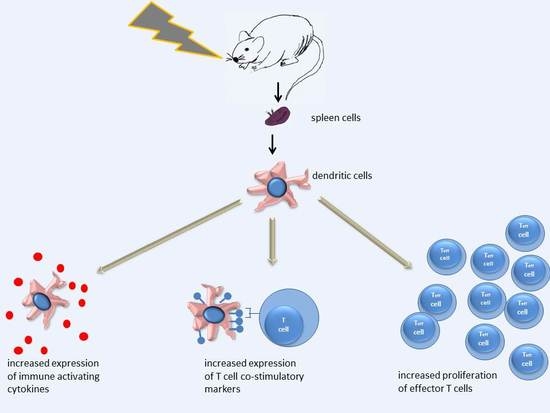
In Vivo Irradiation of Mice Induces Activation of Dendritic Cells
Abstract
:1. Introduction
2. Results
2.1. High-Dose Irradiation Increased the Expression of T Cell Interaction-Related Phenotypical Markers on Splenic DCs
2.2. Low-Dose Irradiation Had Significant Impact on Both Antigen Uptake and Presentation by Splenic DCs
2.3. Irradiation Increased Cytokine Gene Expression in Splenic DCs
2.4. Irradiation Increased the Production of IL-1α and IL-1β by DCs In Vivo
2.5. Irradiated DCs Stimulated Teff and Inhibited Treg Cell Proliferation
3. Discussion
4. Materials and Methods
4.1. Animal Model and Irradiation Procedure
4.2. Isolation of Splenocytes and Dendritic Cells
4.3. Immunophenotyping of DCs
4.4. DC Antigen Uptake and Presentation
4.5. Measuring Cytokine Gene Expression of DCs by qRT-PCR
4.6. In Vivo Cytokine Assay
4.7. T Cell-Proliferation Assay
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Cosimi, A.; Brunstetter, F.H.; Kemmerer, W.T.; Miller, B.N. Cellular immune competence of breast cancer patients receiving radiotherapy. Arch. Surg. 1973, 107, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Bogdandi, E.N.; Balogh, A.; Felgyinszki, N.; Szatmari, T.; Persa, E.; Hildebrandt, G.; Safrany, G.; Lumniczky, K. Effects of low-dose radiation on the immune system of mice after total-body irradiation. Radiat. Res. 2010, 174, 480–489. [Google Scholar] [CrossRef] [PubMed]
- McBride, W.H.; Chiang, C.S.; Olson, J.L.; Wang, C.C.; Hong, J.H.; Pajonk, F.; Dougherty, G.J.; Iwamoto, K.S.; Pervan, M.; Liao, Y.P. A sense of danger from radiation. Radiat. Res. 2004, 162, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Gameiro, S.R.; Jammeh, M.L.; Wattenberg, M.M.; Tsang, K.Y.; Ferrone, S.; Hodge, J.W. Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killing. Oncotarget 2014, 5, 403–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.; Allouch, A.; Martins, I.; Brenner, C.; Modjtahedi, N.; Deutsch, E.; Perfettini, J.L. Modulating Both Tumor Cell Death and Innate Immunity Is Essential for Improving Radiation Therapy Effectiveness. Front. Immunol. 2017, 8, 613. [Google Scholar] [CrossRef] [PubMed]
- Lumniczky, K.; Safrany, G. The impact of radiation therapy on the antitumor immunity: Local effects and systemic consequences. Cancer Lett. 2015, 356, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Theurich, S.; Rothschild, S.I.; Hoffmann, M.; Fabri, M.; Sommer, A.; Garcia-Marquez, M.; Thelen, M.; Schill, C.; Merki, R.; Schmid, T.; et al. Local Tumor Treatment in Combination with Systemic Ipilimumab Immunotherapy Prolongs Overall Survival in Patients with Advanced Malignant Melanoma. Cancer Immunol. Res. 2016, 4, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, A.M.; Simeone, E.; Giannarelli, D.; Muto, P.; Falivene, S.; Borzillo, V.; Giugliano, F.M.; Sandomenico, F.; Petrillo, A.; Curvietto, M.; et al. Abscopal effects of radiotherapy on advanced melanoma patients who progressed after ipilimumab immunotherapy. Oncoimmunology 2014, 3, e28780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Pu, J.; Yu, H.; Liu, Y.; Yan, H.; He, Z.; Feng, X. A Dendritic Cell Vaccine Combined with Radiotherapy Activates the Specific Immune Response in Patients with Esophageal Cancer. J. Immunother. 