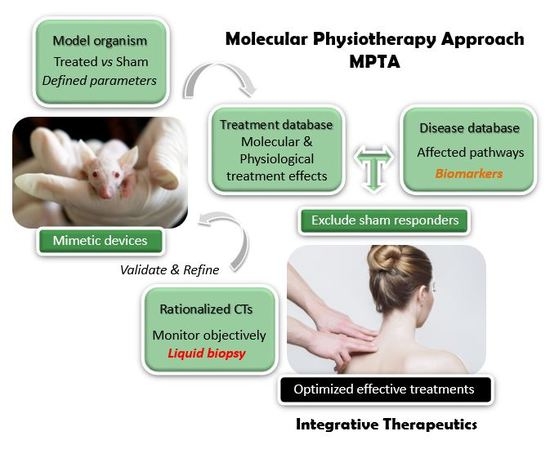
Unraveling the Molecular Determinants of Manual Therapy: An Approach to Integrative Therapeutics for the Treatment of Fibromyalgia and Chronic Fatigue Syndrome/Myalgic Encephalomyelitis
Abstract
:1. Introduction
2. Molecular Determinants of MT: Lessons from Animal Models and Mimetic Devices
2.1. The Neuroimmune Impact of MT
2.2. Effects of MT in Muscle Regeneration
2.3. MT Impact on Pain Relief
3. The Rationale for Using MT to Treat FM and CFS/ME Dysfunctions
4. Future Directions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MT | Manual Therapy |
| FM | Fibromyalgia |
| CFS/ME | Chronic Fatigue Syndrome/Myalgic Encephalomyelitis |
| MPTA | Molecular Physiotherapy Approach |
| CCL | Cyclic Compressive Loading |
| CT | Clinical Trial |
| miR | microRNA |
| EV | Extracellular Vesicle |
| ICD | International Classification of Diseases |
| PBMC | Peripheral Blood Mononuclear Cells |
| DRG | Dorsal Root Ganglion |
| SDH | Spinal cord Dorsal Horn |
References
- Boerma, T.; Harrison, J.; Jakob, R.; Mathers, C.; Schmider, A.; Weber, S. Revising the ICD: Explaining the WHO approach. Lancet 2016, 388, 2476–2477. [Google Scholar] [CrossRef]
- Wolfe, F.; Smythe, H.A.; Yunus, M.B.; Bennett, R.M.; Bombardier, C.; Goldenberg, D.L. The American College of Rheumatology 1990 Criteria for the Classification of fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum 1990, 33, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010, 62, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Jahan, F.; Nanji, K.; Qidwai, W.; Qasim, R. Fibromyalgia syndrome: An overview of pathophysiology, diagnosis and management. Oman Med. J. 2012, 27, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Carruthers, B.M.; Jain, A.K.; De Meirleir, K.L.; Peterson, D.L.; Klimas, N.G.; Lerner, A. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Clinical Working Case Definition, Diagnostic and Treatment Protocols. J. Chronic Fatigue Syndrome 2003, 11, 7–115. [Google Scholar] [CrossRef]
- Carruthers, B.M.; van de Sande, M.I.; De Meirleir, K.L.; Klimas, N.G.; Broderick, G.; Mitchell, T. Myalgic encephalomyelitis: International Consensus Criteria. J. Intern. Med. 2011, 270, 327–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barsky, A.J.; Borus, J.F. Functional somatic syndromes. Ann. Intern. Med. 1999, 130, 910–921. [Google Scholar] [CrossRef] [PubMed]
- Abbi, B.; Natelson, B.H. Is chronic fatigue syndrome the same illness as fibromyalgia: Evaluating the ‘single syndrome’ hypothesis. QJM. 2013, 106, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Natelson, B.H.; Vu, D.; Coplan, J.D.; Mao, X.; Blate, M.; Kang, G.; Soto, E.; Kapusuz, T.; Shungu, D.C. Elevations of Ventricular Lactate Levels Occur in Both Chronic Fatigue Syndrome and Fibromyalgia. Fatigue 2017, 5, 15–20. [Google Scholar] [CrossRef] [PubMed]
- McManimen, S.L.; Jason, L.A. Post-Exertional Malaise in Patients with ME and CFS with Comorbid Fibromyalgia. SRL Neurol. Neurosurg. 2017, 3, 22–27. [Google Scholar] [PubMed]
- Naschitz, J.E.; Rozenbaum, M.; Rosner, I.; Sabo, E.; Priselac, R.M.; Shaviv, N. Cardiovascular response to upright tilt in fibromyalgia differs from that in chronic fatigue syndrome. J. Rheumatol. 2001, 28, 1356–1360. [Google Scholar] [PubMed]
- Naschitz, J.E.; Slobodin, G.; Sharif, D.; Fields, M.; Isseroff, H.; Sabo, E. Electrocardiographic QT interval and cardiovascular reactivity in fibromyalgia differ from chronic fatigue syndrome. Eur. J. Intern Med. 2008, 19, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Korszun, A.; Sackett-Lundeen, L.; Papadopoulos, E.; Brucksch, C.; Masterson, L.; Engelberg, N.C. Melatonin levels in women with fibromyalgia and chronic fatigue syndrome. J. Rheumatol. 1999, 26, 2675–2680. [Google Scholar] [PubMed]
- Crofford, L.J.; Young, E.A.; Engleberg, N.C.; Korszun, A.; Brucksch, C.B.; McClure, L.A. Basal circadian and pulsatile ACTH and cortisol secretion in patients with fibromyalgia and/or chronic fatigue syndrome. Brain Behav. Immun. 2004, 18, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Light, A.R.; Bateman, L.; Jo, D.; Hughen, R.W.; Vanhaitsma, T.A.; White, A.T.; Light, K.C. Gene expression alterations at baseline and following moderate exercise in patients with Chronic Fatigue Syndrome and Fibromyalgia Syndrome. J. Intern. Med. 2012, 271, 64–81. [Google Scholar] [CrossRef] [PubMed]
