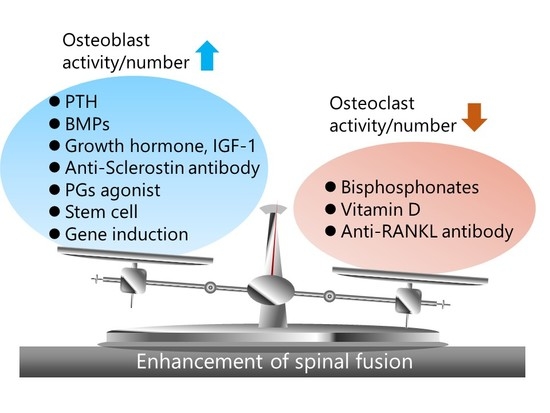
The Biological Enhancement of Spinal Fusion for Spinal Degenerative Disease
Abstract
:1. Introduction
2. Bisphosphonates
2.1. Mechanism of Action
2.2. Experimental Studies in Animal Models of Spinal Fusion
2.3. Clinical Trials for Human Spinal Fusion
2.4. Side Effects
3. Anti-RANKL Monoclonal Antibody
3.1. Mechanism of Action
3.2. Side Effects
3.3. Experimental Studies in Animal Models of Spinal Fusion and Clinical Trials for Human Spinal Fusion
4. PTH
4.1. Mechanism of Action
4.2. Experimental Studies in Animal Models of Spinal Fusion
4.3. Combination Therapy of PTH1-34 and Anti-RANKL Monoclonal Antibody
4.4. Clinical Trials for Human Spinal Fusion
5. BMP
5.1. Mechanism of Action
5.2. Clinical Trials for Human Spinal Fusion
5.3. Side Effects
5.4. Experimental Trials to Both Enhance the Anabolic Effect and Reduce the Side Effects of BMPs
5.5. Carrier Materials for Delivering BMPs
6. Anti-Sclerostin Antibody
6.1. Mechanism of Action
6.2. Experimental Studies in Animal Spinal Fusion Models and Clinical Trials for Human Spinal Fusion
7. Prostaglandins Agonist
7.1. Mechanism of Action
7.2. Experimental Studies in Animal Models of Spinal Fusion and Issues for Clinical Use in Human Spinal Fusion
8. Cell Therapies
8.1. Mechanism of Action and Cell Sources
8.2. Experimental Studies in Animal Spinal Fusion Models, Clinical Trials for Human Spinal Fusion and Issues for Clinical Use
9. Gene Therapies
10. Overview and Future Direction
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| ACS | absorbable collagen sponge |
| ASC | adipose derived stem cell |
| BMA | bone marrow aspiration |
| BMD | bone mineral density |
| BM-MSC | bone marrow-mesenchymal stem cell |
| BMP | bone morphogenetic protein |
| BRONJ | bisphosphonate-related osteonecrosis of the jaw |
| Cbfa1 | core-binding factor subunit alpha-1 |
| COX | Cyclooxygenase |
| FDA | the US Food and Drug Administration |
| ICBG | iliac crest autologous bone grafting |
| OPG | Osteoprotegerin |
| PG | Prostaglandin |
| PKCδ | protein kinase Cδ |
| PTH | parathyroid hormone |
| QOL | quality of life |
| RANKL | receptor activator of nuclear factor κB ligand |
| rhBMP | recombinant human BMP |
| Runx2 | Runt-related transcription factor 2 |
References
- Rajaee, S.S.; Bae, H.W.; Kanim, L.E.; Delamarter, R.B. Spinal fusion in the United States: Analysis of trends from 1998 to 2008. Spine 2012, 37, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Cannada, L.K.; Scherping, S.C.; Yoo, J.U.; Jones, P.K.; Emery, S.E. Pseudoarthrosis of the cervical spine: A comparison of radiographic diagnostic measures. Spine 2003, 28, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Kornblum, M.B.; Fischgrund, J.S.; Herkowitz, H.N.; Abraham, D.A.; Berkower, D.L.; Ditkoff, J.S. Degenerative lumbar spondylolisthesis with spinal stenosis: A prospective long-term study comparing fusion and pseudarthrosis. Spine 2004, 29. [Google Scholar] [CrossRef]
- Makino, T.; Kaito, T.; Fujiwara, H.; Honda, H.; Sakai, Y.; Takenaka, S.; Yoshikawa, H.; Yonenobu, K. Risk Factors for Poor Patient-Reported Quality of Life Outcomes After Posterior Lumbar Interbody Fusion: An Analysis of 2-Year Follow-up. Spine 2017, 42, 1502–1510. [Google Scholar] [CrossRef] [PubMed]
- Makino, T.; Kaito, T.; Fujiwara, H.; Ishii, T.; Iwasaki, M.; Yoshikawa, H.; Yonenobu, K. Does fusion status after posterior lumbar interbody fusion affect patient-based QOL outcomes? An evaluation performed using a patient-based outcome measure. J. Orthop. Sci. 2014, 19, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Phillips, F.M.; Carlson, G.; Emery, S.E.; Bohlman, H.H. Anterior cervical pseudarthrosis. Natural history and treatment. Spine 1997, 22, 1585–1589. [Google Scholar] [CrossRef] [PubMed]
- Lee, R. The outlook for population growth. Science 2011, 333, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Deyo, R.A.; Gray, D.T.; Kreuter, W.; Mirza, S.; Martin, B.I. United States trends in lumbar fusion surgery for degenerative conditions. Spine 2005, 30. [Google Scholar] [CrossRef]
- Datta, H.K.; Ng, W.F.; Walker, J.A.; Tuck, S.P.; Varanasi, S.S. The cell biology of bone metabolism. J. Clin. Pathol. 2008, 61, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, B.P.; Unnanuntana, A.; Cunningham, M.E.; Lane, J.M. The effect of therapies for osteoporosis on spine fusion: A systematic review. Spine J. 2013, 13, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Zipfel, G.J.; Guiot, B.H.; Fessler, R.G. Bone grafting. Neurosurg. Focus 2003, 14, e8. [Google Scholar] [CrossRef] [PubMed]
- Boden, S.D.; Schimandle, J.H.; Hutton, W.C. An experimental lumbar intertransverse process spinal fusion model. Radiographic, histologic, and biomechanical healing characteristics. Spine 1995, 20, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Kalb, S.; Mahan, M.A.; Elhadi, A.M.; Dru, A.; Eales, J.; Lemos, M.; Theodore, N. Pharmacophysiology of bone and spinal fusion. Spine J. 2013, 13, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.L.; Hankenson, K.D. Integration of BMP, Wnt, and notch signaling pathways in osteoblast differentiation. J. Cell. Biochem. 2011, 112, 3491–3501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, F. Building strong bones: Molecular regulation of the osteoblast lineage. Nat. Rev. Mol. Cell Biol. 2011, 13, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Rutkovskiy, A.; Stenslokken, K.O.; Vaage, I.J. Osteoblast Differentiation at a Glance. Med. Sci. Monit. Basic Res. 2016, 22, 95–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeda, K.; Takeshita, S. The role of osteoclast differentiation and function in skeletal homeostasis. J. Biochem. 2016, 159, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, M.C.; Choi, Y. Biology of the RANKL-RANK-OPG System in Immunity, Bone, and Beyond. Front. Immunol. 2014, 5, 511. [Google Scholar] [CrossRef] [PubMed]