2017, 40, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Ruiz, M.E.; Perez-Gracia, J.L.; Rodriguez, I.; Alfaro, C.; Onate, C.; Perez, G.; Gil-Bazo, I.; Benito, A.; Inoges, S.; Lopez-Diaz de Cerio, A.; et al. Combined immunotherapy encompassing intratumoral poly-ICLC, dendritic-cell vaccination and radiotherapy in advanced cancer patients. Ann. Oncol. 2018, 29, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Kesminiene, A.; Cardis, E. Cancer risk from paediatric computed tomography scanning: Implications for radiation protection in medicine. Ann. ICRP 2018. [Google Scholar] [CrossRef] [PubMed]
- Valentin, J. Low-dose extrapolation of radiation-related cancer risk. Ann. ICRP 2005, 35, 1–140. [Google Scholar] [PubMed]
- Pandey, R.; Shankar, B.S.; Sharma, D.; Sainis, K.B. Low dose radiation induced immunomodulation: Effect on macrophages and CD8+ T cells. Int. J. Radiat. Biol. 2005, 81, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Yang, G.; Pan, Z.; Zhao, Y.; Liang, X.; Li, W.; Cai, L. Hormetic Response to Low-Dose Radiation: Focus on the Immune System and Its Clinical Implications. Int. J. Mol. Sci. 2017, 18, 280. [Google Scholar] [CrossRef] [PubMed]
- Candeias, S.M.; Mika, J.; Finnon, P.; Verbiest, T.; Finnon, R.; Brown, N.; Bouffler, S.; Polanska, J.; Badie, C. Low-dose radiation accelerates aging of the T-cell receptor repertoire in CBA/Ca mice. Cell Mol. Life Sci. 2017, 74, 4339–4351. [Google Scholar] [CrossRef] [PubMed]
- Chun, S.H.; Park, G.Y.; Han, Y.K.; Kim, S.D.; Kim, J.S.; Lee, C.G.; Yang, K. Effect of low dose radiation on differentiation of bone marrow cells into dendritic cells. Dose-Response Publ. Int. Hormesis Soc. 2012, 11, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Ina, Y.; Sakai, K. Activation of immunological network by chronic low-dose-rate irradiation in wild-type mouse strains: Analysis of immune cell populations and surface molecules. Int. J. Radiat. Biol. 2005, 81, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Z. Cancer control related to stimulation of immunity by low-dose radiation. Dose-Response Publ. Int. Hormesis Soc. 2006, 5, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Li, W.; Jiang, H.; Liang, X.; Zhao, Y.; Yu, D.; Zhou, L.; Wang, G.; Tian, H.; Han, F.; et al. Low-dose radiation may be a novel approach to enhance the effectiveness of cancer therapeutics. Int. J. Cancer 2016, 139, 2157–2168. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, X.; Li, H.; Niu, C.; Yu, D.; Yang, G.; Liang, X.; Wen, X.; Li, M.; Cui, J. Validating the pivotal role of the immune system in low-dose radiation-induced tumor inhibition in Lewis lung cancer-bearing mice. Cancer Med. 2018, 7, 1338–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balogh, A.; Persa, E.; Bogdandi, E.N.; Benedek, A.; Hegyesi, H.; Safrany, G.; Lumniczky, K. The effect of ionizing radiation on the homeostasis and functional integrity of murine splenic regulatory T cells. Inflamm. Res. 2013, 62, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Cella, M.; Scheidegger, D.; Palmer-Lehmann, K.; Lane, P.; Lanzavecchia, A.; Alber, G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J. Exp. Med. 1996, 184, 747–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dilioglou, S.; Cruse, J.M.; Lewis, R.E. Function of CD80 and CD86 on monocyte- and stem cell-derived dendritic cells. Exp. Mol. Pathol. 