. Oncol. 2015, 10, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Manu, K.A.; Shanmugam, M.K.; Ramachandran, L.; Li, F.; Siveen, K.S.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; Arfuso, F.; Kumar, A.P.; et al. Isorhamnetin augments the anti-tumor effect of capeciatbine through the negative regulation of NF-kappaB signaling cascade in gastric cancer. Cancer Lett. 2015, 363, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Banik, K.; Harsha, C.; Bordoloi, D.; Lalduhsaki Sailo, B.; Sethi, G.; Leong, H.C.; Arfuso, F.; Mishra, S.; Wang, L.; Kumar, A.P.; et al. Therapeutic potential of gambogic acid, a caged xanthone, to target cancer. Cancer Lett. 2018, 416, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Monisha, J.; Jaiswal, A.; Banik, K.; Choudhary, H.; Singh, A.K.; Bordoloi, D.; Kunnumakkara, A.B. Cancer Cell Chemoresistance: A Prime Obstacle in Cancer Therapy. In Cancer Cell Chemoresistance and Chemosensitization; World Scientific: Singapore, 2018; ISBN 978-981-320-856-8. [Google Scholar]
- Bordoloi, D.; Roy, N.K.; Monisha, J.; Padmavathi, G.; Kunnumakkara, A.B. Multi-Targeted Agents in Cancer Cell Chemosensitization: What We Learnt from Curcumin Thus Far. Recent Pat. Anti-Cancer Drug Discov. 2016, 11, 67–97. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Nair, A.S.; Sung, B.; Pandey, M.K.; Aggarwal, B.B. Boswellic acid blocks signal transducers and activators of transcription 3 signaling, proliferation, and survival of multiple myeloma via the protein tyrosine phosphatase SHP-1. Mol. Cancer Res. 2009, 7, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Melnick, S.J. Developmental therapeutics: Review of biologically based CAM therapies for potential application in children with cancer: Part I. J. Pediatr. Hematol. Oncol. 2006, 28, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Deorukhkar, A.; Krishnan, S.; Sethi, G.; Aggarwal, B.B. Back to basics: How natural products can provide the basis for new therapeutics. Expert Opin. Investig. Drugs 2007, 16, 1753–1773. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.H.; Al Zohairy, M.A.; Aly, S.M.; Khan, M.A. Curcumin: A potential candidate in prevention of cancer via modulation of molecular pathways. BioMed Res. Int. 2014, 2014, 761608. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.C.; Pellecchia, M. Apoptosis-based therapies for hematologic malignancies. Blood 2005, 106, 408–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.F.; Weng, C.J.; Sethi, G.; Hu, D.N. Natural bioactives and phytochemicals serve in cancer treatment and prevention. Evid.-Based Complement. Altern. Med. 2013, 2013, 698190. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.H.; Sethi, G.; Kuo, P.L. Novel medicines and strategies in cancer treatment and prevention. BioMed Res. Int. 2014, 2014, 474078. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.S.; Yang, S.F.; Sethi, G.; Hu, D.N. Natural bioactives in cancer treatment and prevention. BioMed Res. Int. 2015, 2015, 182835. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Lee, J.H.; Chai, E.Z.; Kanchi, M.M.; Kar, S.; Arfuso, F.; Dharmarajan, A.; Kumar, A.P.; Ramar, P.S.; Looi, C.Y.; et al. Cancer prevention and therapy through the modulation of transcription factors by bioactive natural compounds. Semin. Cancer Biol. 2016, 40–41, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, A.; Sethi, G. Bioactive natural products in cancer prevention and therapy: Progress and promise. Semin. Cancer Biol. 2016, 40–41, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Yarla, N.S.; Bishayee, A.; Sethi, G.; Reddanna, P.; Kalle, A.M.; Dhananjaya, B.L.; Dowluru, K.S.; Chintala, R.; Duddukuri, G.R. Targeting arachidonic acid pathway by natural products for cancer prevention and therapy. Semin. Cancer Biol. 2016, 40–41, 48–81. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Warrier, S.; Kumar, A.P.; Sethi, G.; Arfuso, F. Potential Role of Natural Compounds as Anti-Angiogenic Agents in Cancer. Curr. Vasc. Pharmacol. 2017, 15, 503–519. [Google Scholar] [CrossRef] [PubMed]
- Singh, S. From exotic spice to modern drug? Cell 2007, 130, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M.; Snader, K.M. Natural products as sources of new drugs over the period 1981–2002. J. Nat. Prod. 2003, 66, 1022–1037. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Bordoloi, D.; Harsha, C.; Banik, K.; Gupta, S.C.; Aggarwal, B.B. Curcumin mediates anticancer effects by modulating multiple cell signaling pathways. Clin. Sci. 2017, 131, 1781–1799. [Google Scholar] [CrossRef] [PubMed]
- Harsha, C.; Banik, K.; Bordoloi, D.; Kunnumakkara, A.B. Antiulcer properties of fruits and vegetables: A mechanism based perspective. Food Chem. Toxicol. 2017, 108 Pt A, 104–119. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Sung, B.; Ravindran, J.; Diagaradjane, P.; Deorukhkar, A.; Dey, S.; Koca, C.; Yadav, V.R.; Tong, Z.; Gelovani, J.G.; et al. γ-tocotrienol inhibits pancreatic tumors and sensitizes them to gemcitabine treatment by modulating the inflammatory microenvironment. Cancer Res. 2010, 70, 8695–8705. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, M.I.; Kontos, C.K.; Halabalaki, M.; Skaltsounis, A.L.; Scorilas, A. Nature promises new anticancer agents: Interplay with the apoptosis-related BCL2 gene family. Anti-Cancer Agents Med. Chem. 2014, 14, 375–399. [Google Scholar] [CrossRef]
- Millimouno, F.M.; Dong, J.; Yang, L.; Li, J.; Li, X. Targeting apoptosis pathways in cancer and perspectives with natural compounds from mother nature. Cancer Prev. Res. 2014, 7, 1081–1107. [Google Scholar] [CrossRef] [PubMed]
- Padmavathi, G.; Rathnakaram, S.R.; Monisha, J.; Bordoloi, D.; Roy, N.K.; Kunnumakkara, A.B. Potential of butein, a tetrahydroxychalcone to obliterate cancer. Phytomedicine 2015, 22, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Flower, A.; Ritchie, A.; Liu, J.; Molassiotis, A.; Yu, H.; Lewith, G. Oral Chinese herbal medicine (CHM) as an adjuvant treatment during chemotherapy for non-small cell lung cancer: A systematic review. Lung Cancer 2010, 68, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.J.; Koh, W.; Kim, B.; Kim, S.H. Are there new therapeutic options for treating lung cancer based on herbal medicines and their metabolites? J. Ethnopharmacol. 2011, 138, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Kannaiyan, R.; Sethi, G. Targeting cell signaling and apoptotic pathways by dietary agents: Role in the prevention and treatment of cancer. Nutr. Cancer 2011, 63, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Nong Wang, J.; Kong, L.D.; Jiang, Q.G.; Tan, R.X. Antidepressant effects of Banxia Houpu decoction, a traditional Chinese medicinal empirical formula. J. Ethnopharmacol. 2000, 73, 277–281. [Google Scholar] [CrossRef]