- Jung, A.; Bisaz, S.; Fleisch, H. The binding of pyrophosphate and two diphosphonates by hydroxyapatite crystals. Calcif. Tissue Res. 1973, 11, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.D. Anti-resorptives in the management of osteoporosis. Best Pract. Res. Clin. Endocrinol. Metab. 2008, 22, 849–868. [Google Scholar] [CrossRef] [PubMed]
- Rodan, G.A.; Fleisch, H.A. Bisphosphonates: Mechanisms of action. J. Clin. Investig. 1996, 97, 2692–2696. [Google Scholar] [CrossRef] [PubMed]
- Lehenkari, P.P.; Kellinsalmi, M.; Napankangas, J.P.; Ylitalo, K.V.; Monkkonen, J.; Rogers, M.J.; Azhayev, A.; Vaananen, H.K.; Hassinen, I.E. Further insight into mechanism of action of clodronate: Inhibition of mitochondrial ADP/ATP translocase by a nonhydrolyzable, adenine-containing metabolite. Mol. Pharmacol. 2002, 61, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, E.; Masson, C.; Laffitte, A.; Chappard, D.; Audran, M. Osteomalacia in a patient with Paget’s bone disease treated with long-term etidronate. Morphologie 2012, 96, 40–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, R.G.; Croucher, P.I.; Rogers, M.J. Bisphosphonates: Pharmacology, mechanisms of action and clinical uses. Osteoporos. Int. 1999, 9 (Suppl. 2), S66–S80. [Google Scholar] [CrossRef] [PubMed]
- Stone, M.A.; Jakoi, A.M.; Iorio, J.A.; Pham, M.H.; Patel, N.N.; Hsieh, P.C.; Liu, J.C.; Acosta, F.L.; Hah, R.; Wang, J.C. Bisphosphonate’s and Intermittent Parathyroid Hormone’s Effect on Human Spinal Fusion: A Systematic Review of the Literature. Asian Spine J. 2017, 11, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Van Beek, E.R.; Lowik, C.W.; Papapoulos, S.E. Bisphosphonates suppress bone resorption by a direct effect on early osteoclast precursors without affecting the osteoclastogenic capacity of osteogenic cells: The role of protein geranylgeranylation in the action of nitrogen-containing bisphosphonates on osteoclast precursors. Bone 2002, 30, 64–70. [Google Scholar] [PubMed]
- McDonald, M.M.; Dulai, S.; Godfrey, C.; Amanat, N.; Sztynda, T.; Little, D.G. Bolus or weekly zoledronic acid administration does not delay endochondral fracture repair but weekly dosing enhances delays in hard callus remodeling. Bone 2008, 43, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.C.; Khan, S.N.; Sandhu, H.S.; Metzl, J.A.; Cammisa, F.P., Jr.; Zheng, F.; Sama, A.A.; Lane, J.M. Alendronate inhibits spine fusion in a rat model. Spine 2005, 30, 2516–2522. [Google Scholar] [CrossRef] [PubMed]
- Lehman, R.A., Jr.; Kuklo, T.R.; Freedman, B.A.; Cowart, J.R.; Mense, M.G.; Riew, K.D. The effect of alendronate sodium on spinal fusion: A rabbit model. Spine J. 2004, 4, 36–43. [Google Scholar] [CrossRef]
- Nakao, S.; Minamide, A.; Kawakami, M.; Boden, S.D.; Yoshida, M. The influence of alendronate on spine fusion in an osteoporotic animal model. Spine 2011, 36, 1446–1452. [Google Scholar] [CrossRef] [PubMed]
- Yasen, M.; Li, X.; Jiang, L.; Yuan, W.; Che, W.; Dong, J. Effect of zoledronic acid on spinal fusion outcomes in an ovariectomized rat model of osteoporosis. J. Orthop. Res. 2015, 33, 1297–1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagahama, K.; Kanayama, M.; Togawa, D.; Hashimoto, T.; Minami, A. Does alendronate disturb the healing process of posterior lumbar interbody fusion? A prospective randomized trial. J. Neurosurg. Spine 2011, 14, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Gasser, J.A.; Ingold, P.; Venturiere, A.; Shen, V.; Green, J.R. Long-term protective effects of zoledronic acid on cancellous and cortical bone in the ovariectomized rat. J. Bone Miner. Res. 2008, 23, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Dai, Z.; Kang, Y.; Lv, G.; Keller, E.T.; Jiang, Y. Effects of zoledronic acid on bone fusion in osteoporotic patients after lumbar fusion. Osteoporos. Int. 2016, 27, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.W.; Huang, K.F.; Hsu, H.T.; Li, H.Y.; Yang, S.S.; Chen, Y.C. Zoledronic acid infusion for lumbar interbody fusion in osteoporosis. J. Surg. Res. 2014, 192, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Buerba, R.A.; Sharma, A.; Ziino, C.; Arzeno, A.; Ajiboye, R.M. Bisphosphonate and Teriparatide Use in Thoracolumbar Spinal Fusion: A Systematic Review and Meta-Analysis of Comparative Studies. Spine 2018. [Google Scholar] [CrossRef] [PubMed]
- Marx, R.E. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: A growing epidemic. J. Oral Maxillofac. Surg. 2003, 61, 1115–1117. [Google Scholar] [CrossRef]
- Santini, D.; Vincenzi, B.; Avvisati, G.; Dicuonzo, G.; Battistoni, F.; Gavasci, M.; Salerno, A.; Denaro, V.; Tonini, G. Pamidronate induces modifications of circulating angiogenetic factors in cancer patients. Clin. Cancer Res. 2002, 8, 1080–1084. [Google Scholar] [CrossRef]
- Rasmusson, L.; Abtahi, J. Bisphosphonate associated osteonecrosis of the jaw: An update on pathophysiology, risk factors, and treatment. Int. J. Dent. 2014, 2014, 471035. [Google Scholar] [CrossRef] [PubMed]