- Cerda-Olmedo, G.; Mena-Duran, A.V.; Monsalve, V.; Oltra, E. Identification of a microRNA signature for the diagnosis of fibromyalgia. PLoS ONE 2015, 10, e0121903. [Google Scholar] [CrossRef] [PubMed]
- Masotti, A.; Baldassarre, A.; Guzzo, M.P.; Iannuccelli, C.; Barbato, C.; Di Franco, M. Circulating microRNA Profiles as Liquid Biopsies for the Characterization and Diagnosis of Fibromyalgia Syndrome. Mol. Neurobiol. 2017, 54, 7129–7136. [Google Scholar] [CrossRef] [PubMed]
- Petty, R.D.; McCarthy, N.E.; Le Dieu, R.; Kerr, J.R. MicroRNAs hsa-miR-99b, hsa-miR-330,hsa-miR-126 and hsa-miR-30c: Potential Diagnostic Biomarkers in Natural Killer(NK) Cells of Patients with Chronic Fatigue Syndrome (CFS)/ Myalgic Encephalomyelitis (ME). PLoS ONE 2016, 11, e0150904. [Google Scholar] [CrossRef] [PubMed]
- Brenu, E.W.; Ashton, K.J.; Batovska, J.; Staines, D.R. Marshall-Gradisnik SM. High-throughput sequencing of plasma microRNA in chronic fatigue syndrome/myalgic encephalomyelitis. PLoS ONE 2014, 9, e102783. [Google Scholar] [CrossRef] [PubMed]
- Kwiatek, R. Treatment of fibromyalgia. Aust. Prescr. 2017, 40, 179–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruun Wyller, V.; Bjørneklett, A.; Brubakk, O.; Festvåg, L.; Follestad, I.; Malt, U. Diagnosis and Treatment of Chronic Fatigue Syndrome/Myalgic Encephalopathy (CFS/ME) [Internet]. Oslo,Norway: Knowledge Centre for the Health Services at The Norwegian Institute of Public Health (NIPH); 2006. Available online: http://www.ncbi.nlm.nih.gov/books/NBK464796/.
- Smith, M.E.; Haney, E.; McDonagh, M.; Pappas, M.; Daeges, M.; Wasson, N.; Fu, R.; Nelson, H.D. Treatment of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Systematic Review for a National Institutes of Health Pathways to Prevention Workshop. Ann. Intern. Med. 2015, 162, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Olivan-Blázquez, B.; Herrera-Mercadal, P.; Puebla-Guedea, M.; Pérez-Yus, M.C.; Andrés, E.; Fayed, N. Efficacy of memantine in the treatment of fibromyalgia: A double-blind, randomised, controlled trial with 6-month follow-up. Pain 2014, 155, 2517–25125. [Google Scholar] [CrossRef] [PubMed]
- Holman, A.J.; Myers, R.R. A randomized, double-blind, placebo-controlled trial of pramipexole, a dopamine agonist, in patients with fibromyalgia receiving concomitant medications. Arthritis Rheum. 2005, 52, 2495–2505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fluge, Ø.; Risa, K.; Lunde, S.; Alme, K.; Rekeland, I.G.; Sapkota, D. B-Lymphocyte Depletion in Myalgic Encephalopathy/ Chronic Fatigue Syndrome. An Open-Label Phase II Study with Rituximab Maintenance Treatment. PLoS ONE 2015, 10, e0129898. [Google Scholar] [CrossRef] [PubMed]
- Eaton, N.; Cabanas, H.; Balinas, C.; Klein, A.; Staines, D.; Marshall-Gradisnik, S. Rituximab impedes natural killer cell function in Chronic Fatigue Syndrome/Myalgic Encephalomyelitis patients: A pilot in vitro investigation. BMC Pharmacol. Toxicol. 2018, 19, 12. [Google Scholar] [CrossRef] [PubMed]
- Strayer, D.R.; Carter, W.A.; Stouch, B.C.; Stevens, S.R.; Bateman, L.; Cimoch, P.J. Chronic Fatigue Syndrome AMP-516 Study Group, Mitchell WM. A. double-blind, placebo-controlled, randomized, clinical trial of the TLR-3 agonist rintatolimod in severe cases of chronic fatigue syndrome. PLoS ONE 2012, 7, e31334. [Google Scholar] [CrossRef] [PubMed]
- Bernardy, K.; Klose, P.; Busch, A.J.; Choy, E.H.; Häuser, W. Cognitive behavioural therapies for fibromyalgia. Cochrane Database Syst. Rev. 2013, 9, CD009796. [Google Scholar] [CrossRef] [PubMed]
- Busch, A.J.; Webber, S.C.; Brachaniec, M.; Bidonde, J.; Bello-Haas, V.D.; Danyliw, A.D. Exercise therapy for fibromyalgia. Curr. Pain Headache Rep. 2011, 15, 358–367. [Google Scholar] [CrossRef] [PubMed]
- White, P.D.; Goldsmith, K.A.; Johnson, A.L.; Potts, L.; Walwyn, R.; DeCesare, J.C.; PACE Trial Management Group. Comparison of adaptive pacing therapy, cognitive behaviour therapy, graded exercise therapy, and specialist medical care for chronic fatigue syndrome (PACE): A randomized trial. Lancet 2011, 377, 823–836. [Google Scholar] [CrossRef]