- Hsu, H.-Y.; Hsu, C.-S. Commonly Used Chinese Herb Formulas with Illustrations; Oriental Healing Arts Institute: Irvine, CA, USA, 1980; ISBN 9780941942034. [Google Scholar]
- Sugaya, A.; Tsuda, T.; Obuchi, T.; Sugaya, E. Effect of Chinese herbal medicine “Hange-Koboku-To” on laryngeal reflex of cats and in other pharmacological tests. Planta Med. 1983, 47, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, K.; Wang, Q.; Seki, H.; Satoh, K.; Takeda, A.; Arai, H.; Sasaki, H. The effects of the traditional chinese medicine, “Banxia Houpo Tang (Hange-Koboku To)” on the swallowing reflex in Parkinson’s disease. Phytomedicine 2000, 7, 259–263. [Google Scholar] [CrossRef]
- Fukushima, M. Profiles of effects of traditional oriental herbal medicines on central nervous systems in humans--assessment of saiboku-to and saiko-ka-ryukotsu-borei-to using EEG and pharmacokinetics of herbal medicine-derived ingredients as indices. Seishin Shinkeigaku Zasshi 1997, 99, 355–369. [Google Scholar] [PubMed]
- Lee, Y.J.; Lee, Y.M.; Lee, C.K.; Jung, J.K.; Han, S.B.; Hong, J.T. Therapeutic applications of compounds in the Magnolia family. Pharmacol. Ther. 2011, 130, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Ha, J.; Park, J.H.; Lee, J.Y.; Lee, Y.S.; Park, H.J.; Choi, J.W.; Masuda, Y.; Nakaya, K.; Lee, K.T. Costunolide triggers apoptosis in human leukemia U937 cells by depleting intracellular thiols. Jpn. J. Cancer Res. 2002, 93, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.S.; Lee, K.H.; Han, M.H.; Lee, H.; Ahn, J.M.; Han, S.B.; Han, G.; Lee, K.; Park, S.K.; Kim, H.M. Antiinflammatory activity of methanol extract isolated from stem bark of Magnolia kobus. Phytother. Res. 2008, 22, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.W.; Tsai, K.; Chin, J.H.; Chan, W.L.; Hong, C.Y. Magnolol attenuates peroxidative damage and improves survival of rats with sepsis. Shock 2000, 13, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Jada, S.; Doma, M.R.; Singh, P.P.; Kumar, S.; Malik, F.; Sharma, A.; Khan, I.A.; Qazi, G.N.; Kumar, H.M. Design and synthesis of novel magnolol derivatives as potential antimicrobial and antiproliferative compounds. Eur. J. Med. Chem. 2012, 51, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Maioli, M.; Basoli, V.; Carta, P.; Fabbri, D.; Dettori, M.A.; Cruciani, S.; Serra, P.A.; Delogu, G. Synthesis of magnolol and honokiol derivatives and their effect against hepatocarcinoma cells. PLoS ONE 2018, 13, e0192178. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Ma, H.; Zhang, T.; Liu, H.; Yu, L.; Li, G.; Li, H.; Hu, M. Magnolol Inhibits the Growth of Non-Small Cell Lung Cancer via Inhibiting Microtubule Polymerization. Cell. Physiol. Biochem. 2017, 42, 1789–1801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.C.; Chen, Y.L.; Lee, C.F.; Hung, C.H.; Chou, T.C. Supplementation of Magnolol Attenuates Skeletal Muscle Atrophy in Bladder Cancer-Bearing Mice Undergoing Chemotherapy via Suppression of FoxO3 Activation and Induction of IGF-1. PLoS ONE 2015, 10, e0143594. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, K.; Gailhouste, L.; Yasukawa, K.; Kosaka, N.; Ochiya, T. A robust screening method for dietary agents that activate tumour-suppressor microRNAs. Sci. Rep. 2015, 5, 14697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKeown, B.T.; McDougall, L.; Catalli, A.; Hurta, R.A. Magnolol causes alterations in the cell cycle in androgen insensitive human prostate cancer cells in vitro by affecting expression of key cell cycle regulatory proteins. Nutr. Cancer 2014, 66, 1154–1164. [Google Scholar] [CrossRef] [PubMed]
- Konoshima, T.; Kozuka, M.; Tokuda, H.; Nishino, H.; Iwashima, A.; Haruna, M.; Ito, K.; Tanabe, M. Studies on inhibitors of skin tumor promotion, IX. Neolignans from Magnolia officinalis. J. Nat. Prod. 1991, 54, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Chang, Y.T.; Liu, J.D.; Yu, C.H.; Ho, Y.S.; Lee, Y.H.; Lee, W.S. Molecular mechanisms of apoptosis induced by magnolol in colon and liver cancer cells. Mol. Carcinog. 2001, 32, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, F.; Wang, X.; Wu, X.; Zhang, B.; Zhang, N.; Wu, W.; Wang, Z.; Weng, H.; Liu, S.; et al. Magnolol inhibits growth of gallbladder cancer cells through the p53 pathway. Cancer Sci. 2015, 106, 1341–1350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S.Y.; Liu, J.D.; Chang, H.C.; Yeh, S.D.; Lin, C.H.; Lee, W.S. Magnolol suppresses proliferation of cultured human colon and liver cancer cells by inhibiting DNA synthesis and activating apoptosis. J. Cell. Biochem. 2002, 84, 532–544. [Google Scholar] [CrossRef] [PubMed]
- Zhai, H.; Nakade, K.; Mitsumoto, Y.; Fukuyama, Y. Honokiol and magnolol induce Ca2+ mobilization in rat cortical neurons and human neuroblastoma SH-SY5Y cells. Eur. J. Pharmacol. 2003, 474, 199–204. [Google Scholar] [CrossRef]
- Ikeda, K.; Sakai, Y.; Nagase, H. Inhibitory effect of magnolol on tumour metastasis in mice. Phytother. Res. 2003, 17, 933–937. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhi, X.; Xu, H. Advances on Semisynthesis, Total Synthesis, and Structure-Activity Relationships of Honokiol and Magnolol Derivatives. Mini Rev. Med. Chem. 2016, 16, 404–426. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, M.J. The Merck Index—An Encyclopedia of Chemicals, Drugs, and Biologicals; Merck and Co. Inc.: Whitehouse Station, NJ, USA, 2006; ISBN 091191000X, ISBN 9780911910001. [Google Scholar]
- Lee, C.W.; Hu, S.C.; Yen, F.L.; Hsu, L.F.; Lee, I.T.; Lin, Z.C.; Tsai, M.H.; Huang, C.L.; Liang, C.J.; Chiang, Y.C. Magnolol Nanoparticles Exhibit Improved Water Solubility and Suppress TNF-alpha-Induced VCAM-1 Expression in Endothelial Cells. J. Biomed. Nanotechnol. 2017, 13, 255–268. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information: PubChem Compound Database. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/72300 (accessed on 5 July 2018).