- Adler, R.A.; El-Hajj Fuleihan, G.; Bauer, D.C.; Camacho, P.M.; Clarke, B.L.; Clines, G.A.; Compston, J.E.; Drake, M.T.; Edwards, B.J.; Favus, M.J.; et al. Managing Osteoporosis in Patients on Long-Term Bisphosphonate Treatment: Report of a Task Force of the American Society for Bone and Mineral Research. J. Bone Miner. Res. 2016, 31, 16–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takayanagi, H. Osteoimmunology: Shared mechanisms and crosstalk between the immune and bone systems. Nat. Rev. Immunol. 2007, 7, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Delmas, P.D. Clinical potential of RANKL inhibition for the management of postmenopausal osteoporosis and other metabolic bone diseases. J. Clin. Densitom. 2008, 11, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Cummings, S.R.; San Martin, J.; McClung, M.R.; Siris, E.S.; Eastell, R.; Reid, I.R.; Delmas, P.; Zoog, H.B.; Austin, M.; Wang, A.; et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N. Engl. J. Med. 2009, 361, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.D.; Pannacciulli, N.; Brown, J.P.; Czerwinski, E.; Nedergaard, B.S.; Bolognese, M.A.; Malouf, J.; Bone, H.G.; Reginster, J.Y.; Singer, A.; et al. Denosumab or Zoledronic Acid in Postmenopausal Women with Osteoporosis Previously Treated with Oral Bisphosphonates. J. Clin. Endocrinol. Metab. 2016, 101, 3163–3170. [Google Scholar] [CrossRef] [PubMed]
- Kostenuik, P.J.; Smith, S.Y.; Samadfam, R.; Jolette, J.; Zhou, L.; Ominsky, M.S. Effects of denosumab, alendronate, or denosumab following alendronate on bone turnover, calcium homeostasis, bone mass and bone strength in ovariectomized cynomolgus monkeys. J. Bone Miner. Res. 2015, 30, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Zebaze, R.M.; Libanati, C.; Austin, M.; Ghasem-Zadeh, A.; Hanley, D.A.; Zanchetta, J.R.; Thomas, T.; Boutroy, S.; Bogado, C.E.; Bilezikian, J.P.; et al. Differing effects of denosumab and alendronate on cortical and trabecular bone. Bone 2014, 59, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Cernes, R.; Barnea, Z.; Biro, A.; Zandman-Goddard, G.; Katzir, Z. Severe Hypocalcemia Following a Single Denosumab Injection. IMAJ 2017, 19, 719–721. [Google Scholar] [PubMed]
- Tateiwa, D.; Outani, H.; Iwasa, S.; Imura, Y.; Tanaka, T.; Oshima, K.; Naka, N.; Araki, N. Atypical femoral fracture associated with bone-modifying agent for bone metastasis of breast cancer: A report of two cases. J. Orthop. Surg. 2017, 25, 2309499017727916. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, H.; Ohba, S.; Yoshida, H.; Saito, K.; Inui, K.; Yasui, R.; Ichikawa, D.; Aiki, M.; Kobayashi, J.; Matsuda, S.; et al. Denosumab-related osteonecrosis of the jaw in a patient with bone metastases of prostate cancer: A case report and literature review. Oncol. Lett. 2017, 14, 127–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.; Chen, C.; Zhang, J.; Ji, X.; Liu, L.; Zhang, G.; Cao, X.; Wang, P. Safety of denosumab in postmenopausal women with osteoporosis or low bone mineral density: A meta-analysis. Int. J. Clin. Exp. Pathol. 2014, 7, 2113–2122. [Google Scholar] [PubMed]
- Hock, J.M.; Gera, I. Effects of continuous and intermittent administration and inhibition of resorption on the anabolic response of bone to parathyroid hormone. J. Bone Miner. Res. 1992, 7, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Podbesek, R.D.; Mawer, E.B.; Zanelli, G.D.; Parsons, J.A.; Reeve, J. Intestinal absorption of calcium in greyhounds: The response to intermittent and continuous administration of human synthetic parathyroid hormone fragment 1–34 (hPTH 1–34). Clin. Sci. 1984, 67, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Tam, C.S.; Heersche, J.N.M.; Murray, T.M.; Parsons, J.A. Parathyroid-Hormone Stimulates the Bone Apposition Rate Independently of Its Resorptive Action—Differential-Effects of Intermittent and Continuous Administration. Endocrinology 1982, 110, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Canalis, E.; Giustina, A.; Bilezikian, J.P. Mechanisms of anabolic therapies for osteoporosis. N. Engl. J. Med. 2007, 357, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Rubin, M.R.; Bilezikian, J.P. The anabolic effects of parathyroid hormone therapy. Clin. Geriatr. Med. 2003, 19, 415–432. [Google Scholar] [CrossRef]
- Horwitz, M.J.; Tedesco, M.B.; Sereika, S.M.; Prebehala, L.; Gundberg, C.M.; Hollis, B.W.; Bisello, A.; Garcia-Ocana, A.; Carneiro, R.M.; Stewart, A.F. A 7-day continuous infusion of PTH or PTHrP suppresses bone formation and uncouples bone turnover. J. Bone Miner. Res. 2011, 26, 2287–2297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, M.; Zeng, G.Q.; Turner, C.H. Biosynthetic human parathyroid hormone (1–34) effects on bone quality in aged ovariectomized rats. Endocrinology 1997, 138, 4330–4337. [Google Scholar] [CrossRef] [PubMed]
- Jerome, C.P.; Burr, D.B.; Van Bibber, T.; Hock, J.M.; Brommage, R. Treatment with human parathyroid hormone (1–34) for 18 months increases cancellous bone volume and improves trabecular architecture in ovariectomized cynomolgus monkeys (Macaca fascicularis). Bone 2001, 28, 150–159. [Google Scholar] [CrossRef]
- Yamane, H.; Takakura, A.; Shimadzu, Y.; Kodama, T.; Lee, J.W.; Isogai, Y.; Ishizuya, T.; Takao-Kawabata, R.; Iimura, T. Acute development of cortical porosity and endosteal naive bone formation from the daily but not weekly short-term administration of PTH in rabbit. PLoS ONE 2017, 12, e0175329. [Google Scholar] [CrossRef] [PubMed]
