The Role of the Primary Cell Wall in Plant Morphogenesis
Abstract
:1. Introduction
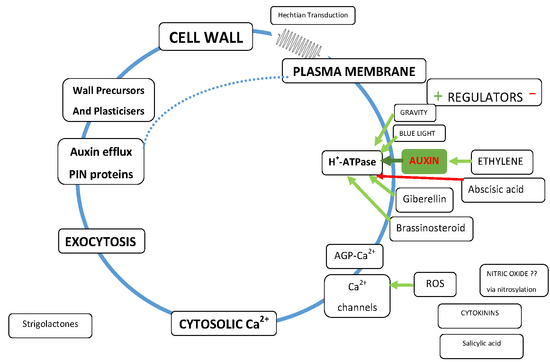
2. Cell Wall Mechanotransduction
- How does it distinguish between the huge forces (mega Pascal range) exerted on the membrane by osmotic pressure and the minute piconewton forces that activate Ca2+ channels and ATPases?
- How does it provide a directional signal in response to anisotropic stress? [26]
- Why does the wall need strong adhesion to the plasma membrane although turgor pressure ensures it? Is the elusive mechanosensor a Boojum or a Snark?
3. Embryogenesis
4. Roots
5. Shoots
6. The Evolution of Morphogenesis
7. Postscript
Funding
Acknowledgments
Conflicts of Interest
References
- Lamport, D.T.A. The Primary Cell Wall; University of Cambridge: Cambridge, UK, 1963; pp. 1–181. [Google Scholar]
- Turing, A.M. The chemical basis of morphogenesis. Philos. Trans. R. Soc. Lond. B 1952, 237, 37–72. [Google Scholar] [CrossRef]
- Thompson, D.W. On Growth and Form; Cambridge University Press: Cambridge, UK, 1917. [Google Scholar]
- Wardlaw, C.W. Phylogeny and Morphogenesis; Macmillan And Co.: Macmillan, London, UK, 1952. [Google Scholar]
- Needham, J. Biochemistry and Morphogenesis; Cambridge University Press: Cambridge, UK, 1942. [Google Scholar]
- Lamport, D.T.A.; Tan, L.; Held, M.A.; Kieliszewksi, M.J. Pollen tube growth and guidance: Occam's razor sharpened on a molecular arabinogalactan glycoprotein Rosetta Stone. New Phytol. 2018, 217, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Qiu, F.; Lamport, D.T.A.; Kieliszewski, M.J. Structure of a hydroxyproline-arabinogalactan polysaccharide from repetitive Ala-Hyp expressed in transgenic Nicotiana tabacum. J. Biol. Chem. 2004, 279, 13156–13165. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Varnai, P.; Lamport, D.T.A.; Yuan, C.; Xu, J.; Qiu, F.; Kieliszewski, M.J. Plant O-Hydroxyproline Arabinogalactans Are Composed of Repeating Trigalactosyl Subunits with Short Bifurcated Side Chains. J. Biol. Chem. 2010, 285, 24575–24583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aspinall, G.O.; Malloy, J.A.; Craig, J.W.T. Extracellular polysaccharides from suspension-cultured sycamore cells. Can. J. Biochem. 1969, 47, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Lamport, D.T.A. Cell wall metabolism. Ann. Rev. Plant Physiol. 1970, 21, 235–270. [Google Scholar] [CrossRef]
- Lamport, D.T.A.; Northcote, D.H. Hydroxyproline in primary cell walls of higher plants. Nature 1960, 188, 665–666. [Google Scholar] [CrossRef]
- Lamport, D.T.A.; Varnai, P. Periplasmic arabinogalactan glycoproteins act as a calcium capacitor that regulates plant growth and development. New Phytol. 2013, 197, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.L.; Cassin, A.M.; Lonsdale, A.; Bacic, A.; Doblin, M.; Schultz, C.J. A Motif and Amino Acid Bias Bioinformatics Pipeline to Identify Hydroxyproline-Rich Glycoproteins. Plant Physiol. 2017, 174, 886–903. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yan, C.; Li, H.; Wu, W.; Liu, Y.; Wang, Y.; Chen, Q.; Ma, H. Bioinformatics Prediction and Evolution Analysis of Arabinogalactan Proteins in the Plant Kingdom. Front. Plant Sci. 2017, 8, 66. [Google Scholar] [CrossRef] [PubMed]
- Pennell, R.I.; Roberts, K. Sexual development in the pea is presaged by altered expression of arabinogalactan protein. Nature 1990, 334, 547–549. [Google Scholar] [CrossRef]
- Pennell, R.I.; Janniche, L.; Scofield, G.N.; Booij, H.; de Vries, S.C.; Roberts, K. Identification of a Transitional Cell State in the Developmental Pathway to Carrot Somatic Embryogenesis. J. Cell Biol. 1992, 119, 1371–1380. [Google Scholar] [CrossRef] [PubMed]