- Jin-Ping, S.; Zai-Kang, T.; Ri-Yan, Z. Study of quality of Hou Po. China J. Chin. Trad. Med. 2000, 25, 466–469. [Google Scholar]
- Tong, Z.-K.; Si, J.-P.; Liu, R. Study on variation and inheritance of phenolic compound concentrations in Magnolia officinalis of different seed sources. For. Res. 2000, 13, 257–261. [Google Scholar]
- Jiang, Y.; Vaysse, J.; Gilard, V.; Balayssac, S.; Dejean, S.; Malet-Martino, M.; David, B.; Fiorini, C.; Barbin, Y. Quality assessment of commercial Magnoliae officinalis Cortex by (1)H-NMR-based metabolomics and HPLC methods. Phytochem. Anal. 2012, 23, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Khoo, C.; Halstead, C.W.; Huynh, T.; Bensoussan, A. Liquid chromatographic determination of honokiol and magnolol in hou po (Magnolia officinalis) as the raw herb and dried aqueous extract. J. AOAC Int. 2007, 90, 1210–1218. [Google Scholar] [PubMed]
- Amorati, R.; Zotova, J.; Baschieri, A.; Valgimigli, L. Antioxidant Activity of Magnolol and Honokiol: Kinetic and Mechanistic Investigations of Their Reaction with Peroxyl Radicals. J. Org. Chem. 2015, 80, 10651–10659. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.Y.; Lai, C.C.; Huang, P.H.; Yang, A.H.; Chiang, S.C.; Huang, P.C.; Tseng, K.W.; Huang, C.H. Magnolol Reduces Renal Ischemia and Reperfusion Injury via Inhibition of Apoptosis. Am. J. Chin. Med. 2017, 45, 1421–1439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, C.M.; Ko, F.N.; Wang, J.P.; Lin, C.N.; Wu, T.S.; Chen, C.C.; Huang, T.F. Antihaemostatic and antithrombotic effect of some antiplatelet agents isolated from Chinese herbs. J. Pharm. Pharmacol. 1991, 43, 667–669. [Google Scholar] [CrossRef] [PubMed]
- Schifano, F.; Guarino, V.; Papanti, D.G.; Baccarin, J.; Orsolini, L.; Corkery, J.M. Is there a potential of misuse for Magnolia officinalis compounds/metabolites? Hum. Psychopharmacol. 2017, 32. [Google Scholar] [CrossRef] [PubMed]
- Hoi, C.P.; Ho, Y.P.; Baum, L.; Chow, A.H. Neuroprotective effect of honokiol and magnolol, compounds from Magnolia officinalis, on beta-amyloid-induced toxicity in PC12 cells. Phytother. Res. 2010, 24, 1538–1542. [Google Scholar] [CrossRef] [PubMed]
- Kou, D.Q.; Jiang, Y.L.; Qin, J.H.; Huang, Y.H. Magnolol attenuates the inflammation and apoptosis through the activation of SIRT1 in experimental stroke rats. Pharmacol. Rep. 2017, 69, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Zhao, R.; Liang, J.C.; Chen, Y. The antidiabetic and hepatoprotective effects of magnolol on diabetic rats induced by high-fat diet and streptozotocin. Acta Pharmaceutica Sinica 2014, 49, 476–481. [Google Scholar] [PubMed]
- Sohn, E.J.; Kim, C.S.; Kim, Y.S.; Jung, D.H.; Jang, D.S.; Lee, Y.M.; Kim, J.S. Effects of magnolol (5,5′-diallyl-2,2′-dihydroxybiphenyl) on diabetic nephropathy in type 2 diabetic Goto-Kakizaki rats. Life Sci. 2007, 80, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Squires, R.F.; Ai, J.; Witt, M.R.; Kahnberg, P.; Saederup, E.; Sterner, O.; Nielsen, M. Honokiol and magnolol increase the number of [3H] muscimol binding sites three-fold in rat forebrain membranes in vitro using a filtration assay, by allosterically increasing the affinities of low-affinity sites. Neurochem. Res. 1999, 24, 1593–1602. [Google Scholar] [CrossRef] [PubMed]
- Garrison, R.; Chambliss, W.G. Effect of a proprietary Magnolia and Phellodendron extract on weight management: A pilot, double-blind, placebo-controlled clinical trial. Altern. Ther. Health Med. 2006, 12, 50–54. [Google Scholar] [PubMed]
- Oikawa, T.; Ito, G.; Koyama, H.; Hanawa, T. Prokinetic effect of a Kampo medicine, Hange-koboku-to (Banxia-houpo-tang), on patients with functional dyspepsia. Phytomedicine 2005, 12, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Chiou, L.C.; Ling, J.Y.; Chang, C.C. Chinese herb constituent beta-eudesmol alleviated the electroshock seizures in mice and electrographic seizures in rat hippocampal slices. Neurosci. Lett. 1997, 231, 171–174. [Google Scholar] [CrossRef]
- Lee, Y.J.; Choi, D.Y.; Han, S.B.; Kim, Y.H.; Kim, K.H.; Hwang, B.Y.; Kang, J.K.; Lee, B.J.; Oh, K.W.; Hong, J.T. Inhibitory effect of ethanol extract of Magnolia officinalis on memory impairment and amyloidogenesis in a transgenic mouse model of Alzheimer’s disease via regulating beta-secretase activity. Phytother. Res. 2012, 26, 1884–1892. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.R.; Chen, H.H.; Ko, C.H.; Chan, M.H. Effects of honokiol and magnolol on acute and inflammatory pain models in mice. Life Sci. 2007, 81, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Kalman, D.S.; Feldman, S.; Feldman, R.; Schwartz, H.I.; Krieger, D.R.; Garrison, R. Effect of a proprietary Magnolia and Phellodendron extract on stress levels in healthy women: A pilot, double-blind, placebo-controlled clinical trial. Nutr. J. 2008, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Mantani, N.; Hisanaga, A.; Kogure, T.; Kita, T.; Shimada, Y.; Terasawa, K. Four cases of panic disorder successfully treated with Kampo (Japanese herbal) medicines: Kami-shoyo-san and Hange-koboku-to. Psychiatry Clin. Neurosci. 2002, 56, 617–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakazawa, T.; Yasuda, T.; Ohsawa, K. Metabolites of orally administered Magnolia officinalis extract in rats and man and its antidepressant-like effects in mice. J. Pharm. Pharmacol. 