- Zebaze, R.; Takao-Kawabata, R.; Peng, Y.; Zadeh, A.G.; Hirano, K.; Yamane, H.; Takakura, A.; Isogai, Y.; Ishizuya, T.; Seeman, E. Increased cortical porosity is associated with daily, not weekly, administration of equivalent doses of teriparatide. Bone 2017, 99, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Graeff, C.; Timm, W.; Nickelsen, T.N.; Farrerons, J.; Marin, F.; Barker, C.; Gluer, C.C.; EUROFORS High Resolution Computed Tomography Substudy Group. Monitoring teriparatide-associated changes in vertebral microstructure by high-resolution CT in vivo: Results from the EUROFORS study. J. Bone Miner. Res. 2007, 22, 1426–1433. [Google Scholar] [CrossRef] [PubMed]
- Neer, R.M.; Arnaud, C.D.; Zanchetta, J.R.; Prince, R.; Gaich, G.A.; Reginster, J.Y.; Hodsman, A.B.; Eriksen, E.F.; Ish-Shalom, S.; Genant, H.K.; et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N. Engl. J. Med. 2001, 344, 1434–1441. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Sugimoto, T.; Nakano, T.; Kishimoto, H.; Ito, M.; Fukunaga, M.; Hagino, H.; Sone, T.; Yoshikawa, H.; Nishizawa, Y.; et al. Randomized Teriparatide [human parathyroid hormone (PTH) 1–34] Once-Weekly Efficacy Research (TOWER) trial for examining the reduction in new vertebral fractures in subjects with primary osteoporosis and high fracture risk. J. Clin. Endocrinol. Metab. 2012, 97, 3097–3106. [Google Scholar] [CrossRef] [PubMed]
- Andreassen, T.T.; Ejersted, C.; Oxlund, H. Intermittent parathyroid hormone (1–34) treatment increases callus formation and mechanical strength of healing rat fractures. J. Bone Miner. Res. 1999, 14, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Kumabe, Y.; Lee, S.Y.; Waki, T.; Iwakura, T.; Takahara, S.; Arakura, M.; Kuroiwa, Y.; Fukui, T.; Matsumoto, T.; Matsushita, T.; et al. Triweekly administration of parathyroid hormone (1–34) accelerates bone healing in a rat refractory fracture model. BMC Musculoskelet. Disord. 2017, 18, 545. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhu, J.; Ma, T.; Liu, C.; Hai, B.; Du, G.; Wang, H.; Li, N.; Leng, H.; Xu, Y.; et al. Comparison of the effects of once-weekly and once-daily rhPTH (1–34) injections on promoting fracture healing in rodents. J. Orthop. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Andreassen, T.T.; Cacciafesta, V. Intermittent parathyroid hormone treatment enhances guided bone regeneration in rat calvarial bone defects. J. Craniofac. Surg. 2004, 15, 424–427. [Google Scholar] [CrossRef] [PubMed]
- Aspenberg, P.; Genant, H.K.; Johansson, T.; Nino, A.J.; See, K.; Krohn, K.; Garcia-Hernandez, P.A.; Recknor, C.P.; Einhorn, T.A.; Dalsky, G.P.; et al. Teriparatide for acceleration of fracture repair in humans: A prospective, randomized, double-blind study of 102 postmenopausal women with distal radial fractures. J. Bone Miner. Res. 2010, 25, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Takahata, M.; Ito, M.; Irie, K.; Abumi, K.; Minami, A. Enhancement of graft bone healing by intermittent administration of human parathyroid hormone (1–34) in a rat spinal arthrodesis model. Bone 2007, 41, 775–785. [Google Scholar] [CrossRef] [PubMed]
- O’Loughlin, P.F.; Cunningham, M.E.; Bukata, S.V.; Tomin, E.; Poynton, A.R.; Doty, S.B.; Sama, A.A.; Lane, J.M. Parathyroid hormone (1–34) augments spinal fusion, fusion mass volume, and fusion mass quality in a rabbit spinal fusion model. Spine 2009, 34, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Lehman, R.A., Jr.; Dmitriev, A.E.; Cardoso, M.J.; Helgeson, M.D.; Christensen, C.L.; Raymond, J.W.; Eckel, T.T.; Riew, K.D. Effect of teriparatide [rhPTH(1,34)] and calcitonin on intertransverse process fusion in a rabbit model. Spine 2010, 35, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Tokuyama, N.; Hirose, J.; Omata, Y.; Yasui, T.; Izawa, N.; Matsumoto, T.; Masuda, H.; Ohmiya, T.; Yasuda, H.; Saito, T.; et al. Individual and combining effects of anti-RANKL monoclonal antibody and teriparatide in ovariectomized mice. Bone Rep. 2015, 2, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, J.N.; Uihlein, A.V.; Lee, H.; Kumbhani, R.; Siwila-Sackman, E.; McKay, E.A.; Burnett-Bowie, S.A.; Neer, R.M.; Leder, B.Z. Teriparatide and denosumab, alone or combined, in women with postmenopausal osteoporosis: The DATA study randomised trial. Lancet 2013, 382, 50–56. [Google Scholar] [CrossRef]
- Kitaguchi, K.; Kashii, M.; Ebina, K.; Kaito, T.; Okada, R.; Makino, T.; Noguchi, T.; Ishimoto, T.; Nakano, T.; Yoshikawa, H. Effects of single or combination therapy of teriparatide and anti-RANKL monoclonal antibody on bone defect regeneration in mice. Bone 2018, 106, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ohtori, S.; Inoue, G.; Orita, S.; Yamauchi, K.; Eguchi, Y.; Ochiai, N.; Kishida, S.; Kuniyoshi, K.; Aoki, Y.; Nakamura, J.; et al. Teriparatide accelerates lumbar posterolateral fusion in women with postmenopausal osteoporosis: Prospective study. Spine 2012, 37, E1464–E1468. [Google Scholar] [CrossRef] [PubMed]
- Ohtori, S.; Inoue, G.; Orita, S.; Yamauchi, K.; Eguchi, Y.; Ochiai, N.; Kishida, S.; Kuniyoshi, K.; Aoki, Y.; Nakamura, J.; et al. Comparison of teriparatide and bisphosphonate treatment to reduce pedicle screw loosening after lumbar spinal fusion surgery in postmenopausal women with osteoporosis from a bone quality perspective. Spine 2013, 38, E487–E492. [Google Scholar] [CrossRef] [PubMed]