- Showalter, A.M. Arabinogalactan-proteins: Structure, expression and function. Cell. Mol. Life Sci. 2001, 58, 1399–1417. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, S.; Jones, B.; Pereira, L.G. Arabinogalactan proteins (AGPs) related to pollen tube guidance into the embryo sac in Arabidopsis. Plant Signal. Behav. 2008, 3, 455–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hepler, P.K. The Cytoskeleton and Its Regulation by Calcium and Protons. Plant Physiol. 2016, 170, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Hecht, K. Studien uber den Vorgang der Plasmolyse; Beitrage zur Biologie der Pflanzen: Berlin, Germany, 1912; p. 17. [Google Scholar]
- Pont-Lezica, R.F.; McNally, J.G.; Pickard, B.G. Wall-to-membrane linkers in onion epidermis: Some hypotheses. Plant Cell Environ. 1993, 16, 111–123. [Google Scholar] [CrossRef]
- Domozych, D.S.; Roberts, R.; Danyow, C.; Flitter, R.; Smith, B. Plasmolysis, Hechtian strand formation, and localized membrane wall adhesions in the desmid Closterium acerosum (Chlorophyta). J. Phycol. 2003, 39, 1194–1206. [Google Scholar] [CrossRef]
- Raimundo, S.C.; Sorensen, I.; Tinaz, B.; Ritter, B.; Rose, J.K.C.; Domozych, D.S. Isolation and manipulation of protoplasts from the unicellular green alga Penium margaritaceum. Plant Methods 2018, 14, 18. [Google Scholar] [CrossRef]
- Buer, C.S.; Weathers, P.J.; Swartzlander, G.A. Changes in Hechtian strands in cold-hardened cells measured by optical microsurgery. Plant Physiol. 2000, 122, 1365–1377. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto-Sugimoto, M.; Higaki, T.; Yaeno, T.; Nagami, A.; Irie, M.; Fujimi, M.; Miyamoto, M.; Akita, K.; Negi, J.; Shirasu, K.; et al. A Munc13-like protein in Arabidopsis mediates H+-ATPase translocation that is essential for stomatal responses. Nat. Commun. 2013, 4, 2215. [Google Scholar] [CrossRef] [PubMed]
- Sampathkumar, A.; Yan, A.; Krupinski, P.; Meyerowitz, E.M. Physical Forces Regulate Plant Development and Morphogenesis. Curr. Biol. 2014, 24, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Serpe, M.D.; Nothnagel, E.A. Arabinogalactan-proteins in the multiple domains of the plant cell surface. Adv. Bot. Res. 1999, 30, 207–289. [Google Scholar]
- Oxley, D.; Bacic, A. Structure of the glycosylphosphatidylinositol anchor of an arabinogalactan protein from Pyrus communis suspension-cultured cells. Proc. Natl. Acad. Sci. USA 1999, 96, 14246–14251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenhaber, B.; Wildpaner, M.; Schultz, C.J.; Borner, G.H.H.; Dupree, P.; Eisenhaber, F. Glycosylphosphatidylinositol lipid anchoring of plant proteins. Sensitive prediction from sequence- and genome-wide studies for Arabidopsis and rice1. Plant Physiol. 2003, 133, 1691–1701. [Google Scholar] [CrossRef] [PubMed]
- Borner, G.H.H.; Lilley, K.S.; Stevens, T.J.; Dupree, P. Identification of glycosylphosphatidylinositol-anchored proteins in Arabidopsis. A proteomic and genomic analysis. Plant Physiol. 2003, 132, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Cross, B.; Ronzon, F.; Roux, B.; Rieu, J.P. Measurement of the Anchorage Force between GPI-Anchored Alkaline Phosphatase and Supported Membranes by AFM Force Spectroscopy. Langmuir 2005, 21, 5149–5153. [Google Scholar] [CrossRef]