2003, 75, 217–227. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Y.Y.; Li, J.; You, X.; Qiu, X.H.; Wang, Y.N.; Gao, F.G. Increased antigen presentation but impaired T cells priming after upregulation of interferon-beta induced by lipopolysaccharides is mediated by upregulation of B7H1 and GITRL. PLoS ONE 2014, 9, e105636. [Google Scholar] [CrossRef] [PubMed]
- Henri, S.; Vremec, D.; Kamath, A.; Waithman, J.; Williams, S.; Benoist, C.; Burnham, K.; Saeland, S.; Handman, E.; Shortman, K. The dendritic cell populations of mouse lymph nodes. J. Immunol. 2001, 167, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Scheicher, C.; Mehlig, M.; Dienes, H.P.; Reske, K. Uptake of microparticle-adsorbed protein antigen by bone marrow-derived dendritic cells results in up-regulation of interleukin-1 alpha and interleukin-12 p40/p35 and triggers prolonged, efficient antigen presentation. Eur. J. Immunol. 1995, 25, 1566–1572. [Google Scholar] [CrossRef] [PubMed]
- Ariyoshi, K.; Takabatake, T.; Shinagawa, M.; Kadono, K.; Daino, K.; Imaoka, T.; Kakinuma, S.; Nishimura, M.; Shimada, Y. Age dependence of hematopoietic progenitor survival and chemokine family gene induction after gamma irradiation in bone marrow tissue in C3H/He mice. Radiat. Res. 2014, 181, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lavender, P.; Watson, J.; Arno, M.; Lehner, T. Stress-activated Dendritic Cells (DC) Induce Dual Interleukin (IL)-15- and IL1beta-mediated Pathways, Which May Elicit CD4+ Memory T Cells and Interferon (IFN)-stimulated Genes. J. Biol. Chem. 2015, 290, 15595–15609. [Google Scholar] [CrossRef] [PubMed]
- Linehan, J.L.; Dileepan, T.; Kashem, S.W.; Kaplan, D.H.; Cleary, P.; Jenkins, M.K. Generation of Th17 cells in response to intranasal infection requires TGF-beta1 from dendritic cells and IL-6 from CD301b+ dendritic cells. Proc. Natl. Acad. Sci. USA 2015, 112, 12782–12787. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda, H.; Cerwenka, A.; Morgan, T.; Dutton, R.W. CD28, IL-2-independent costimulatory pathways for CD8 T lymphocyte activation. J. Immunol. 1999, 163, 1133–1142. [Google Scholar] [PubMed]
- Ossetrova, N.I.; Condliffe, D.P.; Ney, P.H.; Krasnopolsky, K.; Hieber, K.P.; Rahman, A.; Sandgren, D.J. Early-response biomarkers for assessment of radiation exposure in a mouse total-body irradiation model. Health Phys. 2014, 106, 772–786. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, M.; O’Garra, A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010, 10, 170–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujihara, M.; Muroi, M.; Tanamoto, K.; Suzuki, T.; Azuma, H.; Ikeda, H. Molecular mechanisms of macrophage activation and deactivation by lipopolysaccharide: Roles of the receptor complex. Pharmacol. Ther. 2003, 100, 171–194. [Google Scholar] [CrossRef] [PubMed]
- Works, M.G.; Koenig, J.B.; Sapolsky, R.M. Soluble TNF receptor 1-secreting ex vivo-derived dendritic cells reduce injury after stroke. J. Cereb. Blood Flow Metab. 2013, 33, 1376–1385. [Google Scholar] [CrossRef] [PubMed]
