Drug Repurposing of Metabolic Agents in Malignant Glioma
Abstract
:1. Introduction
2. Tumor Metabolism and Glioma
3. Definition and Principle of Drug Repurposing
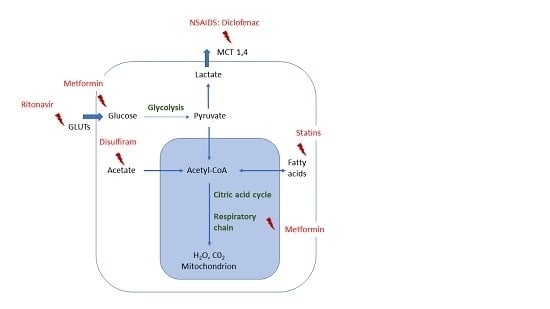
4. Metabolic Drug Repurposing in Glioma
4.1. Metformin
4.2. Statins
4.3. Non-Steroidal Anti-Inflammatory Drugs (NSAID)
4.4. Disulfiram
4.5. Ritonavir
5. Discussion
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| GBM | Glioblastoma |
| NSAR | Non-steroidal anti-inflammator drug |
| IDH | Isocitrate dehydrogenase |
| MGMT | Methylguanine-methyl transferase |
| LOH | Loss of heterocygosity |
References
- Schlegel, U.; Weller, M.; Westphal, M. Neuroonkologie, 2nd ed.; Thieme: Stuttgart, Germany, 2003; ISBN 3-13-109062-6. [Google Scholar]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K. Who Classification of Tumours of the Central Nervous System, 4th ed.; IARC Press: Lyon, Frence, 2016. [Google Scholar]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Gorlia, T.; Erridge, S.C.; Perry, J.; Hong, Y.K.; Aldape, K.D.; Lhermitte, B.; Pietsch, T.; Grujicic, D.; et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated mgmt promoter (centric eortc 26071-22072 study): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2014, 15, 1100–1108. [Google Scholar] [CrossRef]
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: A randomized clinical trial. JAMA 2017, 318, 2306–2316. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Parsons, D.W.; Jin, G.; McLendon, R.; Rasheed, B.A.; Yuan, W.; Kos, I.; Batinic-Haberle, I.; Jones, S.; Riggins, G.J.; et al. IDH1 and IDH2 mutations in gliomas. N. Engl. J. Med. 2009, 360, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Hegi, M.E.; Diserens, A.C.; Gorlia, T.; Hamou, M.F.; de Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. Mgmt gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Perry, A.; Borell, T.J.; Lee, H.K.; O’Fallon, J.; Hosek, S.M.; Kimmel, D.; Yates, A.; Burger, P.C.; Scheithauer, B.W.; et al. Alterations of chromosome arms 1p and 19q as predictors of survival in oligodendrogliomas, astrocytomas, and mixed oligoastrocytomas. J. Clin. Oncol. 2000, 18, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Buckner, J.C. Factors influencing survival in high-grade gliomas. Semin. Oncol. 2003, 30, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Merkel, A.; Soeldner, D.; Wendl, C.; Urkan, D.; Kuramatsu, J.B.; Seliger, C.; Proescholdt, M.; Eyupoglu, I.Y.; Hau, P.; Uhl, M. Early postoperative tumor progression predicts clinical outcome in glioblastoma-implication for clinical trials. J. Neuro-Oncol. 2017, 132, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Adeberg, S.; Bernhardt, D.; Ben Harrabi, S.; Bostel, T.; Mohr, A.; Koelsche, C.; Diehl, C.; Rieken, S.; Debus, J. Metformin influences progression in diabetic glioblastoma patients. Strahlenther. Onkol. 2015, 191, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Chambless, L.B.; Parker, S.L.; Hassam-Malani, L.; McGirt, M.J.; Thompson, R.C. Type 2 diabetes mellitus and obesity are independent risk factors for poor outcome in patients with high-grade glioma. J. Neuro-Oncol. 2012, 106, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Chinot, O.L.; de La Motte Rouge, T.; Moore, N.; Zeaiter, A.; Das, A.; Phillips, H.; Modrusan, Z.; Cloughesy, T. Avaglio: Phase 3 trial of bevacizumab plus temozolomide and radiotherapy in newly diagnosed glioblastoma multiforme. Adv. Ther. 2011, 28, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; Butowski, N.; Tran, D.D.; Recht, L.D.; Lim, M.; Hirte, H.; Ashby, L.; Mechtler, L.; Goldlust, S.A.; Iwamoto, F.; et al. Rindopepimut with temozolomide for patients with newly diagnosed, egfrviii-expressing glioblastoma (ACT IV): A randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017, 18, 1373–1385. [Google Scholar] [CrossRef]