- Dufort, C.C.; Paszek, M.J.; Weaver, V.M. Balancing forces: Architectural control of mechanotransduction. Nat. Rev. Mol. Cell Biol. 2011, 12, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Murthy, S.E.; Dubin, A.E.; Patapoutian, A. Piezos thrive under pressure: Mechanically activated ion channels in health and disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Eberhard, S.; Pattathil, S.; Warder, C.; Glushka, J.; Yuan, C.; Hao, Z.; Zhu, X.; Avci, U.; Miller, J.S.; et al. An Arabidopsis Cell Wall Proteoglycan Consists of Pectin and Arabinoxylan Covalently Linked to an Arabinogalactan Protein. Plant Cell 2013, 25, 270–287. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.W.; Roca-Cusachs, P.; Sheetz, M.P. Stretchy Proteins on Stretchy Substrates: The Important Elements of Integrin-Mediated Rigidity Sensing. Dev. Cell 2010, 19, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Sukharev, S.; Sachs, F. Molecular force transduction by ion channels—Diversity and unifying principles. J. Cell Sci. 2012, 125, 3075–3083. [Google Scholar] [CrossRef] [PubMed]
- Yusko, E.C.; Asbury, C.L. Force is a signal that cells cannot ignore. Mol. Biol. Cell 2014, 25, 3717–3725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falhof, J.; Pedersen, J.T.; Fuglsang, J.T.; Palmgren, M. Plasma Membrane H+-ATPase Regulation in the Center of Plant Physiology. Mol. Plant 2016, 9, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Villalobos, D.; Diaz-Moeeno, M.; van der Schuren, A.; Tamaki, T.; Kang, Y.H.; Gujas, B.; Novak, O.; Jaspert, N.; Li, Z.; Wolf, S.; et al. The Effects of High Steady State Auxin Levels on Root Cell Elongation in Brachypodium. Plant Cell 2016, 28, 1009–1024. [Google Scholar] [CrossRef] [PubMed]
- Cieslak, M.; Runions, A.; Prusinkiewicz, P. Auxin-driven patterning with unidirectional fluxes. J. Exp. Bot. 2015, 66, 5083–5102. [Google Scholar] [CrossRef] [PubMed]
- Leyser, O. Auxin Signaling. Plant Physiol. 2018, 176, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Habets, M.E.; Offringa, R. PIN-driven polar auxin transport in plant developmental plasticity: A key target for environmental and endogenous signals. New Phytol. 2014, 203, 362–377. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Takahashi, K.; Inoue, S.; Kinoshita, T. Abscisic Acid Suppresses Hypocotyl Elongation by Dephosphorylating Plasma Membrane H+-ATPase in Arabidopsis thaliana. Plant Cell Physiol. 2014, 55, 845–853. [Google Scholar] [CrossRef] [PubMed]
- De Vries, J.; Curtis, B.A.; Gould, S.B.; Archibald, J.M. Embryophyte stress signaling evolved in the algal progenitors of land plants. Proc. Natl. Acad. Sci. USA 2018, 115, E3471–E3486. [Google Scholar] [CrossRef] [PubMed]
- Folta, K.M.; Lieg, E.G.; Durham, T.; Spalding, E.P. Primary Inhibition of Hypocotyl Growth and Phototropism Depend Differently on Phototropin Mediated Increases in Cytoplasmic Calcium Induced by Blue Light. Plant Physiol. 2003, 133, 1464–1470. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Qi, Z.; Berkowitz, G.A. Teaching an Old Hormone New Tricks: Cytosolic Ca2+ Elevation Involvement in Plant Brassinosteroid Signal Transduction Cascades. Plant Physiol. 2013, 163, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Swarup, R.; Perry, P.; Hagenbeek, D.; Van Der Straeten, D.; Beemster, G.T.S.; Sandberg, G.; Bhalerao, R.; Ljung, K.; Bennett, M. Ethylene Upregulates Auxin Biosynthesis in Arabidopsis Seedlings to Enhance Inhibition of Root Cell Elongation. Plant Cell 2007, 19, 2186–2196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muday, G.K.; Rahman, A.; Binder, B.M. Auxin and ethylene: Collaborators or competitors? Trends Plant Sci. 2012, 17, 1360–1385. [Google Scholar] [CrossRef] [PubMed]