- Ohtori, S.; Orita, S.; Yamauchi, K.; Eguchi, Y.; Ochiai, N.; Kuniyoshi, K.; Aoki, Y.; Nakamura, J.; Miyagi, M.; Suzuki, M.; et al. More than 6 Months of Teriparatide Treatment Was More Effective for Bone Union than Shorter Treatment Following Lumbar Posterolateral Fusion Surgery. Asian Spine J. 2015, 9, 573–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, P.G.; Ji, G.Y.; Shin, D.A.; Ha, Y.; Yoon, D.H.; Kim, K.N. An effect comparison of teriparatide and bisphosphonate on posterior lumbar interbody fusion in patients with osteoporosis: A prospective cohort study and preliminary data. Eur. Spine J. 2017, 26, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Ebata, S.; Takahashi, J.; Hasegawa, T.; Mukaiyama, K.; Isogai, Y.; Ohba, T.; Shibata, Y.; Ojima, T.; Yamagata, Z.; Matsuyama, Y.; et al. Role of Weekly Teriparatide Administration in Osseous Union Enhancement within Six Months after Posterior or Transforaminal Lumbar Interbody Fusion for Osteoporosis-Associated Lumbar Degenerative Disorders: A Multicenter, Prospective Randomized Study. J. Bone Joint Surg. Am. Vol. 2017, 99, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Urist, M.R. Bone: Formation by autoinduction. Science 1965, 150, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Wozney, J.M.; Rosen, V.; Celeste, A.J.; Mitsock, L.M.; Whitters, M.J.; Kriz, R.W.; Hewick, R.M.; Wang, E.A. Novel regulators of bone formation: Molecular clones and activities. Science 1988, 242, 1528–1534. [Google Scholar] [CrossRef] [PubMed]
- Lissenberg-Thunnissen, S.N.; de Gorter, D.J.; Sier, C.F.; Schipper, I.B. Use and efficacy of bone morphogenetic proteins in fracture healing. Int. Orthop. 2011, 35, 1271–1280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bragdon, B.; Moseychuk, O.; Saldanha, S.; King, D.; Julian, J.; Nohe, A. Bone morphogenetic proteins: A critical review. Cell. Signal. 2011, 23, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Pneumaticos, S.G.; Triantafyllopoulos, G.K.; Basdra, E.K.; Papavassiliou, A.G. Segmental bone defects: From cellular and molecular pathways to the development of novel biological treatments. J. Cell. Mol. Med. 2010, 14, 2561–2569. [Google Scholar] [CrossRef] [PubMed]
- Mahendra, A.; Maclean, A.D. Available biological treatments for complex non-unions. Injury 2007, 38 (Suppl. 4), S7–S12. [Google Scholar] [CrossRef]
- Burkus, J.K.; Heim, S.E.; Gornet, M.F.; Zdeblick, T.A. Is INFUSE bone graft superior to autograft bone? An integrated analysis of clinical trials using the LT-CAGE lumbar tapered fusion device. J. Spinal Disord. Tech. 2003, 16, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Burkus, J.K.; Sandhu, H.S.; Gornet, M.F.; Longley, M.C. Use of rhBMP-2 in combination with structural cortical allografts: Clinical and radiographic outcomes in anterior lumbar spinal surgery. J. Bone Joint Surg. Am. Vol. 2005, 87, 1205–1212. [Google Scholar] [CrossRef]
- Simmonds, M.C.; Brown, J.V.; Heirs, M.K.; Higgins, J.P.; Mannion, R.J.; Rodgers, M.A.; Stewart, L.A. Safety and effectiveness of recombinant human bone morphogenetic protein-2 for spinal fusion: A meta-analysis of individual-participant data. Ann. Intern. Med. 2013, 158, 877–889. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.I.; Lurie, J.D.; Tosteson, A.N.; Deyo, R.A.; Farrokhi, F.R.; Mirza, S.K. Use of bone morphogenetic protein among patients undergoing fusion for degenerative diagnoses in the United States, 2002 to 2012. Spine J. 2015, 15, 692–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McClellan, J.W.; Mulconrey, D.S.; Forbes, R.J.; Fullmer, N. Vertebral bone resorption after transforaminal lumbar interbody fusion with bone morphogenetic protein (rhBMP-2). Clin. Spine Surg. 2006, 19, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Shields, L.B.; Raque, G.H.; Glassman, S.D.; Campbell, M.; Vitaz, T.; Harpring, J.; Shields, C.B. Adverse effects associated with high-dose recombinant human bone morphogenetic protein-2 use in anterior cervical spine fusion. Spine 2006, 31, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Seeherman, H.; Wozney, J.M. Delivery of bone morphogenetic proteins for orthopedic tissue regeneration. Cytokine Growth Factor Rev. 2005, 16, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Boden, S.D.; Martin, G.J., Jr.; Morone, M.A.; Ugbo, J.L.; Moskovitz, P.A. Posterolateral lumbar intertransverse process spine arthrodesis with recombinant human bone morphogenetic protein 2/hydroxyapatite-tricalcium phosphate after laminectomy in the nonhuman primate. Spine 1999, 24, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Kaito, T.; Morimoto, T.; Mori, Y.; Kanayama, S.; Makino, T.; Takenaka, S.; Sakai, Y.; Otsuru, S.; Yoshioka, Y.; Yoshikawa, H. BMP-2/7 heterodimer strongly induces bone regeneration in the absence of increased soft tissue inflammation. Spine J. 2018, 18, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, T.; Kaito, T.; Kashii, M.; Matsuo, Y.; Sugiura, T.; Iwasaki, M.; Yoshikawa, H. Effect of Intermittent Administration of Teriparatide (Parathyroid Hormone 1–34) on Bone Morphogenetic Protein-Induced Bone Formation in a Rat Model of Spinal Fusion. J. Bone Joint Surg. Am. Vol. 2014, 96, e107. [Google Scholar] [CrossRef] [PubMed]