- Macatonia, S.E.; Hosken, N.A.; Litton, M.; Vieira, P.; Hsieh, C.S.; Culpepper, J.A.; Wysocka, M.; Trinchieri, G.; Murphy, K.M.; O’Garra, A. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J. Immunol. 1995, 154, 5071–5079. [Google Scholar] [PubMed]
- Rutella, S.; Danese, S.; Leone, G. Tolerogenic dendritic cells: Cytokine modulation comes of age. Blood 2006, 108, 1435–1440. [Google Scholar] [CrossRef] [PubMed]
- De Saint-Vis, B.; Fugier-Vivier, I.; Massacrier, C.; Gaillard, C.; Vanbervliet, B.; Ait-Yahia, S.; Banchereau, J.; Liu, Y.J.; Lebecque, S.; Caux, C. The cytokine profile expressed by human dendritic cells is dependent on cell subtype and mode of activation. J. Immunol. 1998, 160, 1666–1676. [Google Scholar] [PubMed]
- Morelli, A.E.; Zahorchak, A.F.; Larregina, A.T.; Colvin, B.L.; Logar, A.J.; Takayama, T.; Falo, L.D.; Thomson, A.W. Cytokine production by mouse myeloid dendritic cells in relation to differentiation and terminal maturation induced by lipopolysaccharide or CD40 ligation. Blood 2001, 98, 1512–1523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugihara, T.; Murano, H.; Nakamura, M.; Tanaka, K. In vivo partial bystander study in a mouse model by chronic medium-dose-rate gamma-ray irradiation. Radiat. Res. 2013, 179, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Chajon, E.; Castelli, J.; Marsiglia, H.; De Crevoisier, R. The synergistic effect of radiotherapy and immunotherapy: A promising but not simple partnership. Crit. Rev. Oncol. Hematol. 2017, 111, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Esposito, A.; Criscitiello, C.; Curigliano, G. Immune checkpoint inhibitors with radiotherapy and locoregional treatment: Synergism and potential clinical implications. Curr. Opin. Oncol. 2015, 27, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, D.; Franzese, C.; Navarria, P.; Ascolese, A.M.; De Rose, F.; Del Vecchio, M.; Santoro, A.; Scorsetti, M. Radiotherapy and immunotherapy: Can this combination change the prognosis of patients with melanoma brain metastases? Cancer Treat. Rev. 2016, 50, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Sharma, A.; von Boehmer, L.; Surace, L.; Knuth, A.; van den Broek, M. Radiotherapy supports protective tumor-specific immunity. Oncoimmunology 2012, 1, 1610–1611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tajima, G.; Delisle, A.J.; Hoang, K.; O’Leary, F.M.; Ikeda, K.; Hanschen, M.; Stoecklein, V.M.; Lederer, J.A. Immune system phenotyping of radiation and radiation combined injury in outbred mice. Radiat. Res. 2013, 179, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Merrick, A.; Errington, F.; Milward, K.; O’Donnell, D.; Harrington, K.; Bateman, A.; Pandha, H.; Vile, R.; Morrison, E.; Selby, P.; et al. Immunosuppressive effects of radiation on human dendritic cells: Reduced IL-12 production on activation and impairment of naive T-cell priming. Br. J. Cancer 2005, 92, 1450–1458. [Google Scholar] [CrossRef] [PubMed]