- Sato, E.M.; Hijazi, H.; Bennett, M.J.; Vissenjberg, K.; Swarup, R. New insights into root gravitropic signaling. J. Exp. Bot. 2015, 66, 2155–2165. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Ito, T.; Fukazawa, J.; Takahashi, Y. Gibberellin Induces an Increase in Cytosolic Ca2+ via a DELLA-Independent Signaling Pathway. Plant Physiol. 2017, 175, 1536–1542. [Google Scholar] [CrossRef] [PubMed]
- Monshausen, G.B.; Miller, N.D.; Murphy, A.S.; Gilroy, J.S. Dynamics of auxin-dependent Ca2+ and pH signaling in root growth revealed by integrating high-resolution imaging with automated computer vision-based analysis. Plant J. 2011, 65, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, C.; Fujii, N.; Miyazawa, Y.; Kamada, M.; Kasahara, H.; Osada, I.; Shimazu, T.; Fusejima, Y.; Higashibata, A.; Yamazaki, T.; et al. The gravity-induced re-localization of auxin efflux carrier CsPIN1 in cucumber seedlings: Spaceflight experiments for immunohistochemical microscopy. Microgravity 2016, 2, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Domingos, P.; Prado, A.M.; Wong, A.; Gehring, C.; Feijo, J.A. Nitric Oxide: A Multitasked Signaling Gas in Plants. Mol. Plant 2015, 8, 506–520. [Google Scholar] [CrossRef] [PubMed]
- Pucciariello, O.; Legris, M.; Rojas, C.C.; Iglesias, M.J.; Hernando, C.E.; Dezar, C.; Vazquez, M.; Yanovsky, M.J.; Finlayson, S.A.; Prat, S.; et al. Rewiring of auxin signaling under persistent shade. Proc. Natl. Acad. Sci. USA 2018, 115, 5612–5617. [Google Scholar] [CrossRef] [PubMed]
- Foreman, J.; Demidchik, V.; Bothwell, J.H.F.; Mylona, P.; Miedema, H.; Torres, M.A.; Linstead, P.; Costa, S.; Brownlee, C.; Jones, J.D.G.; et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 2003, 422, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Proseus, T.E.; Boyer, J.S. Calcium pectate chemistry controls growth rate of Chara coralline. J. Exp. Bot. 2006, 57, 3989–4002. [Google Scholar] [CrossRef] [PubMed]
- Levesque-Tremblay, G.; Pelloux, J.; Braybrook, S.A.; Muller, K. Tuning of pectin methylesterification: Consequences for cell wall biomechanics and development. Planta 2015, 242, 791–811. [Google Scholar] [CrossRef] [PubMed]
- Van den Bulck, K.; Swennen, K.; Loosveld, A.M.; Christophe, M.; Brijs, K.; Proost, P.; Van Damme, J.; Campenhout, S.; Mort, A.; Delcour, J.A. Isolation of cereal arabinogalactan-peptides and structural comparison of their carbohydrate and peptide moieties. J. Cereal Sci. 2005, 41, 59–67. [Google Scholar] [CrossRef]
- Braybrook, S.A.; Peaucell, A. Mechano-Chemical Aspects of Organ Formation in Arabidopsis thaliana: The Relationship between Auxin and Pectin. PLoS ONE 2013, 8, e57813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cosgrove, D.J. Catalysts of plant cell wall loosening. F100Research 2016, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.J. Diffuse Growth of Plant Cell Walls. Plant Physiol. 2018, 176, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Heyn, A.N.J. The physiology of cell elongation. Bot. Rev. 1940, 6, 515–574. [Google Scholar] [CrossRef]
- Ding, J.P.; Pickard, B.G. Mechanosensory calcium-selective cation channels in epidermal cells. Plant J. 1993, 3, 83–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, K.; Hayashi, K.; Kinoshita, T. Auxin Activates the Plasma Membrane H+-ATPase by Phosphorylation during Hypocotyl Elongation in Arabidopsis. Plant Physiol. 2012, 159, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Mazhab-Jafari, M.T.; Rohou, A.; Schmidt, C.; Bueler, S.A.; Benlekbir, S.; Robinson, C.V.; Rubinstein, J.L. Atomic model for the membrane-embedded VO motor of a eukaryotic V-ATPase. Nature 2016, 539, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Lane, N. Power, Sex, Suicide: Mitochondria and the Meaning of Life; Oxford University Press: Cambridge, MA, USA, 2005. [Google Scholar]