2003, 55, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.H.; Chou, C.J.; Chen, C.F. Pharmacokinetics and brain distribution of magnolol in the rat after intravenous bolus injection. J. Pharm. Pharmacol. 1996, 48, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Duan, X.; Yang, G.; Zhang, X.; Deng, L.; Zheng, H.; Deng, C.; Wen, J.; Wang, N.; Peng, C.; et al. Honokiol crosses BBB and BCSFB, and inhibits brain tumor growth in rat 9L intracerebral gliosarcoma model and human U251 xenograft glioma model. PLoS ONE 2011, 6, e18490. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.H.; Jawan, B.; Sun, C.K.; Goto, S.; Lin, Y.C.; Hung, C.T.; Pan, M.C.; Hsu, L.W.; Cheng, Y.F.; Lai, C.Y.; et al. High concentration of magnolol induces hepatotoxicity under serum-reduced conditions. Phytomedicine 2010, 17, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, X.; Cui, W.; Zhang, X.; Li, N.; Chen, J.; Wong, A.W.; Roberts, A. Evaluation of short-term and subchronic toxicity of magnolia bark extract in rats. Regul. Toxicol. Pharmacol. 2007, 49, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Coppola, M.; Mondola, R. Potential use of Magnolia officinalis bark polyphenols in the treatment of cannabis dependence. Med. Hypotheses 2014, 83, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Kim, S.Y.; Zhao, Y.Z.; Sohn, D.H. Honokiol reduces oxidative stress, c-jun-NH2-terminal kinase phosphorylation and protects against glycochenodeoxycholic acid-induced apoptosis in primary cultured rat hepatocytes. Planta Med. 2006, 72, 661–664. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wang, X. Cytochrome C-mediated apoptosis. Annu. Rev. Biochem. 2004, 73, 87–106. [Google Scholar] [CrossRef] [PubMed]
- Ledgerwood, E.C.; Morison, I.M. Targeting the apoptosome for cancer therapy. Clin. Cancer Res. 2009, 15, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Taylor, R.C.; Cullen, S.P.; Martin, S.J. Apoptosis: Controlled demolition at the cellular level. Nat. Rev. Mol. Cell Biol. 2008, 9, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Fischer, U.; Janssen, K.; Schulze-Osthoff, K. Cutting-edge apoptosis-based therapeutics: A panacea for cancer? BioDrugs 2007, 21, 273–297. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.E.; Hsieh, M.T.; Tsai, T.H.; Hsu, S.L. Effector mechanism of magnolol-induced apoptosis in human lung squamous carcinoma CH27 cells. Br. J. Pharmacol. 2003, 138, 193–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, F.; Wang, T.; Wu, Y.F.; Gu, Y.; Xu, X.L.; Zheng, S.; Hu, X. Honokiol: A potent chemotherapy candidate for human colorectal carcinoma. World J. Gastroenterol. 2004, 10, 3459–3463. [Google Scholar] [CrossRef] [PubMed]
- Battle, T.E.; Arbiser, J.; Frank, D.A. The natural product honokiol induces caspase-dependent apoptosis in B-cell chronic lymphocytic leukemia (B-CLL) cells. Blood 2005, 106, 690–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.H.; Chen, Y.; Tung, P.Y.; Wu, J.C.; Chen, K.H.; Wu, J.M.; Wang, S.M. Mechanisms for the magnolol-induced cell death of CGTH W-2 thyroid carcinoma cells. J. Cell. Biochem. 2007, 101, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Hahm, E.R.; Arlotti, J.A.; Marynowski, S.W.; Singh, S.V. Honokiol, a constituent of oriental medicinal herb magnolia officinalis, inhibits growth of PC-3 xenografts in vivo in association with apoptosis induction. Clin. Cancer Res. 2008, 14, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Park, S.S.; Lee, U.S.; Kim, W.J.; Moon, S.K. Signaling pathway for TNF-alpha-induced MMP-9 expression: Mediation through p38 MAP kinase, and inhibition by anti-cancer molecule magnolol in human urinary bladder cancer 5637 cells. Int. Immunopharmacol. 2008, 8, 1821–1826. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zang, C.; Emde, A.; Planas-Silva, M.D.; Rosche, M.; Kuhnl, A.; Schulz, C.O.; Elstner, E.; Possinger, K.; Eucker, J. Anti-tumor effect of honokiol alone and in combination with other anti-cancer agents in breast cancer. Eur. J. Pharmacol. 2008, 591, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.C.; Liu, Y.C.; Liang, Y.C.; Ho, Y.S.; Lee, W.S. Magnolol inhibits human glioblastoma cell proliferation through upregulation of p21/Cip1. J. Agric. Food Chem. 2009, 57, 7331–7337. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Aggarwal, B.B. Nuclear transcription factor-kappaB as a target for cancer drug development. Leukemia 2002, 16, 1053–1068. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Eckmann, L.; Greten, T.F.; Park, J.M.; Li, Z.W.; Egan, L.J.; Kagnoff, M.F.; Karin, M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 2004, 118, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Pikarsky, E.; Porat, R.M.; Stein, I.; Abramovitch, R.; Amit, S.; Kasem, S.; Gutkovich-Pyest, E.; Urieli-Shoval, S.; Galun, E.; Ben-Neriah, Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature 2004, 431, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Sethi, G.; Tergaonkar, V. Potential pharmacological control of the NF-kappaB pathway. Trends Pharmacol. Sci. 2009, 30, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Sethi, G.; Shanmugam, M.K.; Ramachandran, L.; Kumar, A.P.; Tergaonkar, V. Multifaceted link between cancer and inflammation. Biosci. Rep. 2012, 32, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Chai, E.Z.; Siveen, K.S.; Shanmugam, M.K.; Arfuso, F.; Sethi, G. Analysis of the intricate relationship between chronic inflammation and cancer. Biochem. J. 2015, 468, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, J.; Arfuso, F.