- Malecka, A.; Wang, Q.; Shah, S.; Sutavani, R.V.; Spendlove, I.; Ramage, J.M.; Greensmith, J.; Franks, H.A.; Gough, M.J.; Saalbach, A.; et al. Stromal fibroblasts support dendritic cells to maintain IL-23/Th17 responses after exposure to ionizing radiation. J. Leukoc. Biol. 2016, 100, 381–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frey, B.; Hehlgans, S.; Rodel, F.; Gaipl, U.S. Modulation of inflammation by low and high doses of ionizing radiation: Implications for benign and malign diseases. Cancer Lett. 2015, 368, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Ventura, J.; Lobachevsky, P.N.; Palazzolo, J.S.; Forrester, H.; Haynes, N.M.; Ivashkevich, A.; Stevenson, A.W.; Hall, C.J.; Ntargaras, A.; Kotsaris, V.; et al. Localized Synchrotron Irradiation of Mouse Skin Induces Persistent Systemic Genotoxic and Immune Responses. Cancer Res. 2017, 77, 6389–6399. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Whitton, J.L. Cutting edge: Re-evaluating the in vivo cytokine responses of CD8+ T cells during primary and secondary viral infections. J. Immunol. 2005, 174, 5936–5940. [Google Scholar] [CrossRef] [PubMed]
- Mayer, K.D.; Mohrs, K.; Reiley, W.; Wittmer, S.; Kohlmeier, J.E.; Pearl, J.E.; Cooper, A.M.; Johnson, L.L.; Woodland, D.L.; Mohrs, M. Cutting edge: T-bet and IL-27R are critical for in vivo IFN-gamma production by CD8 T cells during infection. J. Immunol. 2008, 180, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, H.; Takahashi, K.; Monzen, S.; Kashiwakura, I. Differential Induction from X-irradiated Human Peripheral Blood Monocytes to Dendritic Cells. J. Radiat. Res. 2008, 49, 293–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, Y.P.; Wang, C.C.; Butterfield, L.H.; Economou, J.S.; Ribas, A.; Meng, W.S.; Iwamoto, K.S.; McBride, W.H. Ionizing radiation affects human MART-1 melanoma antigen processing and presentation by dendritic cells. J. Immunol. 2004, 173, 2462–2469. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.D.; Chen, Z.D.; Xing, Y. Gamma irradiation of human dendritic cells influences proliferation and cytokine profile of T cells in autologous mixed lymphocyte reaction. Cell Biol. Int. 2004, 28, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, Q.J.; Yang, S.; Li, Y.F.; El-Gamil, M.; Rosenberg, S.A.; Robbins, P.F. Irradiation enhances human T-cell function by upregulating CD70 expression on antigen-presenting cells in vitro. J. Immunother. 2011, 34, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Jahns, J.; Anderegg, U.; Saalbach, A.; Rosin, B.; Patties, I.; Glasow, A.; Kamprad, M.; Scholz, M.; Hildebrandt, G. Influence of low dose irradiation on differentiation, maturation and T-cell activation of human dendritic cells. Mutat. Res. 2011, 709–710, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Shigematsu, A.; Adachi, Y.; Koike-Kiriyama, N.; Suzuki, Y.; Iwasaki, M.; Koike, Y.; Nakano, K.; Mukaide, H.; Imamura, M.; Ikehara, S. Effects of low-dose irradiation on enhancement of immunity by dendritic cells. J. Radiat. Res. 2007, 48, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, H.; Kashiwakura, I. Impairment of mature dendritic cells derived from X-irradiated human monocytes depends on the type of maturation stimulus used. Radiat. Res. 2012, 178, 280–288. [Google Scholar] [CrossRef] [PubMed]