- Sharpe, M.; Goldsmith, K.A.; Johnson, A.L.; Chalder, T.; Walker, J.; White, P.D. Rehabilitative treatments for chronic fatigue syndrome: Long-term follow-up from the PACE trial. Lancet Psychiatry. 2015, 2, 1067–1074. [Google Scholar] [CrossRef]
- Twisk, F. PACE: CBT and GET are not rehabilitative therapies. Lancet Psychiatry 2016, 3, e6. [Google Scholar] [CrossRef]
- Wilshire, C.E.; Kindlon, T.; Courtney, R.; Matthees, A.; Tuller, D.; Geraghty, K. Rethinking the treatment of chronic fatigue syndrome-a reanalysis and evaluation of findings from a recent major trial of graded exercise and CBT. BMC Psychol. 2018, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Eller-Smith, O.C.; Nicol, A.L.; Christianson, J.A. Potential Mechanisms Underlying Centralized Pain and Emerging Therapeutic Interventions. Front. Cell. Neurosci. 2018, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Wang, F.Y.; Feng, C.Q.; Yang, X.F.; Sun, Y.H. Massage therapy for fibromyalgia: A systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2014, 9, e89304. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.L.; Matsutani, L.A.; Marques, A.P. Effectiveness of different styles of massage therapy in fibromyalgia: A systematic review and meta-analysis. Man Ther. 2015, 20, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Alraek, T.; Lee, M.S.; Choi, T.Y.; Cao, H.; Liu, J. Complementary and alternative medicine for patients with chronic fatigue syndrome: A systematic review. BMC Complement Altern. Med. 2011, 11, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Field, T.M.; Sunshine, W.; Hernandez-Reif, M.; Quintino, O.; Schanberg, S.; Kuhn, C. Massage therapy effects on depression and somatic symptoms in chronic fatigue syndrome. J. Chronic Fatigue Syndr. 1997, 3, 43–51. [Google Scholar] [CrossRef]
- Wang, J.H.; Chai, T.Q.; Lin, G.H.; Luo, L. Effects of the intelligent-turtle massage on the physical symptoms and immune functions in patients with chronic fatigue syndrome. J. Tradit. Chin. Med. 2009, 29, 24–28. [Google Scholar] [CrossRef]
- Waters-Banker, C.; Butterfield, T.A.; Dupont-Versteegden, E. Immunomodulatory effects of massage on nonperturbed skeletal muscle in rats. J. Appl. Physiol. 2014, 116, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Major, B.; Rattazzi, L.; Brod, S.; Pilipović, I.; Leposavić, G.; D’Acquisto, F. Massage-like stroking boosts the immune system in mice. Sci. Rep. 2015, 5, 10913. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.W.; Dodge, T.R.; Hammond, M.A.; Wallace, J.M.; Zhou, F.C.; Yokota, H. Physical weight loading induces expression of tryptophan hydroxylase 2 in the brain stem. PLoS ONE 2014, 9, e85095. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Duan, W.; Wan, R.; Guo, Z. Prophylactic activation of neuroprotective stress response pathways by dietary and behavioral manipulations. NeuroRx 2004, 1, 111–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cezar, C.A.; Roche, E.T.; Vandenburgh, H.H.; Duda, G.N.; Walsh, C.J.; Mooney, D.J. Biologic-free mechanically induced muscle regeneration. Proc. Natl. Acad. Sci. USA 2016, 113, 1534–1539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, B.F.; Hamilton, K.L.; Majeed, Z.R.; Abshire, S.M.; Confides, A.L.; Hayek, A.M. Enhanced skeletal muscle regrowth and remodelling in massaged and contralateral non-massaged hindlimb. J. Physiol. 2018, 596, 83–103. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, J.S.; Hajira, A.; Lopez, M.A.; Boriek, A.M. Genome-wide Mechanosensitive MicroRNA (MechanomiR) Screen Uncovers Dysregulation of Their Regulatory Networks in the mdm Mouse Model of Muscular Dystrophy. J. Biol. Chem. 2015, 290, 24986–25011. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.; Bianco, A.; Paoli, A.; Palma, A. The Relation between Stretching Typology and Stretching Duration: The Effects on Range of Motion. Int. J. Sports Med. 2018, 39, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Cafarelli, E.; Flint, F. The role of massage in preparation for and recovery from exercise. An overview. Sports Med. 1992, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Field, T.; Diego, M.; Hernandez-Reif, M. Moderate pressure is essential for massage therapy effects. Int. J. Neurosci. 