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; Kumar, A.P.; Ahn, K.S.; Sethi, G. NF-kappaB in cancer therapy. Arch. Toxicol. 2015, 89, 711–731. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Harikumar, K.B.; Ahn, K.S.; Badmaev, V.; Aggarwal, B.B. Modification of cysteine residue in p65 subunit of nuclear factor-kappaB (NF-kappaB) by picroliv suppresses NF-kappaB-regulated gene products and potentiates apoptosis. Cancer Res. 2008, 68, 8861–8870. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Ichikawa, H.; Anand, P.; Mohankumar, C.J.; Hema, P.S.; Nair, M.S.; Aggarwal, B.B. Coronarin D, a labdane diterpene, inhibits both constitutive and inducible nuclear factor-kappa B pathway activation, leading to potentiation of apoptosis, inhibition of invasion, and suppression of osteoclastogenesis. Mol. Cancer Ther. 2008, 7, 3306–3317. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Nair, A.S.; Ahn, K.S.; Pandey, M.K.; Yi, Z.; Liu, M.; Aggarwal, B.B. Gossypin, a pentahydroxy glucosyl flavone, inhibits the transforming growth factor beta-activated kinase-1-mediated NF-kappaB activation pathway, leading to potentiation of apoptosis, suppression of invasion, and abrogation of osteoclastogenesis. Blood 2007, 109, 5112–5121. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cao, W.; Zhang, B.; Liu, Y.Q.; Wang, Z.Y.; Wu, Y.P.; Yu, X.J.; Zhang, X.D.; Ming, P.H.; Zhou, G.B.; et al. The natural compound magnolol inhibits invasion and exhibits potential in human breast cancer therapy. Sci. Rep. 2013, 3, 3098. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.S.; Sethi, G.; Shishodia, S.; Sung, B.; Arbiser, J.L.; Aggarwal, B.B. Honokiol potentiates apoptosis, suppresses osteoclastogenesis, and inhibits invasion through modulation of nuclear factor-kappaB activation pathway. Mol. Cancer Res. 2006, 4, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.U.; Kim, M.H.; Kim, H.M.; Jeong, H.J. Anticancer potential of magnolol for lung cancer treatment. Arch. Pharm. Res. 2011, 34, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Park, H.J.; Chung, H.J.; Min, H.Y.; Park, E.J.; Lee, M.A.; Shin, Y.; Lee, S.K. Wnt/beta-catenin signaling mediates the antitumor activity of magnolol in colorectal cancer cells. Mol. Pharmacol. 2012, 82, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Li, H.B.; Yi, X.; Gao, J.M.; Ying, X.X.; Guan, H.Q.; Li, J.C. Magnolol-induced H460 cells death via autophagy but not apoptosis. Arch. Pharm. Res. 2007, 30, 1566–1574. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Guru, S.K.; Pathania, A.S.; Kumar, A.; Bhushan, S.; Malik, F. Autophagy triggered by magnolol derivative negatively regulates angiogenesis. Cell Death Dis. 2013, 4, e889. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Cho, Y.H.; Park, K.; Kim, E.J.; Jung, K.H.; Park, S.S.; Kim, W.J.; Moon, S.K. Magnolol elicits activation of the extracellular signal-regulated kinase pathway by inducing p27KIP1-mediated G2/M-phase cell cycle arrest in human urinary bladder cancer 5637 cells. Biochem. Pharmacol. 2008, 75, 2289–2300. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.C.; Lee, C.F.; Huang, W.H.; Chou, T.C. Magnolol suppresses hypoxia-induced angiogenesis via inhibition of HIF-1alpha/VEGF signaling pathway in human bladder cancer cells. Biochem. Pharmacol. 2013, 85, 1278–1287. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.C.; Tu, S.H.; Huang, C.S.; Chen, C.S.; Ho, C.T.; Lin, H.W.; Lee, C.H.; Chang, H.W.; Chang, C.H.; Wu, C.H.; et al. Human breast cancer cell metastasis is attenuated by lysyl oxidase inhibitors through down-regulation of focal adhesion kinase and the paxillin-signaling pathway. Breast Cancer Res. Treat. 2012, 134, 989–1004. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Yuan, X.; Zhang, B.; Wang, S.; Chen, Q. Screening active anti-breast cancer compounds from Cortex Magnolia officinalis by 2D LC-MS. J. Sep. Sci. 2013, 36, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Bi, Y.; Yang, C.; Yang, J.; Jiang, Y.; Meng, F.; Yu, B.; Khan, M.; Ma, T.; Yang, H. Magnolol induces apoptosis in MCF-7 human breast cancer cells through G2/M phase arrest and caspase-independent pathway. Die Pharm. 2013, 68, 755–762. [Google Scholar]
- Li, M.; Hu, S.; Chen, X.; Wang, R.; Bai, X. Research on major antitumor active components in Zi-Cao-Cheng-Qi decoction based on hollow fiber cell fishing with high performance liquid chromatography. J. Pharm. Biomed. Anal. 2018, 149, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Nabekura, T.; Hiroi, T.; Kawasaki, T.; Uwai, Y. Effects of natural nuclear factor-kappa B inhibitors on anticancer drug efflux transporter human P-glycoprotein. Biomed. Pharmacother. 2015, 70, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Syu, W.J.; Shen, C.C.; Lu, J.J.; Lee, G.H.; Sun, C.M. Antimicrobial and cytotoxic activities of neolignans from Magnolia officinalis. Chem. Biodivers. 2004, 1, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.H.; Ren, H.Y.; Shen, J.X.; Zhang, X.Y.; Ye, H.M.; Shen, D.Y. Magnolol suppresses the proliferation and invasion of cholangiocarcinoma cells via inhibiting the NF-kappaB signaling pathway. Biomed. Pharmacother. 