- Lamport, D.T.A.; Kieliszewksi, M.J.; Showalter, A.M. Salt-stress upregulates periplasmic arabinogalactan-proteins: Using salt-stress to analyse AGP function. New Phytol. 2006, 169, 479–492. [Google Scholar] [CrossRef] [PubMed]
- Heisler, G.; Ohno, C.; Das, P.; Sieber, P.; Reddy, G.V.; Long, J.A.; Meyerowitz, E.M. Patterns of Auxin Transport and Gene Expression during Primordium Development Revealed by Live Imaging of the Arabidopsis Inflorescence Meristem. Curr. Biol. 2005, 15, 1899–1911. [Google Scholar] [CrossRef] [PubMed]
- Rayle, D.L.; Cleland, R.E. The acid growth theory of auxin-induced cell elongation is alive and well. Plant Physiol. 1992, 99, 1271–1274. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature 1961, 191, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Moller, B.; Weijers, D. Auxin Control of Embryo Patterning. Cold Spring Harb. Perspect. Biol. 2009, 1, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Arber, A. The Natural Philosophy of Plant Form; Cambridge University Press: Cambridge, UK, 1950. [Google Scholar]
- Steward, F.C.; Mapes, M.O.; Smith, J. Growth and organised development of cultured cells. I. Growth and division of freely suspended cells. Am. J. Bot. 1958, 45, 693–703. [Google Scholar] [CrossRef]
- Steward, F.C.; Mapes, M.O.; Mears, K. Growth and organised development of cultured cells. II Organisation in cultures grown from freely suspended cells. Am. J. Bot. 1958, 45, 705–708. [Google Scholar] [CrossRef]
- Dolan, L.; Janmaat, K.; Willemsen, V.; Linstead, P.; Poethig, S.; Roberts, K.; Scheres, B. Cellular organisation of the Arabidopsis thaliana root. Development 1993, 119, 71–84. [Google Scholar] [PubMed]
- Kidner, C.; Sundaresan, V.; Roberts, K.; Dolan, L. Clonal analysis of the Arabidopsis root confirms that position, not lineage, determines cell fate. Planta 2000, 211, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Zhao, J. Localization of arabinogalactan proteins in egg cells, zygotes, and two-celled proembryos and effects of β-D-glucosyl Yariv reagent on egg cell fertilization and zygote division in Nicotiana tabacum L. J. Exp. Bot. 2006, 57, 2061–2074. [Google Scholar] [CrossRef] [PubMed]
- Rubery, P.H.; Sheldrake, A.R. Carrier-mediated Auxin Transport. Planta 1974, 118, 101–121. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Ueda, J.; Komaki, M.K.; Bell, C.J.; Shimura, Y. Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell 1991, 3, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Sun, M.X. The suspensor as a model system to study the mechanism of cell fate specification during early embryogenesis. Plant Reprod. 2018, 31, 59–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pennell, R.I.; Janniche, L.; Kjellbom, P.; Scofield, G.N.; Peart, J.M.; Roberts, K. Developmental regulation of a plasma membrane arabinogalactan protein epitope in oilseed rape flowers. Plant Cell 1991, 3, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Pickett-Heaps, J.D.; Northcote, D.H. Organisation of microtubules and endoplasmic reticulum during mitosis and cytokinesis in wheat meristems. J. Cell Sci. 1966, 1, 109–120. [Google Scholar] [PubMed]