2010, 120, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Field, T.; Hernandez-Reif, M.; Diego, M.; Schanberg, S.; Kuhn, C. Cortisol decreases and serotonin and dopamine increase following massage therapy. Int. J. Neurosci. 2005, 115, 1397–1413. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Chaudhry, I.; Reid, M.B.; Boriek, A.M. Distinct signaling pathways are activated in response to mechanical stress applied axially and transversely to skeletal muscle fibers. J. Biol. Chem. 2002, 277, 46493–46503. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Boriek, A.M. Mechanical stress activates the nuclear factor-kappaB pathway in skeletal muscle fibers: A possible role in Duchenne muscular dystrophy. FASEB J. 2003, 17, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Martinez, C.O.; McHale, M.J.; Wells, J.T.; Ochoa, O.; Michalek, J.E.; McManus, L.M. Regulation of skeletal muscle regeneration by CCR2-activating chemokines is directly related to macrophage recruitment. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R832–R842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pillon, N.J.; Bilan, P.J.; Fink, L.N.; Klip, A. Cross-talk between skeletal muscle and immune cells: Muscle-derived mediators and metabolic implications. Am. J. Physiol. Endocrinol. MeTable. 2013, 304, E453–E465. [Google Scholar] [CrossRef] [PubMed]
- Cousens, L.; Najafian, N.; Martin, W.D.; De Groot, A.S. Tregitope: Immunomodulation powerhouse. Hum. Immunol. 2014, 75, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Schröder, B. The multifaceted roles of the invariant chain CD74--More than just a chaperone. Biochim Biophys. Acta. 2016, 1863, 1269–1281. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kovacs-Nolan, J.; Yang, C.; Archbold, T.; Fan, M.Z.; Mine, Y. Hen egg lysozyme attenuates inflammation and modulates local gene expression in a porcine model of dextran sodium sulfate (DSS)-induced colitis. J. Agric. Food Chem. 2009, 57, 2233–2240. [Google Scholar] [CrossRef] [PubMed]
- Saclier, M.; Cuvellier, S.; Magnan, M. Monocyte/macrophage interactions with myogenic precursor cells during skeletal muscle regeneration. FEBS J. 2013, 280, 4118e4130. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Huang, D.; Ransohoff, R.M.; Zhou, L. Acute skeletal muscle injury: CCL2 expression by both monocytes and injured muscle is required for repair. FASEB J. 2011, 25, 3344–3355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dodge, T.; Wanis, M.; Ayoub, R.; Zhao, L.; Watts, N.B.; Bhattacharya, A. Mechanical loading, damping, and load-driven bone formation in mouse tibiae. Bone 2012, 51, 810–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Hamamura, K.; Turner, C.H.; Yokota, H. Lengthening of mouse hindlimbs with joint loading. J. Bone Miner. MeTable 2010, 28, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Beneficial effects of moderate voluntary physical exercise and its biological mechanisms on brain health. Neurosci. Bull. 2008, 24, 265–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, K.; Lee, S.; Hong, Y.; Park, S.; Choi, J.; Chang, K.T.; Kim, J.H.; Hong, Y. Therapeutic physical exercise in neural injury: Friend or foe? J. Phys. Ther. Sci. 2015, 27, 3933–3935. [Google Scholar] [CrossRef] [PubMed]
- Ottenhof, K.W.; Sild, M.; Lévesque, M.L.; Ruhé, H.G.; Booij, L. TPH2 polymorphisms across the spectrum of psychiatric morbidity: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2018, 92, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Matthes, S.; Mosienko, V.; Bashammakh, S.; Alenina, N.; Bader, M. Tryptophan hydroxylase as novel target for the treatment of depressive disorders. Pharmacology 2010, 85, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Švob Štrac, D.; Pivac, N.; Mück-Šeler, D. The serotonergic system and cognitive function. Transl. Neurosci. 2016, 7, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Borselli, C.; Storrie, H.; Benesch-Lee, F.; Shvartsman, D.; Cezar, C.; Lichtman, J.W. Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors. Proc. Natl. Acad. Sci. USA 2010, 107, 3287–3292. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, R. Mechano-biology of skeletal muscle hypertrophy and regeneration: Possible mechanism of stretch-induced activation of resident myogenic stem cells. Anim. Sci. J. 2010, 81, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Li, H.; Zhai, L.; Zou, X.; Meng, J.; Zhong, R.; Li, C.; Wang, H.; Zhang, Y.; Zhu, D. MicroRNA-431 accelerates muscle regeneration and ameliorates muscular dystrophy by targeting Pax7 in mice. Nat. Commun. 2015, 6, 7713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.; Murashov, A.K. MicroRNA-431 regulates axon regeneration in mature sensory neurons by targeting the Wnt antagonist Kremen1. Front. Mol. Neurosci. 2013, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Luan, Z.; Liu, Y.; Stuhlmiller, T.J.; Marquez, J.; García-Castro, M.I. SUMOylation of Pax7 is essential for neural crest and muscle development. Cell Mol. Life Sci. 2013, 70, 1793–1806. [Google Scholar] [CrossRef] [PubMed]
- Griffiths-Jones, S. The microRNA Registry. Nucleic Acids Res. 2004, 32, D109–D111. [Google Scholar] [CrossRef] [PubMed]
- Tétreault, N.; De Guire, V. miRNAs: Their discovery, biogenesis and mechanism of action. Clin. Biochem. 2013, 46, 842–845. [Google Scholar] [CrossRef] [PubMed]
- Ladoux, B.; Nelson, W.J.; Yan, J.; Mège, R.M. The mechanotransduction machinery at work at adherens junctions. Integr. Biol. 2015, 7, 1109–1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Crawford, M.; Higuita-Castro, N.; Nana-Sinkam, P.; Ghadiali, S.N. miR-146a regulates mechanotransduction and pressure-induced inflammation in small airway epithelium. FASEB J. 2012, 26, 3351–3364. [Google Scholar] [CrossRef] [PubMed]
- Kobus, K.; Kopycinska, J.; Kozlowska-Wiechowska, A.; Urasinska, E.; Kempinska-Podhorodecka, A.; Haas, T.L. Angiogenesis within the duodenum of patients with cirrhosis is modulated by mechanosensitive Kruppel-like factor 2 and microRNA-126. Liver Int. 2012, 32, 1222–1232. [Google Scholar] [CrossRef] [PubMed]
- Drummond, M.J.; McCarthy, J.J.; Sinha, M.; Spratt, H.M.; Volpi, E.; Esser, K.A. Aging and microRNA expression in human skeletal muscle: A microarray and bioinformatics analysis. Physiol. Genomics 2011, 43, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Von Frey, M. On the Use of Stimulus Hairs. J. Neurosci. Methods 1896, 177, 71–131. [Google Scholar]
- Gregory, N.S.; Harris, A.L.; Robinson, C.R.; Dougherty, P.M.; Fuchs, P.N.; Sluka, K.A. An overview of animal models of pain: Disease models and outcome measures. J. Pain 2013, 14, 1255–1269. [Google Scholar] [CrossRef] [PubMed]
- DeSantana, J.M.; da Cruz, K.M.; Sluka, K.A. Animal models of fibromyalgia. Arthritis Res. Ther. 2013, 15, 222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasui, M.; Yoshimura, T.; Takeuchi, S.; Tokizane, K.; Tsuda, M.; Inoue, K. A chronic fatigue syndrome model demonstrates mechanical allodynia and muscular hyperalgesia via spinal microglial activation. Glia 2014, 62, 1407–1417. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, B.T.; Frakes, E.P.; Kasuya, J.; Hammond, D.L.; Kitamoto, T. Changes in expression of sensory organ-specific microRNAs in rat dorsal root ganglia in association with mechanical hypersensitivity induced by spinal nerve ligation. Neuroscience 2009, 164, 711–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.R.; Chen, K.H.; Yang, C.H.; Huang, H.W.; Sheen-Chen, S.M. Intrathecal miR-183 delivery suppresses mechanical allodynia in mononeuropathic rats. Eur. J. Neurosci. 2014, 39, 1682–1689. [Google Scholar] [CrossRef] [PubMed]
- Tam Tam, S.; Bastian, I.; Zhou, X.F.; Vander Hoek, M.; Michael, M.Z.; Gibbins, I.L. MicroRNA-143 expression in dorsal root ganglion neurons. Cell Tissue Res. 2011, 346, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Bali, K.K.; Selvaraj, D.; Satagopam, V.P.; Lu, J.; Schneider, R.; Kuner, R. Genome-wide identification and functional analyses of microRNA signatures associated with cancer pain. EMBO Mol. Med. 2013, 5, 1740–1758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Schack, D.; Agostino, M.J.; Murray, B.S.; Li, Y.; Reddy, P.S.; Chen, J. Dynamic changes in the microRNA expression profile reveal multiple regulatory mechanisms in the spinal nerve ligation model of neuropathic pain. PLoS ONE 2011, 6, e17670. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Dubner, R. Interactions between the immune and nervous systems in pain. Nat. Med. 2010, 16, 1267–1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brenu, E.W.; Ashton, K.J.; van Driel, M.; Staines, D.R.; Peterson, D.; Atkinson, G.M. Cytotoxic lymphocyte microRNAs as prospective biomarkers for chronic fatigue syndrome/myalgic encephalomyelitis. J. Affect. 2012, 141, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Nakasa, T.; Miyaki, S.; Ishikawa, M.; Deie, M.; Adachi, N. Expression of MicroRNA-146a in osteoarthritis cartilage. Arthritis Rheum 2009, 60, 1035–1341. [Google Scholar] [CrossRef] [PubMed]