2017, 94, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.F.; Lee, T.S.; Lin, S.Y.; Hsu, S.P.; Juan, S.H.; Hsu, Y.H.; Zhong, W.B.; Lee, W.S. Involvement of Ras/Raf-1/ERK actions in the magnolol-induced upregulation of p21 and cell-cycle arrest in colon cancer cells. Mol. Carcinog. 2007, 46, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B.; Lee, M.S.; Cha, E.Y.; Lee, J.S.; Sul, J.Y.; Song, I.S.; Kim, J.Y. Magnolol-induced apoptosis in HCT-116 colon cancer cells is associated with the AMP-activated protein kinase signaling pathway. Biol. Pharm. Bull. 2012, 35, 1614–1620. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.C.; Lee, W.S. P27/Kip1 is responsible for magnolol-induced U373 apoptosis in vitro and in vivo. J. Agric. Food Chem. 2013, 61, 2811–2819. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Ikeda, K.; Sakai, Y. Inhibitory effect of magnolol and honokiol from Magnolia obovata on human fibrosarcoma HT-1080. Invasiveness in vitro. Planta Med. 2001, 67, 705–708. [Google Scholar] [CrossRef] [PubMed]
- Rasul, A.; Yu, B.; Khan, M.; Zhang, K.; Iqbal, F.; Ma, T.; Yang, H. Magnolol, a natural compound, induces apoptosis of SGC-7901 human gastric adenocarcinoma cells via the mitochondrial and PI3K/Akt signaling pathways. Int. J. Oncol. 2012, 40, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.C.; Hueng, D.Y.; Huang, H.Y.; Chen, J.Y.; Chen, Y. Magnolol and honokiol exert a synergistic anti-tumor effect through autophagy and apoptosis in human glioblastomas. Oncotarget 2016, 7, 29116–29130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.C.; Tsao, M.J.; Chiu, C.Y.; Kan, P.C.; Chen, Y. Magnolol Inhibits Human Glioblastoma Cell Migration by Regulating N-Cadherin. J. Neuropathol. Exp. Neurol. 2018, 77, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Hamasaki, Y.; Kobayashi, I.; Zaitu, M.; Tsuji, K.; Kita, M.; Hayasaki, R.; Muro, E.; Yamamoto, S.; Matsuo, M.; Ichimaru, T.; et al. Magnolol inhibits leukotriene synthesis in rat basophilic leukemia-2H3 cells. Planta Med. 1999, 65, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.B.; Wang, C.Y.; Ho, K.J.; Lu, F.J.; Chang, T.C.; Lee, W.S. Magnolol induces apoptosis in human leukemia cells via cytochrome c release and caspase activation. Anti-Cancer Drugs 2003, 14, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Ikai, T.; Akao, Y.; Nakagawa, Y.; Ohguchi, K.; Sakai, Y.; Nozawa, Y. Magnolol-induced apoptosis is mediated via the intrinsic pathway with release of AIF from mitochondria in U937 cells. Biol. Pharm. Bull. 2006, 29, 2498–2501. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, J.; Jin, W.; Guo, X.; Li, N.; Ma, T.; Huo, Q.; Wu, C. Transglycosylation of neolignans by enzymatic synthesis and evaluation of their antitumor activity. J. South. Med. Univ. 2015, 35, 1570–1574. [Google Scholar]
- Li, H.M.; Zhao, S.R.; Huo, Q.; Ma, T.; Liu, H.; Lee, J.K.; Hong, Y.S.; Wu, C.Z. A new dimeric neolignan from Magnolia grandiflora L. seeds. Arch. Pharm. Res. 2015, 38, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Y.J.; Jin, B.F.; Su, X.J.; Tao, Y.W.; She, Z.G.; Lin, Y.C. 1H and 13C NMR assignments for two lignans from the heartwood of Streblus asper. Magnet. Reson. Chem. 2008, 46, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Di Micco, S.; Pulvirenti, L.; Bruno, I.; Terracciano, S.; Russo, A.; Vaccaro, M.C.; Ruggiero, D.; Muccilli, V.; Cardullo, N.; Tringali, C.; et al. Identification by Inverse Virtual Screening of magnolol-based scaffold as new tankyrase-2 inhibitors. Bioorg. Med. Chem. 2018, 26, 3953–3957. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tong, Y.; Yang, X.; Li, F.; Zheng, L.; Liu, W.; Wu, J.; Ou, R.; Zhang, G.; Hu, M.; et al. Novel histone deacetylase inhibitors derived from Magnolia officinalis significantly enhance TRAIL-induced apoptosis in non-small cell lung cancer. Pharmacol. Res. 2016, 111, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.R.; Chong, I.W.; Chen, Y.H.; Hwang, J.J.; Yin, W.H.; Chen, H.L.; Chou, S.H.; Chiu, C.C.; Liu, P.L. Magnolol induces apoptosis via caspase-independent pathways in non-small cell lung cancer cells. Arch. Pharm. Res. 2014, 37, 548–557. [Google Scholar] [CrossRef] [PubMed]
- You, Q.; Li, M.; Jiao, G. Magnolol induces apoptosis via activation of both mitochondrial and death receptor pathways in A375-S2 cells. Arch. Pharm. Res. 2009, 32, 1789–1794. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.F.; Chou, C.T.; Liang, W.Z.; Kuo, C.C.; Wang, J.L.; Hao, L.J.; Jan, C.R. The effect of magnolol on Ca(2+) homeostasis and its related physiology in human oral cancer cells. Arch. Oral Biol. 2018, 89, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Chuang, T.C.; Hsu, S.C.; Cheng, Y.T.; Shao, W.S.; Wu, K.; Fang, G.S.; Ou, C.C.; Wang, V. Magnolol down-regulates HER2 gene expression, leading to inhibition of HER2-mediated metastatic potential in ovarian cancer cells. Cancer Lett. 2011, 311, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Han, H.K.; Van Anh, L.T. Modulation of P-glycoprotein expression by honokiol, magnolol and 4-O-methylhonokiol, the bioactive components of Magnolia officinalis. Anticancer Res. 2012, 32, 4445–4452. [Google Scholar] [PubMed]
- McKeown, B.T.; Hurta, R.A. Magnolol affects expression of IGF-1 and associated binding proteins in human prostate cancer cells in vitro. Anticancer Res. 2014, 34, 6333–6338. [Google Scholar] [PubMed]