- Hutterer, M.; Nowosielski, M.; Haybaeck, J.; Embacher, S.; Stockhammer, F.; Gotwald, T.; Holzner, B.; Capper, D.; Preusser, M.; Marosi, C.; et al. A single-arm phase ii austrian/german multicenter trial on continuous daily sunitinib in primary glioblastoma at first recurrence (surge 01-07). Neuro-Oncology 2014, 16, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Moeckel, S.; Meyer, K.; Leukel, P.; Heudorfer, F.; Seliger, C.; Stangl, C.; Bogdahn, U.; Proescholdt, M.; Brawanski, A.; Vollmann-Zwerenz, A.; et al. Response-predictive gene expression profiling of glioma progenitor cells in vitro. PLoS ONE 2014, 9, e108632. [Google Scholar] [CrossRef] [PubMed]
- Moeckel, S.; Vollmann-Zwerenz, A.; Proescholdt, M.; Brawanski, A.; Riemenschneider, M.J.; Bogdahn, U.; Bosserhoff, A.K.; Spang, R.; Hau, P. Validation study: Response-predictive gene expression profiling of glioma progenitor cells in vitro. PLoS ONE 2016, 11, e0151312. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O.; Wind, F.; Negelein, E. The metabolism of tumors in the body. J. Gen. Physiol. 1927, 8, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Seliger, C.; Leukel, P.; Moeckel, S.; Jachnik, B.; Lottaz, C.; Kreutz, M.; Brawanski, A.; Proescholdt, M.; Bogdahn, U.; Bosserhoff, A.K.; et al. Lactate-modulated induction of thbs-1 activates transforming growth factor (tgf)-beta2 and migration of glioma cells in vitro. PLoS ONE 2014, 8, e78935. [Google Scholar] [CrossRef]
- Chirasani, S.R.; Leukel, P.; Gottfried, E.; Hochrein, J.; Stadler, K.; Neumann, B.; Oefner, P.J.; Gronwald, W.; Bogdahn, U.; Hau, P.; et al. Diclofenac inhibits lactate formation and efficiently counteracts local immune suppression in a murine glioma model. Int. J. Cancer 2013, 132, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Langedijk, J.; Mantel-Teeuwisse, A.K.; Slijkerman, D.S.; Schutjens, M.H. Drug repositioning and repurposing: Terminology and definitions in literature. Drug Discov. Today 2015, 20, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Galie, N.; Ghofrani, H.A.; Torbicki, A.; Barst, R.J.; Rubin, L.J.; Badesch, D.; Fleming, T.; Parpia, T.; Burgess, G.; Branzi, A.; et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N. Engl. J. Med. 2005, 353, 2148–2157. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, I.; Lue, T.F.; Padma-Nathan, H.; Rosen, R.C.; Steers, W.D.; Wicker, P.A. Oral sildenafil in the treatment of erectile dysfunction. Sildenafil study group. N. Engl. J. Med. 1998, 338, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Singhal, S.; Mehta, J.; Desikan, R.; Ayers, D.; Roberson, P.; Eddlemon, P.; Munshi, N.; Anaissie, E.; Wilson, C.; Dhodapkar, M.; et al. Antitumor activity of thalidomide in refractory multiple myeloma. N. Engl. J. Med 1999, 341, 1565–1571. [Google Scholar] [CrossRef] [PubMed]
- Jick, S.S.; Kaye, J.A.; Vasilakis-Scaramozza, C.; Garcia Rodriguez, L.A.; Ruigomez, A.; Meier, C.R.; Schlienger, R.G.; Black, C.; Jick, H. Validity of the general practice research database. Pharmacotherapy 2003, 23, 686–689. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.F.; Nordestgaard, B.G.; Bojesen, S.E. Statin use and reduced cancer-related mortality. N. Engl. J. Med. 2012, 367, 1792–1802. [Google Scholar] [CrossRef] [PubMed]
- Happold, C.; Gorlia, T.; Chinot, O.; Gilbert, M.R.; Nabors, L.B.; Wick, W.; Pugh, S.L.; Hegi, M.; Cloughesy, T.; Roth, P.; et al. Does valproic acid or levetiracetam improve survival in glioblastoma? A pooled analysis of prospective clinical trials in newly diagnosed glioblastoma. J. Clin. Oncol. 2016, 34, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Suissa, S.; Azoulay, L. Metformin and the risk of cancer: Time-related biases in observational studies. Diabetes Care 2012, 35, 2665–2673. [Google Scholar] [CrossRef] [PubMed]
- Ramos, E.M.; Hoffman, D.; Junkins, H.A.; Maglott, D.; Phan, L.; Sherry, S.T.; Feolo, M.; Hindorff, L.A. Phenotype-genotype integrator (phegeni): Synthesizing genome-wide association study (gwas) data with existing genomic resources. Eur. J. Hum. Genet. 2014, 22, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Welter, D.; MacArthur, J.; Morales, J.; Burdett, T.; Hall, P.; Junkins, H.; Klemm, A.; Flicek, P.; Manolio, T.; Hindorff, L.; et al. The nhgri gwas catalog, a curated resource of snp-trait associations. Nucleic Acids Res. 2014, 42, D1001–D1006. [Google Scholar] [CrossRef] [PubMed]