- Nakasa, T.; Miyaki, S.; Okubo, A.; Hashimoto, M.; Nishida, K.; Ochi, M. Expression of microRNA-146 in rheumatoid arthritis synovial tissue. Arthritis Rheum 2008, 58, 1284–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taganov, K.D.; Boldin, M.P.; Chang, K.J.; Baltimore, D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl. Acad. Sci. USA 2006, 103, 12481–12486. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gibson, G.; Kim, J.S.; Kroin, J.; Xu, S.; van Wijnen, A.J.; Im, H.J. MicroRNA-146a is linked to pain-related pathophysiology of osteoarthritis. Gene 2011, 480, 34–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjersing, J.L.; Lundborg, C.; Bokarewa, M.I.; Mannerkorpi, K. Profile of cerebrospinal microRNAs in fibromyalgia. PLoS ONE 2013, 8, e78762. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Nakasa, T.; Tanaka, N.; Ishikawa, M.; Yamada, K.; Yamasaki, K. Responses of microRNAs 124a and 223 following spinal cord injury in mice. Spinal Cord 2010, 48, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Bhalala, O.G.; Pan, L.; Sahni, V.; McGuire, T.L.; Gruner, K.; Tourtellotte, W.G. MicroRNA-21 regulates astrocytic response following spinal cord injury. J. Neurosci. 2012, 32, 17935–17947. [Google Scholar] [CrossRef] [PubMed]
- Poh, K.W.; Yeo, J.F.; Ong, W.Y. MicroRNA changes in the mouse prefrontal cortex after inflammatory pain. Eur. J. Pain 2011, 15, 1–12. [Google Scholar] [CrossRef]
- Simeoli, R.; Montague, K.; Jones, H.R.; Castaldi, L.; Chambers, D.; Kelleher, J.H. Exosomal cargo including microRNA regulates sensory neuron to macrophage communication after nerve trauma. Nat. Commun. 2017, 8, 1778. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Zhang, H.; Fang, M.; Zhang, Y.; Lu, N.; Zhu, Q. Analgesic effects of Chinese Tuina massage in a rat model of pain. Exp. Ther. Med. 2016, 11, 1367–1374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terry, R.; Perry, R.; Ernst, E. An overview of systematic reviews of complementary and alternative medicine for fibromyalgia. Clin. Rheumatol. 2012, 31, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Kalichman, L. Massage therapy for fibromyalgia symptoms. Rheumatol. Int. 2010, 30, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Winkelmann, A.; Häuser, W.; Friedel, E.; Moog-Egan, M.; Seeger, D.; Settan, M. Physiotherapy and physical therapies for fibromyalgia syndrome. Systematic review, meta-analysis and guideline. Schmerz 2012, 26, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Terhorst, L.; Schneider, M.J.; Kim, K.H.; Goozdich, L.M.; Stilley, C.S. Complementary and alternative medicine in the treatment of pain in fibromyalgia: A systematic review of randomized controlled trials. J. Manip. Physiol. Ther. 2011, 34, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Bronfort, G.; Haas, M.; Evans, R.; Leininger, B.; Triano, J. Effectiveness of manual therapies: The UK evidence report. Chiropr. Osteopat. 2010, 18, 3. [Google Scholar] [CrossRef] [PubMed]
- Crawford, C.; Boyd, C.; Paat, C.F.; Price, A.; Xenakis, L.; Yang, E. Evidence for Massage Therapy (EMT) Working Group. The Impact of Massage Therapy on Function in Pain Populations-A Systematic Review and Meta-Analysis of Randomized Controlled Trials: Part, I., Patients Experiencing Pain in the General Population. Pain Med. 2016, 17, 1353–1375. [Google Scholar] [CrossRef] [PubMed]
- Dupuy, O.; Douzi, W.; Theurot, D.; Bosquet, L.; Dugué, B. An Evidence-Based Approach for Choosing Post-exercise Recovery Techniques to Reduce Markers of Muscle Damage, Soreness, Fatigue, and Inflammation: A Systematic Review with Meta-Analysis. Front. Physiol. 2018, 9, 403. [Google Scholar] [CrossRef] [PubMed]