- Lee, D.H.; Szczepanski, M.J.; Lee, Y.J. Magnolol induces apoptosis via inhibiting the EGFR/PI3K/Akt signaling pathway in human prostate cancer cells. J. Cell. Biochem. 2009, 106, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.S.; Park, K.K. Magnolol suppresses metastasis via inhibition of invasion, migration, and matrix metalloproteinase-2/-9 activities in PC-3 human prostate carcinoma cells. Biosci. Biotechnol. Biochem. 2010, 74, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.H.; Chan, C.W.; Fang, J.Y.; Shih, Y.M.; Liu, Y.W.; Wang, T.V.; Chen, C.Y. 2-O-Methylmagnolol upregulates the long non-coding RNA, GAS5, and enhances apoptosis in skin cancer cells. Cell Death Dis. 2017, 8, e2638. [Google Scholar] [CrossRef] [PubMed]
- Kuo, D.H.; Lai, Y.S.; Lo, C.Y.; Cheng, A.C.; Wu, H.; Pan, M.H. Inhibitory effect of magnolol on TPA-induced skin inflammation and tumor promotion in mice. J. Agric. Food Chem. 2010, 58, 5777–5783. [Google Scholar] [CrossRef] [PubMed]
- Chilampalli, C.; Guillermo, R.; Zhang, X.; Kaushik, R.S.; Young, A.; Zeman, D.; Hildreth, M.B.; Fahmy, H.; Dwivedi, C. Effects of magnolol on UVB-induced skin cancer development in mice and its possible mechanism of action. BMC Cancer 2011, 11, 456. [Google Scholar] [CrossRef] [PubMed]
- Chilampalli, C.; Zhang, X.; Kaushik, R.S.; Young, A.; Zeman, D.; Hildreth, M.B.; Fahmy, H.; Dwivedi, C. Chemopreventive effects of combination of honokiol and magnolol with alpha-santalol on skin cancer developments. Drug Discov. Ther. 2013, 7, 109–115. [Google Scholar] [PubMed]
- Khwairakpam, A.D.; Monisha, J.; Banik, K.; Choudhary, H.; Sharma, A.; Bordoloi, D.; Kunnumakkara, A.B. Chemoresistance in Brain Cancer and Different Chemosensitization Approaches. In Cancer Cell Chemoresistance and Chemosensitization; World Scientific: Singapore, 2018; pp. 107–127. ISBN 978-981-320-856-8. [Google Scholar]
- Singh, A.K.; Roy, N.K.; Anip, A.; Banik, K.; Monisha, J.; Bordoloi, D.; Kunnumakkara, A.B. Different methods to inhibit chemoresistance in Hepatocellular carcinoma. In Cancer Cell Chemoresistance and Chemosensitization; World Scientific: Singapore, 2018; pp. 378–398. ISBN 978-981-320-856-8. [Google Scholar]
- Padmavathi, G.; Monisha, J.; Banik, K.; Thakur, K.K.; Choudhary, H.; Bordoloi, D.; Kunnumakkara, A.B. Different chemosensitization approaches to overcome chemoresistance in prostate cancer. In Cancer Cell Chemoresistance and Chemosensitization; World Scientific: Singapore, 2018; pp. 583–613. ISBN 978-981-320-856-8. [Google Scholar]
- Javadi, M.; Roy, N.K.; Sharma, A.; Banik, K.; Ganesan, P.; Bordoloi, D.; Kunnumakkara, A. Chemoresistance and chemosensitization in Melanoma. In Cancer Cell Chemoresistance and Chemosensitization; World Scientific: Singapore, 2018; pp. 479–527. ISBN 978-981-320-856-8. [Google Scholar]
- Kapoor, V.K.; McMichael, A.J. Gallbladder cancer: An ‘Indian’ disease. Natl. Med. J. India 2003, 16, 209–213. [Google Scholar] [PubMed]
- Banik, K.; Sailo, B.L.; Thakur, K.K.; Jaiswal, A.; Monisha, J.; Bordoloi, D.; Kunnumakkara, A.B. Potential of different chemosensitizers to overcome chemoresistance in cervical cancer. In Cancer Cell Chemoresistance and Chemosensitization; World Scientific: Singapore, 2018; pp. 163–179. ISBN 978-981-320-856-8. [Google Scholar]
- Sailo, B.L.; Bordoloi, D.; Banik, K.; Khwairakpam, A.D.; Roy, N.K.; Prakash, J.; Kunnumakkara, A.B. Therapeutic strategies for chemosensitization of renal cancer. In Cancer Cell Chemoresistance and Chemosensitization; World Scientific: Singapore, 2018; pp. 615–639. ISBN 978-981-320-856-8. [Google Scholar]



| Cancer | Models | Mechanism(s) of Action | References |
|---|---|---|---|
| Bladder cancer | In vivo | ↓Myostatin, activin A formation, FoxO3, ubiquitin ligases MuRF-1 & MAFbx/atrogin-1 | [53] |
| In vitro | ↑p27Kip1 ↓cyclin -B1/CDC2 | [122] | |
| In vitro | ↓MMP-9 | [103] | |
| In vitro | ↓HIF-1α/VEGF-dependent angiogenesis pathways | [123] | |
| In vivo | ↓HIF-1α/VEGF-dependent angiogenesis pathways | [123] | |
| Breast cancer | In vitro | ↑miR-200c & E-cadherin | [54] |
| In vitro | ↓LOX | [124] | |
| In vitro | ↓Cell growth | [125] | |
| In vitro | ↑Cell cycle arrest at G2/M phase, ROS, release of cyt-c, AIF, Bax, p21 & p53 ↓MMP, Bcl-2, cyclin-B1 & CDK-1 | [126] | |
| In vitro | ↓MMP-9 & NF-κB activity | [116] | |
| In vivo | ↓MMP-9 & NF-κB activity | [116] | |
| Cervical cancer | In vitro | ↓Cell survival | [127] |
| In vitro | ↓P-gp & MDR | [128] | |
| In vitro | ↑Cell cytotoxicity | [129] | |
| Cholangiocarcinoma | In vitro | ↓PCNA, Ki67, MMP-2,-7,-9, cyclin-D1, p-IκBα & p-P65 ↑Cell cycle arrest in G1 phase | [130] |
| In vivo | ↓Tumor growth | [130] | |
| Colon cancer | In vitro | ↑Cytosolic free Ca(2+); translocation of cyt-c; caspase-3, -8, & - 9 ↓Bcl-2 | [57] |
| In vitro | ↓DNA synthesis ↑cell cycle arrest at G0/G1 phase | [59] | |
| In vivo | ↓Tumor growth ↑p21 | [59] | |
| In vivo | ↑ERK phosphorylation, p21 ↓thymidine incorporation | [131] | |
| In vitro | ↓β-catenin, MMP-7, uPA & c-myc | [109] | |
| In vivo | ↓Invasion & motility of tumor cells | [109] | |
| In vitro | ↑p53, Bax & AMPK activation ↓Bcl-2 | [132] | |
| In vitro | ↑Apoptosis & p27Cip1 protein | [133] | |
| Fibrosarcoma | In vitro | ↓MMP-9 | [134] |