- Kaito, T.; Morimoto, T.; Kanayama, S.; Otsuru, S.; Kashii, M.; Makino, T.; Kitaguchi, K.; Furuya, M.; Chijimatsu, R.; Ebina, K.; et al. Modeling and remodeling effects of intermittent administration of teriparatide (parathyroid hormone 1–34) on bone morphogenetic protein-induced bone in a rat spinal fusion model. Bone Rep. 2016, 5, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Kaito, T.; Johnson, J.; Ellerman, J.; Tian, H.; Aydogan, M.; Chatsrinopkun, M.; Ngo, S.; Choi, C.; Wang, J.C. Synergistic effect of bone morphogenetic proteins 2 and 7 by ex vivo gene therapy in a rat spinal fusion model. J. Bone Joint Surg. Am. Vol. 2013, 95, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Kaneko, E.; Maeda, J.; Ueno, N. Mesoderm induction by BMP-4 and -7 heterodimers. Biochem. Biophys. Res. Commun. 1997, 232, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Valera, E.; Isaacs, M.J.; Kawakami, Y.; Izpisua Belmonte, J.C.; Choe, S. BMP-2/6 heterodimer is more effective than BMP-2 or BMP-6 homodimers as inductor of differentiation of human embryonic stem cells. PLoS ONE 2010, 5, e11167. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wu, G. The signaling and functions of heterodimeric bone morphogenetic proteins. Cytokine Growth Factor Rev. 2012, 23, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, M.J.; Kawakami, Y.; Allendorph, G.P.; Yoon, B.H.; Izpisua Belmonte, J.C.; Choe, S. Bone morphogenetic protein-2 and -6 heterodimer illustrates the nature of ligand-receptor assembly. Mol. Endocrinol. 2010, 24, 1469–1477. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Kim, J.; Cheng, C.; Rawlins, B.A.; Boachie-Adjei, O.; Crystal, R.G.; Hidaka, C. Noggin regulation of bone morphogenetic protein (BMP) 2/7 heterodimer activity in vitro. Bone 2006, 39, 61–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Bialy, I.; Jiskoot, W.; Reza Nejadnik, M. Formulation, Delivery and Stability of Bone Morphogenetic Proteins for Effective Bone Regeneration. Pharm. Res. 2017, 34, 1152–1170. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, G.; Bialorucki, C.; Yildirim-Ayan, E. Nanofibrous yet injectable polycaprolactone-collagen bone tissue scaffold with osteoprogenitor cells and controlled release of bone morphogenetic protein-2. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 51, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Ben-David, D.; Srouji, S.; Shapira-Schweitzer, K.; Kossover, O.; Ivanir, E.; Kuhn, G.; Muller, R.; Seliktar, D.; Livne, E. Low dose BMP-2 treatment for bone repair using a PEGylated fibrinogen hydrogel matrix. Biomaterials 2013, 34, 2902–2910. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.U.; Lim, H.C.; Hong, J.Y.; Lee, J.S.; Jung, U.W.; Choi, S.H. Bone regenerative efficacy of biphasic calcium phosphate collagen composite as a carrier of rhBMP-2. Clin. Oral Implants Res. 2016, 27, e91–e99. [Google Scholar] [CrossRef] [PubMed]
- Compton, J.T.; Lee, F.Y. A review of osteocyte function and the emerging importance of sclerostin. J. Bone Joint Surg. Am. Vol. 2014, 96, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Poole, K.E.; van Bezooijen, R.L.; Loveridge, N.; Hamersma, H.; Papapoulos, S.E.; Lowik, C.W.; Reeve, J. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J. 2005, 19, 1842–1844. [Google Scholar] [CrossRef] [PubMed]
- Weivoda, M.M.; Youssef, S.J.; Oursler, M.J. Sclerostin expression and functions beyond the osteocyte. Bone 2017, 96, 45–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balemans, W.; Ebeling, M.; Patel, N.; Van Hul, E.; Olson, P.; Dioszegi, M.; Lacza, C.; Wuyts, W.; Van Den Ende, J.; Willems, P.; et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum. Mol. Genet. 2001, 10, 537–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunkow, M.E.; Gardner, J.C.; Van Ness, J.; Paeper, B.W.; Kovacevich, B.R.; Proll, S.; Skonier, J.E.; Zhao, L.; Sabo, P.J.; Fu, Y.; et al. Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am. J. Hum. Genet. 2001, 68, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Van Bezooijen, R.L.; Deruiter, M.C.; Vilain, N.; Monteiro, R.M.; Visser, A.; van der Wee-Pals, L.; van Munsteren, C.J.; Hogendoorn, P.C.; Aguet, M.; Mummery, C.L.; et al. SOST expression is restricted to the great arteries during embryonic and neonatal cardiovascular development. Dev. Dyn. 2007, 236, 606–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ott, S.M. Sclerostin and Wnt signaling—The pathway to bone strength. J. Clin. Endocrinol. Metab. 2005, 90, 6741–6743. [Google Scholar] [CrossRef] [PubMed]
- Ten Dijke, P.; Krause, C.; de Gorter, D.J.; Lowik, C.W.; van Bezooijen, R.L. Osteocyte-derived sclerostin inhibits bone formation: Its role in bone morphogenetic protein and Wnt signaling. J. Bone Joint Surg. Am. Vol. 2008, 90 (Suppl. 1), 31–35. [Google Scholar] [CrossRef] [PubMed]
- Winkler, D.G.; Sutherland, M.K.; Geoghegan, J.C.; Yu, C.; Hayes, T.; Skonier, J.E.; Shpektor, D.; Jonas, M.; Kovacevich, B.R.; Staehling-Hampton, K.; et al. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J. 2003, 22, 6267–6276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cosman, F.; Crittenden, D.B.; Adachi, J.D.; Binkley, N.; Czerwinski, E.; Ferrari, S.; Hofbauer, L.C.; Lau, E.; Lewiecki, E.M.; Miyauchi, A.; et al. Romosozumab Treatment in Postmenopausal Women with Osteoporosis. N. Engl. J. Med. 2016, 375, 1532–1543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Cao, Y.; Zhang, S.; Zhang, W.; Zhang, B.; Tang, Q.; Li, Z.; Wu, J. Romosozumab treatment in postmenopausal women with osteoporosis: A meta-analysis of randomized controlled trials. Climacteric 2018, 21, 1–7. [Google Scholar] [CrossRef] [PubMed]