- Crane, J.D.; Ogborn, D.I.; Cupido, C.; Melov, S.; Hubbard, A.; Bourgeois, J.M. Massage therapy attenuates inflammatory signaling after exercise-induced muscle damage. Sci. Transl. Med. 2012, 4, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Tsilioni, I.; Russell, I.J.; Stewart, J.M.; Gleason, R.M.; Theoharides, T.C. Neuropeptides CRH, SP, HK-1, and Inflammatory Cytokines IL-6 and TNF Are Increased in Serum of Patients with Fibromyalgia Syndrome, Implicating Mast Cells. J. Pharmacol. Exp. Ther. 2016, 356, 664–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patarca, R.; Klimas, N.G.; Lugtendorf, S.; Antoni, M.; Fletcher, M.A. Dysregulated expression of tumor necrosis factor in chronic fatigue syndrome: Interrelations with cellular sources and patterns of soluble immune mediator expression. Clin. Infect. Dis. 1994, 18, S43–S53. [Google Scholar] [CrossRef]
- Delextrat, A.; Hippocrate, A. Leddington-Wright, S.; Clarke, N.D. Including stretches to a massage routine improves recovery from official matches in basketball players. J. Strength Cond. Res. 2014, 28, 716–727. [Google Scholar] [CrossRef] [PubMed]
- Rowe, P.C.; Fontaine, K.R.; Violand, R.L. Neuromuscular strain as a contributor to cognitive and other symptoms in chronic fatigue syndrome: Hypothesis and conceptual model. Front. Physiol. 2013, 4, 115. [Google Scholar] [CrossRef] [PubMed]
- Rowe, P.C.; Fontaine, K.R.; Lauver, M.; Jasion, S.E.; Marden, C.L.; Moni, M. Neuromuscular Strain Increases Symptom Intensity in Chronic Fatigue Syndrome. PLoS ONE 2016, 11, e0159386. [Google Scholar] [CrossRef] [PubMed]
- Goats, G.C. Massage–the scientific basis of an ancient art: Part 2. Physiological and therapeutic effects. Br. J. Sports Med. 1994, 28, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Vallelunga, A.; Berlingieri, C.; Ragusa, M.; Purrello, M.; Stabile, M.R.; Calabrese, M.C. Physical rehabilitation modulates microRNAs involved in multiple sclerosis: A case report. Clin. Case Rep. 2017, 5, 2040–2043. [Google Scholar] [CrossRef] [PubMed]
- Bjersing, J.L.; Bokarewa, M.I.; Mannerkorpi, K. Profile of circulating microRNAs in fibromyalgia and their relation to symptom severity: An exploratory study. Rheumatol. Int. 2015, 35, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Citak-Karakaya, I.; Akbayrak, T.; Demirtürk, F.; Ekici, G.; Bakar, Y. Short and long-term results of connective tissue manipulation and combined ultrasound therapy in patients with fibromyalgia. J. Manipulative. Physiol. Ther. 2006, 29, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Sagar, S.M.; Dryden, T.; Wong, R.K. Massage therapy for cancer patients: A reciprocal relationship between body and mind. Curr. Oncol. 2007, 14, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, J.; Sullivan, S.J.; Seaborne, D.E. The effect of two intensities of massage on H.-reflex amplitude. Phys. Ther. 1992, 72, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Lidbeck, J. Central hyperexcitability in chronic musculoskeletal pain: A conceptual breakthrough with multiple clinical implications. Pain Res. Manag. 2002, 7, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Langevin, H.M.; Bouffard, N.A.; Badger, G.J.; Iatridis, J.C.; Howe, A.K. Dynamic fibroblast cytoskeletal response to subcutaneous tissue stretch ex vivo and in vivo. Am. J. Physiol. Cell Physiol. 2005, 288, C747–C756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaada, B.; Torsteinbø, O. Increase of plasma beta-endorphins in connective tissue massage. Gen. Pharmacol. 1989, 20, 487–489. [Google Scholar] [CrossRef]
- Roberts, L. Effects of patterns of pressure application on resting electromyography during massage. Int. J. Ther. Massage Bodyw. 2011, 4, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Rullman, E.; Mekjavic, I.B.; Fischer, H.; Eiken, O. PlanHab (Planetary Habitat Simulation): The combined and separate effects of 21 days bed rest and hypoxic confinement on human skeletal muscle miRNA expression. Physiol. Rep. 2016, 4, E12753. [Google Scholar] [CrossRef] [PubMed]
- Berk, L.S.; Felten, D.L.; Tan, S.A.; Bittman, B.B.; Westengard, J. Modulation of neuroimmune parameters during the eustress of humor-associated mirthful laughter. Altern. Ther. Health Med. 2001, 7, 62–72. [Google Scholar] [PubMed]