- Schaefer, E.; Belcram, K.; Uyttewaal, M.; Duroc, Y.; Goussot, M.; Legland, D.; Laruelle, O.; de Tauzia-Moreau, M.L.; Pastuglia, M.; Bouchez, D. The preprophase band of microtubules controls the robustness of division orientation in plants. Science 2017, 356, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Cannon, M.C.; Terneus, K.; Hall, Q.; Wang, Y.; Wegenhart, B.L.; Chen, L.; Lamport, D.T.A.; Chen, Y.; Kieliszewski, M.J. Self-assembly of the plant cell wall requires an extensin scaffold. Proc. Natl. Acad. Sci. USA 2008, 105, 2226–2231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamant, O.; Heisler, M.G.; Jonnson, H.; Krupinski, P.; Uyttewaal, M.; Bokov, P.; Corson, F.; Sahlin, P.; Boudaoud, A.; Meyerowitz, E.M.; et al. Developmental patterning by mechanical signals in Arabidopsis. Science 2008, 322, 1650–1654. [Google Scholar] [CrossRef] [PubMed]
- Geshi, N.; Johansen, J.N.; Dilokpimol, A.; Rolland, A.; Belcram, K.; Verger, S.; Kotake, T.; Tsumuraya, Y.; Kaneko, S.; Tryfona, T.; et al. A galactosyltransferase acting on arabinogalactan protein glycans is essential for embryo development in Arabidopsis. Plant J. 2013, 76, 128–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basu, D.; Liang, Y.; Liu, X.; Himmeldirk, K.; Faik, A.; Kieliszewski, M.; Held, M.A.; Showalter, A.M. Functional identification of a hydroxyproline-O-galactosyltransferase specific for arabinogalactan protein biosynthesis in Arabidopsis. J. Biol. Chem. 2013, 288, 10132–10143. [Google Scholar] [CrossRef] [PubMed]
- Dolan, L.; Linstead, P.; Roberts, K. An AGP epitope distinguishes a central metaxylem initial from other vascular initials in the Arabidopsis root. Protoplasma 1995, 189, 149–155. [Google Scholar] [CrossRef]
- Olatunji, D.; Geelen, D.; Verstraeten, I. Control of Endogenous Auxin Levels in Plant Root Development. Int. J. Mol. Sci. 2017, 18, 2587. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Kieliszewski, M.J.; Showalter, A.M. Overexpression of tomato LeAGP-1 arabinogalactan-protein promotes lateral branching and hampers reproductive development. Plant J. 2004, 40, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Feraru, E.; Feraru, M.I.; Kleine-Vehn, J.; Martinie, A.; Mouille, G.; Vanneste, S.; Vernhettes, S.; Runions, J.; Friml, J. PIN Polarity Maintenance by the Cell Wall in Arabidopsis. Curr. Biol. 2011, 21, 338–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leitz, G.; Kang, B.H.; Schoenwaelder, M.E.A.; Staehelin, L.A. Statolith Sedimentation Kinetics and Force Transduction to the Cortical Endoplasmic Reticulum in Gravity-Sensing Arabidopsis Columella Cells. Plant Cell 2009, 21, 843–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caspar, T.; Pickard, B.G. Gravitropism in a starchless mutant of Arabidopsis. Planta 1989, 177, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Berut, A.; Chauvet, H.; Legue, V.; Moulia, B.; Pouliquen, V.; Forterre, Y. Gravisensors in plant cells behave like an active granular liquid. Proc. Natl. Acad. Sci. USA 2018, 115, 5123–5128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Firn, R.D.; Wagstaff, C.; Digby, J. The use of mutants to probe models of gravitropism. J. Exp. Bot. 2000, 51, 1323–1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Everdeen, D.S.; Kiefer, S.; Willard, J.J.; Muldoon, E.P.; Dey, P.M.; Li, X.B.; Lamport, D.T.A. Enzymic crosslinkage of monomeric extensin precursors in vitro. Plant Physiol. 1988, 87, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Cleland, R.E.; Karlsnes, A. A possible role for hydroxyproline-containing proteins in the cessation of cell elongation. Plant Physiol. 1967, 42, 669–671. [Google Scholar] [CrossRef] [PubMed]