| Gallbladder cancer | In vitro | ↑Cell cycle arrest at G0 /G1 phase, p53 & p21 ↓cyclin -D1, CDC25A, & CDK-2 | [58] |
| In vivo | ↓Tumor growth ↑cell cycle arrest at G0 /G1 phase, p53 & p21 ↓cyclin -D1, CDC25A & CDK-2 | [58] | |
| Gastric cancer | In vitro | ↓PI3K/AKTsignaling pathways | [135] |
| Glioblastoma | In vitro | ↓Cyclin-A, -D1 & CDK-2, -4& -6 | [136] |
| In vitro | ↓Tumor growth ↑apoptosis | [136] | |
| In vitro | ↑Cell cycle arrest at G0 /G1 phase& p21/Cip1 ↓cyclins -A & -D1& DNA synthesis | [105] | |
| In vitro | ↑p27Kip1 & apoptosis | [133] | |
| In vivo | ↑p27Kip1 & apoptosis | [133] | |
| In vitro | ↓myosin light chain phosphatase & N-cadherin | [137] | |
| Kidney cancer | In vitro | ↓Cell survival | [127] |
| In vivo | ↓Tumor growth, invasion & metastasis | [61] | |
| Leukemia | In vivo | ↓LTs, PLA2, 5-LO, LTC4 synthase & LTA4 hydrolase | [138] |
| In vitro | ↑Bax & cleavage of caspase-3, ↓PI3K/AKT pathway | [121] | |
| In vitro | ↑Apoptosis, cyt-c release, caspase-9,-3 &-2 & cleaved PARP | [139] | |
| In vitro | ↓ERK signal transduction &Bcl-2 protein ↑AIF | [140] | |
| Liver cancer | In vitro | ↓Cell viability | [51] |
| In vitro | ↓Cell survival | [127] | |
| In vitro | ↓Cell proliferation | [141] | |
| In vitro | ↓Cell viability | [142] | |
| In vitro | ↑Cytosolic free Ca (2+), translocation of cyt-c, caspase-3, -8, & -9 ↓Bcl-2 | [57] | |
| In vitro | ↓DNA synthesis ↑cell cycle arrest at G0/G1 phase& apoptosis | [59] | |
| In vivo | ↓Tumor growth, invasion & metastasis | [61] | |
| In vitro | ↑Cell cytotoxicity | [129] | |
| In vitro | ↑Cell cytotoxicity | [143] | |
| Lung cancer | In vitro | ↑Cell cycle arrest in M phase, polymerization of microtubule, apoptosis via p53-independent pathway & autophgy via ↓AKT/mTOR | [52] |
| In vivo | ↓Tumor growth | [52] | |
| In vitro | ↓Cell proliferation | [144] | |
| In vitro | ↑Cell apoptosis cell cycle arrest in G0/G1 phase, TRAIL-R2 (DR5), Bax, caspase-3, & cleaved PARP | [145] | |
| In vivo | ↓Tumor growth | [145] | |
| In vitro | ↑Bad, Bcl-XS, & caspase-9, -3 & -6↓Bcl-xL | [98] | |
| In vivo | ↓Tumor growth, invasion & metastasis | [61] | |
| In vitro | ↓NF-κB activation | [117] | |
| In vitro | ↑Autophagy ↓PI3K/PTEN/AKT pathway | [120] | |
| In vitro | ↑Caspase-3 & cleavage of PARP↓NF-κB/Rel A | [118] | |
| In vitro | ↑Release of Bid, Bax & cyt-c from mitochondria ↑PI3K/AKT & ERK1/2 | [146] | |
| Melanoma | In vitro | ↑Casapase-3, -8, -9 activities | [147] |
| Neuroblastoma | In vivo | ↑Cytosolic free Ca (2+); via PLC-mediated pathway | [60] |
| Oral cancer | In vitro | ↑Ca (2+) influx via PKC-sensitive store-operated Ca (2+) entry & ↑Ca (2+) release from ER in a PLC-associated manner | [148] |
| Ovarian cancer | In vitro | ↑Cell cytotoxicity | [129] |
| In vitro | ↓PI3K/AKT/mTOR-signaling, ↑PARP cleavage, caspase-3 activation | [149] | |
| In vitro | ↓P-gp | [150] | |
| Prostate cancer | In vitro | ↓IGF-1, IGFBP-5, p-IGF-1R & ↑IGFBP-3, IGF-1R | [151] |
| In vitro | ↑Cell cytotoxicity, ↓cyclins -A,- B1,-D1 & -E, ↓CDK-2 & -4 | [55] | |
| In vitro | ↓Inhibiting the EGFR/PI3K/AKT signaling, ↑cyt-c release, Bax | [152] | |
| In vitro | ↓MMP-2 & MMP-9 | [153] | |
| In vitro | ↑Autophagy; ↓cell proliferation, migration, invasion & tube formation | [121] | |
| Skin cancer | In vitro | ↑GAS5 & apoptosis | [154] |
| In vivo | ↓Tumor growth | [56] | |
| In vivo | ↓ERK-1/2; MAPK; PI3K/AKT, iNOS & COX-2 | [155] | |
| In vivo | ↑Cleavage of caspase-8 & PARP, p21 & G2/M phase cell cycle arrest | [156] | |
| In vitro | ↑G2/M phase cell cycle arrest, Cip/p21, cleavage of caspase-8 & PARP, ↓cyclin -B1, -A, CDK-4, CDC2 | [156] | |
| In vivo | ↓Cell viability & proliferation↑apoptosis | [157] | |
| In vitro | ↓Cell proliferation, Bax & Bcl-2 ↑apoptosis & caspases-3, 8, 9 | [147] | |
| Spleen cancer | In vivo | ↓Tumor growth, invasion & metastasis | [61] |
| Thyroid cancer | In vitro | ↑Apoptosis via the cyt-c/caspase-3/PARP/AIF & PTEN/AKT/caspase-9/PARP pathways & necrosis via PARP activation | [101] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ranaware, A.M.; Banik, K.; Deshpande, V.; Padmavathi, G.; Roy, N.K.; Sethi, G.; Fan, L.; Kumar, A.P.; Kunnumakkara, A.B. Magnolol: A Neolignan from the Magnolia Family for the Prevention and Treatment of Cancer. Int. J. Mol. Sci. 2018, 19, 2362. https://doi.org/10.3390/ijms19082362
Ranaware AM, Banik K, Deshpande V, Padmavathi G, Roy NK, Sethi G, Fan L, Kumar AP, Kunnumakkara AB. Magnolol: A Neolignan from the Magnolia Family for the Prevention and Treatment of Cancer. International Journal of Molecular Sciences. 2018; 19(8):2362. https://doi.org/10.3390/ijms19082362
Chicago/Turabian StyleRanaware, Abhishek Manoj, Kishore Banik, Vishwas Deshpande, Ganesan Padmavathi, Nand Kishor Roy, Gautam Sethi, Lu Fan, Alan Prem Kumar, and Ajaikumar B. Kunnumakkara. 2018. "Magnolol: A Neolignan from the Magnolia Family for the Prevention and Treatment of Cancer" International Journal of Molecular Sciences 19, no. 8: 2362. https://doi.org/10.3390/ijms19082362
APA StyleRanaware, A. M., Banik, K., Deshpande, V., Padmavathi, G., Roy, N. K., Sethi, G., Fan, L., Kumar, A. P., & Kunnumakkara, A. B. (2018). Magnolol: A Neolignan from the Magnolia Family for the Prevention and Treatment of Cancer. International Journal of Molecular Sciences, 19(8), 2362. https://doi.org/10.3390/ijms19082362