- Fang, C.Y.; Reibel, D.K.; Longacre, M.L.; Rosenzweig, S.; Campbell, D.E.; Douglas, S.D. Enhanced psychosocial well-being following participation in a mindfulness-based stress reduction program is associated with increased natural killer cell activity. J. Altern. Complement. Med. 2010, 16, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Niemi, A.K. Review of Randomized Controlled Trials of Massage in Preterm Infants. Children 2017, 4. [Google Scholar] [CrossRef] [PubMed]
- McLean, S.; Naish, R.; Reed, L.; Urry, S.; Vicenzino, B. A pilot study of the manual force levels required to produce manipulation induced hypoalgesia. Clin. Biomech. 2002, 17, 304–308. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Zhang, Z.; Peng, W. miRDDCR: A miRNA-based method to comprehensively infer drug-disease causal relationships. Sci. Rep. 2017, 7, 15921. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.M.; Kroemer, G.; Zitvogel, L. Extracellular vesicles: Masters of intercellular communication and potential clinical interventions. J. Clin. Investig. 2016, 126, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Farasyn, A. Release of myofascial pain with deep cross-friction named “roptrotherapy”. Int. J. Ther. Massage Bodyw. 2010, 3, 36–37. [Google Scholar] [PubMed]
- Oltra, E. Relevance of splicing on tumor-released exosome landscape: Implications in cancer therapeutics. Front. Endocrinol. 2014, 5, 194. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Morales, M.; Olea, N.; Ruíz, C.; del Castilo Jde, D.; Martínez, M.; Lorenzo, C. Massage after exercise–responses of immunologic and endocrine markers: A randomized single-blind placebo-controlled study. J. Strength Cond. Res. 2009, 23, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Corrales, A.; Campano-Cuevas, E.; Castillo-Dalí, G.; Torres-Lagares, D.; Gutiérrez-Pérez, J.L. Ability of salivary biomarkers in the prognostic of systemic and buccal inflammation. J. Clin. Exp. Dent. 2017, 9, e716–e722. [Google Scholar] [CrossRef] [PubMed]

| Organism | Treatment | Parameter | Markers | Tissues/Cells Affected | Contral. | Cites |
|---|---|---|---|---|---|---|
| Rattus norvegicus | CCL mimetic device | Pressure | CCR2, ILT3, CD74, LYZ2/CD68 & CD163 | Immune/skeletal muscle | Yes | [40] |
| Mus musculus | Massage-like stroking | Massage | T cell numbers/noradrenaline levels | Immune/endocrine | Unknown | [41] |
| Rattus norvegicus | Electro-mechanical loading system | Knee loading | Tph2, Sim1, Pet1 | Brain stem | Unknown | [42,43] |
| Mus musculus | Ferrogels driven by external magnets | Pressure | Intramuscular [O2] | Immune/skeletal muscle | No | [44] |
| Rattus norvegicus | CCL mimetic device | Pressure | Anabolism/Pax7 | Skeletal muscle | Yes | [45] |
| Mus musculus (mdm) | Ex vivo mechanical stretch | Stretching | MechanomiRs/microRNA machinery | Skeletal muscle | Unknown | [46] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espejo, J.A.; García-Escudero, M.; Oltra, E. Unraveling the Molecular Determinants of Manual Therapy: An Approach to Integrative Therapeutics for the Treatment of Fibromyalgia and Chronic Fatigue Syndrome/Myalgic Encephalomyelitis. Int. J. Mol. Sci. 2018, 19, 2673. https://doi.org/10.3390/ijms19092673
Espejo JA, García-Escudero M, Oltra E. Unraveling the Molecular Determinants of Manual Therapy: An Approach to Integrative Therapeutics for the Treatment of Fibromyalgia and Chronic Fatigue Syndrome/Myalgic Encephalomyelitis. International Journal of Molecular Sciences. 2018; 19(9):2673. https://doi.org/10.3390/ijms19092673
Chicago/Turabian StyleEspejo, José Andrés, María García-Escudero, and Elisa Oltra. 2018. "Unraveling the Molecular Determinants of Manual Therapy: An Approach to Integrative Therapeutics for the Treatment of Fibromyalgia and Chronic Fatigue Syndrome/Myalgic Encephalomyelitis" International Journal of Molecular Sciences 19, no. 9: 2673. https://doi.org/10.3390/ijms19092673
APA StyleEspejo, J. A., García-Escudero, M., & Oltra, E. (2018). Unraveling the Molecular Determinants of Manual Therapy: An Approach to Integrative Therapeutics for the Treatment of Fibromyalgia and Chronic Fatigue Syndrome/Myalgic Encephalomyelitis. International Journal of Molecular Sciences, 19(9), 2673. https://doi.org/10.3390/ijms19092673