- Epstein, L.; Lamport, D.T.A. An intramolecular linkage involving isodityrosine in extension. Phytochemistry 1984, 23, 1241–1246. [Google Scholar] [CrossRef]
- Held, M.A.; Tan, L.; Kamyab, A.; Hare, M.; Shpak, E.; Kieliszewksi, M.J. Di-isodityrosine is the intermolecular cross-link of isodityrosine-rich extensin analogs cross-linked in vitro. J. Biol. Chem. 2004, 279, 55474–55482. [Google Scholar] [CrossRef] [PubMed]
- Friml, J.; Wisniewski, J.P.; Benkova, E.; Mengen, K.; Palme, K. Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 2002, 415, 806–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knox, J.P.; Day, S.; Roberts, K. A set of cell surface glycoproteins forms an early marker of cell position but not cell type, in the root apical meristem of Daucus carota L. Development 1989, 106, 47–56. [Google Scholar]
- Keller, B.; Lamb, C.J. Specific expression of a novel cell wall hydroxyproline-rich glycoprotein gene in lateral root initiation. Genes Dev. 1989, 3, 1639–1646. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Men, S.; Fischer, U.; Stepanova, A.N.; Alonso, J.M.; Ljung, K.; Grebe, M. Local auxin biosynthesis modulates gradient-directed planar polarity in Arabidopsis. Nat. Cell Biol. 2009, 11, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Adler, I.; Barabe, D.; Jean, R.V. A History of the Study of Phyllotaxis. Ann. Bot. 1997, 80, 231–244. [Google Scholar] [CrossRef] [Green Version]
- Vernoux, T.; Besnard, F.; Traas, J. Auxin at the Shoot Apical Meristem. Cold Spring Harb. Perspect. Biol. 2010, 2, a001487. [Google Scholar] [CrossRef] [PubMed]
- Coen, E.S.; Meyerowitz, E.M. The war of the whorls: Genetic interactions controlling flower development. Nature 1991, 353, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Taoka, K.; Ohki, I.; Tsuji, H.; Kojima, C.; Shimamoto, K. Structure and function of florigen and the receptor complex. Trends Plant Sci. 2013, 18, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Luschnig, C.; Vert, G. The dynamics of plant plasma membrane proteins: PINs and beyond. Development 2014, 141, 2924–2938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balla, J.; Medvedova, Z.; Kalousek, P.; Matiješèuková, N.; Friml, J.; Reinohl, V.; Prochazka, S. Auxin flow-mediated competition between axillary buds to restore apical dominance. Sci. Rep. 2016, 6, 35955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.D.; Tan, L.; Showalter, A.M.; Lamport, D.T.A.; Kieliszewski, M.J. Tomato LeAGP-1 arabinogalactan-protein purified from transgenic tobacco corroborates the Hyp contiguity hypothesis. Plant J. 2002, 31, 431–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wardlaw, C.W. Evidence relating to the diffusion reaction theory of morphogenesis. New Phytol. 1955, 54, 39–48. [Google Scholar] [CrossRef]
- Jalbout, A.F.; Leif, A.; Adamowicz, L.; Polt, R.; Apponi, A.J.; Ziurys, L.M. Sugar Synthesis from a Gas-Phase Formose Reaction. Astrobiology 2007, 7, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Margulis, L. Origin of Eukaryotic Cells; Yale University Press: New Haven, CT, USA, 1970. [Google Scholar]
- Liu, X.; Wolfe, R.; Welch, L.R.; Domozych, D.S.; Popper, Z.A.; Showalter, A.M. Bioinformatic Identification and Analysis of Extensins in the Plant Kingdom. PLoS ONE 2016, 11, e0150177. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K. Crystalline glycoprotein cell walls of algae: Their structure, composition and assembly. Philos. Trans. R. Soc. Lond. B 1974, 268, 129–146. [Google Scholar] [CrossRef]