- McClung, M.R. Sclerostin antibodies in osteoporosis: Latest evidence and therapeutic potential. Ther. Adv. Musculoskelet. Dis. 2017, 9, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Zhang, C.-Q.; Chai, Y.-M.; Li, X.-L. Systemic administration of sclerostin monoclonal antibody accelerates fracture healing in the femoral osteotomy model of young rats. Int. Immunopharmacol. 2015, 24, 7–13. [Google Scholar]
- Liu, Y.; Rui, Y.; Cheng, T.Y.; Huang, S.; Xu, L.; Meng, F.; Lee, W.Y.; Zhang, T.; Li, N.; Li, C.; et al. Effects of Sclerostin Antibody on the Healing of Femoral Fractures in Ovariectomised Rats. Calcif. Tissue Int. 2016, 98, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Suen, P.K.; He, Y.X.; Chow, D.H.; Huang, L.; Li, C.; Ke, H.Z.; Ominsky, M.S.; Qin, L. Sclerostin monoclonal antibody enhanced bone fracture healing in an open osteotomy model in rats. J. Orthop. Res. 2014, 32, 997–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suen, P.K.; Zhu, T.Y.; Chow, D.H.; Huang, L.; Zheng, L.Z.; Qin, L. Sclerostin Antibody Treatment Increases Bone Formation, Bone Mass, and Bone Strength of Intact Bones in Adult Male Rats. Sci. Rep. 2015, 5, 15632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virk, M.S.; Alaee, F.; Tang, H.; Ominsky, M.S.; Ke, H.Z.; Lieberman, J.R. Systemic administration of sclerostin antibody enhances bone repair in a critical-sized femoral defect in a rat model. J. Bone Joint Surg. Am. Vol. 2013, 95, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Fracon, R.N.; Teofilo, J.M.; Satin, R.B.; Lamano, T. Prostaglandins and bone: Potential risks and benefits related to the use of nonsteroidal anti-inflammatory drugs in clinical dentistry. J. Oral Sci. 2008, 50, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Hadi, O.; Parvizi, J.; Austin, M.S.; Viscusi, E.; Einhorn, T. Nonsteroidal anti-inflammatory drugs in orthopaedics. J. Bone Joint Surg. Am. Vol. 2009, 91, 2020–2027. [Google Scholar]
- Yoshida, K.; Oida, H.; Kobayashi, T.; Maruyama, T.; Tanaka, M.; Katayama, T.; Yamaguchi, K.; Segi, E.; Tsuboyama, T.; Matsushita, M.; et al. Stimulation of bone formation and prevention of bone loss by prostaglandin E EP4 receptor activation. Proc. Natl. Acad. Sci. USA 2002, 99, 4580–4585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumont, A.S.; Verma, S.; Dumont, R.J.; Hurlbert, R.J. Nonsteroidal anti-inflammatory drugs and bone metabolism in spinal fusion surgery: A pharmacological quandary. J. Pharmacol. Toxicol. Methods 2000, 43, 31–39. [Google Scholar] [CrossRef]
- Graham, S.; Gamie, Z.; Polyzois, I.; Narvani, A.A.; Tzafetta, K.; Tsiridis, E.; Helioti, M.; Mantalaris, A.; Tsiridis, E. Prostaglandin EP2 and EP4 receptor agonists in bone formation and bone healing: In vivo and in vitro evidence. Expert Opin. Investig. Drugs 2009, 18, 746–766. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Imai, Y.; Ohta, Y.; Takaoka, K. Prostaglandin E2 EP4 agonist (ONO-4819) accelerates BMP-induced osteoblastic differentiation. Bone 2007, 41, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Namikawa, T.; Terai, H.; Hoshino, M.; Kato, M.; Toyoda, H.; Yano, K.; Nakamura, H.; Takaoka, K. Enhancing effects of a prostaglandin EP4 receptor agonist on recombinant human bone morphogenetic protein-2 mediated spine fusion in a rabbit model. Spine 2007, 32, 2294–2299. [Google Scholar] [CrossRef] [PubMed]
- Pagkalos, J.; Leonidou, A.; As-Sultany, M.; Heliotis, M.; Mantalaris, A.; Tsiridis, E. Prostaglandin E(2) receptors as potential bone anabolic targets—Selective EP4 receptor agonists. Curr. Mol. Pharmacol. 2012, 5, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Kanayama, S.; Kaito, T.; Kitaguchi, K.; Ishiguro, H.; Hashimoto, K.; Chijimatsu, R.; Otsuru, S.; Takenaka, S.; Makino, T.; Sakai, Y.; et al. ONO-1301 Enhances in vitro Osteoblast Differentiation and in vivo Bone Formation Induced by Bone Morphogenetic Protein. Spine 2017, 43, E616–E624. [Google Scholar] [CrossRef] [PubMed]
- Barba, M.; Cicione, C.; Bernardini, C.; Campana, V.; Pagano, E.; Michetti, F.; Logroscino, G.; Lattanzi, W. Spinal fusion in the next generation: Gene and cell therapy approaches. Sci. World J. 2014, 2014, 406159. [Google Scholar] [CrossRef] [PubMed]
- Khashan, M.; Inoue, S.; Berven, S.H. Cell based therapies as compared to autologous bone grafts for spinal arthrodesis. Spine 2013, 38, 1885–1891. [Google Scholar] [CrossRef] [PubMed]
- Barbanti Brodano, G.; Terzi, S.; Trombi, L.; Griffoni, C.; Valtieri, M.; Boriani, S.; Magli, M.C. Mesenchymal stem cells derived from vertebrae (vMSCs) show best biological properties. Eur. Spine J. 2013, 22 (Suppl. 6), S979–S984. [Google Scholar] [CrossRef] [PubMed]