- De Lastic, A.L.; Rodi, M.; Mouzaki, A. Effect of dendritic cell state and antigen-presentation conditions on resulting T-cell phenotypes and Th cytokine profiles. Immunobiology 2016, 221, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Teitz-Tennenbaum, S.; Li, Q.; Okuyama, R.; Davis, M.A.; Sun, R.; Whitfield, J.; Knibbs, R.N.; Stoolman, L.M.; Chang, A.E. Mechanisms involved in radiation enhancement of intratumoral dendritic cell therapy. J. Immunother. 2008, 31, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Hauser, S.H.; Calorini, L.; Wazer, D.E.; Gattoni-Celli, S. Radiation-enhanced expression of major histocompatibility complex class I antigen H-2Db in B16 melanoma cells. Cancer Res. 1993, 53, 1952–1955. [Google Scholar] [PubMed]
- Reits, E.A.; Hodge, J.W.; Herberts, C.A.; Groothuis, T.A.; Chakraborty, M.; Wansley, E.K.; Camphausen, K.; Luiten, R.M.; de Ru, A.H.; Neijssen, J.; et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J. Exp. Med. 2006, 203, 1259–1271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.Z. Nonlinear dose-response relationship in the immune system following exposure to ionizing radiation: Mechanisms and implications. Nonlinearity Biol. Toxicol. Med. 2003, 1, 71–92. [Google Scholar] [CrossRef] [PubMed]





| Treatment | CD40+ | CD80+ | CD86+ | B7-H1+ | DEC205+ |
|---|---|---|---|---|---|
| 0 Gy DC | 1.00 (±0.37) | 1.00 (±0.05) | 1.00 (±0.42) | 1.00 (±0.12) | 1.00 (±0.24) |
| 0 Gy DC + LPS | 3.45 (±2.88) | 1.54 (±0.39) * | 2.35 (±1.90) | 1.25 (±0.13) | 1.09 (±0.37) |
| 2 Gy DC | 2.82 (±0.87) ** | 1.08 (±0.62) | 1.50 (±0.80) | 1.02 (±0.19) | 1.38 (±0.26) |
| 2 Gy DC + LPS | 2.95 (±1.38) * | 1.56 (±0.46) | 2.18 (±1.89) | 1.29 (±0.18) | 1.27 (±0.39) |
| Name (Product Length) | Sequence |
|---|---|
| 18S RNA (199 bp) | forward: 5′-CGCGGTTCTATTTTGTTGGT-3′ |
| reverse: 5′-AGTCGGCATCGTTTATGGTC-3′ | |
| IL-1α (161 bp) | forward: 5′-CCCGTCCTTAAAGCTGTCTG -3′ |
| reverse: 5′-AATTGGAATCCAGGGGAAAC -3′ | |
| IL-1β (230 bp) | forward: 5′-GCCCATCCTCTGTGACTCAT -3′ |
| reverse: 5′-AGGCCACAGGTATTTTGTCG -3′ | |
| IL-6 (134 bp) | forward: 5′-ATCCAGTTGCCTTCTTGGGACTGA-3′ |
| reverse: 5′-TAAGCCTCCGACTTGTGAAGTGGT-3′ | |
| IL-10 (119 bp) | forward: 5′-GGGTTGCCAAGCCTTATCGGAAAT-3′ |
| reverse: 5′-TCTTCAGCTTCTCACCCAGGGAAT-3′ | |
| IL-12 (210 bp) | forward: 5′-GAAGTCCAATGCAAAGGCGGGAAT-3 |
| reverse: 5′-AAAG-CCAACCAAGCAGAAGACAGC-3′ | |
| TNFα (143 bp) | forward: 5′-CCAACGGCATGGATCTCAAAGACA-3′ |
| reverse: 5′-TGAGATAGCAAATCGGCTGACGGT-3′ |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Persa, E.; Szatmári, T.; Sáfrány, G.; Lumniczky, K. In Vivo Irradiation of Mice Induces Activation of Dendritic Cells. Int. J. Mol. Sci. 2018, 19, 2391. https://doi.org/10.3390/ijms19082391
Persa E, Szatmári T, Sáfrány G, Lumniczky K. In Vivo Irradiation of Mice Induces Activation of Dendritic Cells. International Journal of Molecular Sciences. 2018; 19(8):2391. https://doi.org/10.3390/ijms19082391
Chicago/Turabian StylePersa, Eszter, Tünde Szatmári, Géza Sáfrány, and Katalin Lumniczky. 2018. "In Vivo Irradiation of Mice Induces Activation of Dendritic Cells" International Journal of Molecular Sciences 19, no. 8: 2391. https://doi.org/10.3390/ijms19082391
APA StylePersa, E., Szatmári, T., Sáfrány, G., & Lumniczky, K. (2018). In Vivo Irradiation of Mice Induces Activation of Dendritic Cells. International Journal of Molecular Sciences, 19(8), 2391. https://doi.org/10.3390/ijms19082391