- Kerr, T.; Bailey, I.W. The cambium and its derivative tissues. X Structure, optical properties and chemical composition of the so-called middle lamella. J. Arnold Arbor. 1934, 15, 327–349. [Google Scholar]
- De Smet, I.; Voss, U.; Lau, S.; Wilson, M.; Shao, N.; Timme, R.E.; Swarup, R.; Kerr, I.; Hodgman, C.; Bock, R.; et al. Unraveling the Evolution of Auxin Signaling. Plant Physiol. 2010, 110, 168161. [Google Scholar] [CrossRef] [PubMed]
- Inoue, N.; Yamada, S.; Nagata, Y.; Shimmen, T. Rhizoid Differentiation in Spirogyra: Position Sensing by Terminal Cells. Plant Cell Physiol. 2002, 43, 479–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, D.H.; Lamport, D.T.A.; Miller, M. Hydroxyproline heterooligosaccharides in Chlamydomonas. Science 1972, 176, 918–920. [Google Scholar] [CrossRef] [PubMed]
- Lamport, D.T.A.; Miller, D.H. Hydroxyproline arabinosides in the plant kingdom. Plant Physiol. 1971, 48, 454–456. [Google Scholar] [CrossRef] [PubMed]
- Buglass, S.; Lamport, D.T.A.; Xu, J.; Tan, L.; Kieliszewksi, M.J. Origin of the Land Plants: Is Coleochaete their closest living relative? The writing is on the wall. In Proceedings of the 11th Cell Wall Meeting, Copenhagen, Denmark, 12–17 August 2007; p. 130. [Google Scholar]
- Delwiche, C.F.; Cooper, E.D. The Evolutionary Origin of a Terrestrial Flora. Curr. Biol. 2015, 2015 25, R899–R910. [Google Scholar] [CrossRef]
- Kaplan, D.R.; Cooke, T.J. The genius of Wilhelm Hofmeister: The origin of causal-analytical research in plant development. Am. J. Bot. 1996, 83, 1647–1660. [Google Scholar] [CrossRef]
- Kenrick, P.; Strullu-Derrien, C. The Origin and Early Evolution of Roots. Plant Physiol. 2014, 166, 570–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chrispeels, M.J.; Varner, J.E. Biographical Memoirs; The National Academy of Sciences: Washington, DC, USA, 1997; pp. 353–371. [Google Scholar]











| System | Action | Adhesion Strength pN | References |
|---|---|---|---|
| Integrin-RGD binding | Binding | ~10 | [35] |
| GPI-proteins | Adhesion | 103–350 pN | [31] |
| Protein e.g., talin | Unfolding | 5 pN | [32] |
| Cation channels | Open | 4 pN (to open) | [36] |
| Auxin Efflux Protein | Major Location or Function | Efflux Orientation | References |
|---|---|---|---|
| PIN 1 | Outer protoderm and lower cells of proembryo | Rootward | [71] |
| PIN 2 | Root epidermis and lateral root cap; regulates gravitropism | Shootward | [26] [42] |
| PIN 3 | Gravitropism | Rootward | [26] |
| PIN 4 | Hypophysis | [71] | |
| PIN 5 | ER | Cytosol | [42] |
| PIN 6 | ER | Cytosol | [42] |
| PIN 7 | Suspensor | Apical towards embryo Rootward | [80] |
| PIN 8 | ER | cytosol | [42] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamport, D.T.A.; Tan, L.; Held, M.; Kieliszewski, M.J. The Role of the Primary Cell Wall in Plant Morphogenesis. Int. J. Mol. Sci. 2018, 19, 2674. https://doi.org/10.3390/ijms19092674
Lamport DTA, Tan L, Held M, Kieliszewski MJ. The Role of the Primary Cell Wall in Plant Morphogenesis. International Journal of Molecular Sciences. 2018; 19(9):2674. https://doi.org/10.3390/ijms19092674
Chicago/Turabian StyleLamport, Derek T. A., Li Tan, Michael Held, and Marcia J. Kieliszewski. 2018. "The Role of the Primary Cell Wall in Plant Morphogenesis" International Journal of Molecular Sciences 19, no. 9: 2674. https://doi.org/10.3390/ijms19092674
APA StyleLamport, D. T. A., Tan, L., Held, M., & Kieliszewski, M. J. (2018). The Role of the Primary Cell Wall in Plant Morphogenesis. International Journal of Molecular Sciences, 19(9), 2674. https://doi.org/10.3390/ijms19092674