- Risbud, M.V.; Shapiro, I.M.; Guttapalli, A.; Di Martino, A.; Danielson, K.G.; Beiner, J.M.; Hillibrand, A.; Albert, T.J.; Anderson, D.G.; Vaccaro, A.R. Osteogenic potential of adult human stem cells of the lumbar vertebral body and the iliac crest. Spine 2006, 31, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Beederman, M.; Lamplot, J.D.; Nan, G.; Wang, J.; Liu, X.; Yin, L.; Li, R.; Shui, W.; Zhang, H.; Kim, S.H.; et al. BMP signaling in mesenchymal stem cell differentiation and bone formation. J. Biomed. Sci. Eng. 2013, 6, 32–52. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T.; Iizuka, H.; Tsutsumi, S.; Kayakabe, M.; Takagishi, K. Evaluation of posterolateral spinal fusion using mesenchymal stem cells: Differences with or without osteogenic differentiation. Spine 2007, 15, 2432–2436. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Dong, Y.; Hong, Y.; Guang, Q.; Chen, X. Evaluation of Anterior Vertebral Interbody Fusion Using Osteogenic Mesenchymal Stem Cells Transplanted in Collagen Sponge. Clin. Spine Surg. 2016, 29, E201–E207. [Google Scholar] [CrossRef] [PubMed]
- Skovrlj, B.; Guzman, J.Z.; Al Maaieh, M.; Cho, S.K.; Iatridis, J.C.; Qureshi, S.A. Cellular bone matrices: Viable stem cell-containing bone graft substitutes. Spine J. 2014, 14, 2763–2772. [Google Scholar] [CrossRef] [PubMed]
- Parekkadan, B.; Milwid, J.M. Mesenchymal stem cells as therapeutics. Annu. Rev. Biomed. Eng. 2010, 12, 87–117. [Google Scholar] [CrossRef] [PubMed]
- Muschler, G.F.; Midura, R.J. Connective tissue progenitors: Practical concepts for clinical applications. Clin. Orthop. Relat. Res. 2002, 395, 66–80. [Google Scholar] [CrossRef]
- Livingston, T.L.; Gordon, S.; Archambault, M.; Kadiyala, S.; McIntosh, K.; Smith, A.; Peter, S.J. Mesenchymal stem cells combined with biphasic calcium phosphate ceramics promote bone regeneration. J. Mater. Sci. Mater. Med. 2003, 14, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Yousef, M.A.A.; La Maida, G.A.; Misaggi, B. Long-term Radiological and Clinical Outcomes after Using Bone Marrow Mesenchymal Stem Cells Concentrate Obtained with Selective Retention Cell Technology in Posterolateral Spinal Fusion. Spine 2017, 42, 1871–1879. [Google Scholar] [CrossRef] [PubMed]
- Lou, X. Induced Pluripotent Stem Cells as a new Strategy for Osteogenesis and Bone Regeneration. Stem Cell Rev. 2015, 11, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Douglas, J.T.; Rivera, A.A.; Lyons, G.R.; Lott, P.F.; Wang, D.; Zayzafoon, M.; Siegal, G.P.; Cao, X.; Theiss, S.M. Ex vivo transfer of the Hoxc-8-interacting domain of Smad1 by a tropism-modified adenoviral vector results in efficient bone formation in a rabbit model of spinal fusion. J. Spinal Disord. Tech. 2010, 23, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.S.; Zhang, X.; Soo, C.; Hsu, T.; Napoli, A.; Aghaloo, T.; Wu, B.M.; Tsou, P.; Ting, K.; Wang, J.C. The osteoinductive properties of Nell-1 in a rat spinal fusion model. Spine J. 2007, 7, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Viggeswarapu, M.; Boden, S.D.; Liu, Y.; Hair, G.A.; Louis-Ugbo, J.; Murakami, H.; Kim, H.S.; Mayr, M.T.; Hutton, W.C.; Titus, L. Adenoviral delivery of LIM mineralization protein-1 induces new-bone formation in vitro and in vivo. J. Bone Joint Surg. Am. Vol. 2001, 83, 364–376. [Google Scholar] [CrossRef]


| Mechanism of Action | Effect on Bone Metabolism | Clinical Trials for Human Spinal Fusion | Effect on Fusion in Animal Models | Effect on Fusion in Human | |
|---|---|---|---|---|---|
| Bisphosphonates | Involved in osteoclasts and induction of apoptosis of osteoclasts | Inhibition of bone resorption | Yes | Yes | Controversial |
| Anti-RANKL monoclonal antibody | Prevention of the interaction between RANKL and RANK receptor on osteoclasts and osteoclast precursors by binding RANKL | Inhibition of bone resorption | No | N/A | N/A |
| PTH1-34 | Stimulation of osteoblast differentiation by intermittent PTH (PTH1-34) | Activation of bone formation (intermittent PTH1-34) Activation of bone resorption (continuous PTH1-34) | Yes | Yes | Yes |
| BMPs | Activation of Runx2 expression and induction of osteoblast differentiation | Activation of bone formation | Yes | Yes | Yes |
| Anti-sclerostin antibody | Inhibition of sclerostin which interferes BMP and Wnt signaling | Activation of bone formation | No | N/A | N/A |
| Prostaglandins agonist | Activation of Runx2 expression | Activation of bone formation | No | Yes (combined use with BMP) | N/A |
| Stem cell | Induction of mesenchymal stem cells (bone marrow stem cells, adipose-derived stem cells, and bone marrow aspiration) | Supplementation of cell source for osteoblast | Yes | Yes | Yes |
| Gene therapy | Delivery of osteoinductive genes locally around the sites of fusion | Activation of bone formation | No | N/A | N/A |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makino, T.; Tsukazaki, H.; Ukon, Y.; Tateiwa, D.; Yoshikawa, H.; Kaito, T. The Biological Enhancement of Spinal Fusion for Spinal Degenerative Disease. Int. J. Mol. Sci. 2018, 19, 2430. https://doi.org/10.3390/ijms19082430
Makino T, Tsukazaki H, Ukon Y, Tateiwa D, Yoshikawa H, Kaito T. The Biological Enhancement of Spinal Fusion for Spinal Degenerative Disease. International Journal of Molecular Sciences. 2018; 19(8):2430. https://doi.org/10.3390/ijms19082430
Chicago/Turabian StyleMakino, Takahiro, Hiroyuki Tsukazaki, Yuichiro Ukon, Daisuke Tateiwa, Hideki Yoshikawa, and Takashi Kaito. 2018. "The Biological Enhancement of Spinal Fusion for Spinal Degenerative Disease" International Journal of Molecular Sciences 19, no. 8: 2430. https://doi.org/10.3390/ijms19082430
APA StyleMakino, T., Tsukazaki, H., Ukon, Y., Tateiwa, D., Yoshikawa, H., & Kaito, T. (2018). The Biological Enhancement of Spinal Fusion for Spinal Degenerative Disease. International Journal of Molecular Sciences, 19(8), 2430. https://doi.org/10.3390/ijms19082430