- Uhlen, M.; Oksvold, P.; Fagerberg, L.; Lundberg, E.; Jonasson, K.; Forsberg, M.; Zwahlen, M.; Kampf, C.; Wester, K.; Hober, S.; et al. Towards a knowledge-based human protein atlas. Nat. Biotechnol. 2010, 28, 1248–1250. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Pinto, S.M.; Getnet, D.; Nirujogi, R.S.; Manda, S.S.; Chaerkady, R.; Madugundu, A.K.; Kelkar, D.S.; Isserlin, R.; Jain, S.; et al. A draft map of the human proteome. Nature 2014, 509, 575–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilhelm, M.; Schlegl, J.; Hahne, H.; Gholami, A.M.; Lieberenz, M.; Savitski, M.M.; Ziegler, E.; Butzmann, L.; Gessulat, S.; Marx, H.; et al. Mass-spectrometry-based draft of the human proteome. Nature 2014, 509, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Magrane, M.; UniProt, C. Uniprot knowledgebase: A hub of integrated protein data. Database 2011, 2011, bar009. [Google Scholar] [CrossRef] [PubMed]
- Bulusu, K.C.; Tym, J.E.; Coker, E.A.; Schierz, A.C.; Al-Lazikani, B. Cansar: Updated cancer research and drug discovery knowledgebase. Nucleic Acids Res. 2014, 42, D1040–D1047. [Google Scholar] [CrossRef] [PubMed]
- Safran, M.; Dalah, I.; Alexander, J.; Rosen, N.; Iny Stein, T.; Shmoish, M.; Nativ, N.; Bahir, I.; Doniger, T.; Krug, H.; et al. Genecards version 3: The human gene integrator. Database 2010, 2010, baq020. [Google Scholar] [CrossRef] [PubMed]
- Rappaport, N.; Twik, M.; Nativ, N.; Stelzer, G.; Bahir, I.; Stein, T.I.; Safran, M.; Lancet, D. Malacards: A comprehensive automatically-mined database of human diseases. Curr. Protoc. Bioinform. 2014, 47, 1.24.1–1.24.19. [Google Scholar] [CrossRef] [PubMed]
- Pinero, J.; Queralt-Rosinach, N.; Bravo, A.; Deu-Pons, J.; Bauer-Mehren, A.; Baron, M.; Sanz, F.; Furlong, L.I. Disgenet: A discovery platform for the dynamical exploration of human diseases and their genes. Database 2015, 2015, bav028. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Knox, C.; Guo, A.C.; Shrivastava, S.; Hassanali, M.; Stothard, P.; Chang, Z.; Woolsey, J. Drugbank: A comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res. 2006, 34, D668–D672. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Fang, H.; Reagan, K.; Xu, X.; Mendrick, D.L.; Slikker, W., Jr.; Tong, W. In silico drug repositioning: What we need to know. Drug Discov. Today 2013, 18, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Gerber, D.E.; Minna, J.D. Alk inhibition for non-small cell lung cancer: From discovery to therapy in record time. Cancer Cell 2010, 18, 548–551. [Google Scholar] [CrossRef] [PubMed]
- Wurth, R.; Thellung, S.; Bajetto, A.; Mazzanti, M.; Florio, T.; Barbieri, F. Drug-repositioning opportunities for cancer therapy: Novel molecular targets for known compounds. Drug Discov. Today 2016, 21, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Sung, B.; Prasad, S.; Webb, L.J.; Aggarwal, B.B. Cancer drug discovery by repurposing: Teaching new tricks to old dogs. Trends Pharmacol. Sci. 2013, 34, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, C.; Jacobs, T.F. Metformin; StatPearls: Treasure Island, FL, USA, 2018. [Google Scholar]
- Evans, J.M.; Donnelly, L.A.; Emslie-Smith, A.M.; Alessi, D.R.; Morris, A.D. Metformin and reduced risk of cancer in diabetic patients. BMJ 2005, 330, 1304–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birsoy, K.; Sabatini, D.M.; Possemato, R. Untuning the tumor metabolic machine: Targeting cancer metabolism: A bedside lesson. Nat. Med. 2012, 18, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Isakovic, A.; Harhaji, L.; Stevanovic, D.; Markovic, Z.; Sumarac-Dumanovic, M.; Starcevic, V.; Micic, D.; Trajkovic, V. Dual antiglioma action of metformin: Cell cycle arrest and mitochondria-dependent apoptosis. Cell. Mol. Life Sci. 2007, 64, 1290–1302. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Sunayama, J.; Okada, M.; Watanabe, E.; Seino, S.; Shibuya, K.; Suzuki, K.; Narita, Y.; Shibui, S.; Kayama, T.; et al. Glioma-initiating cell elimination by metformin activation of FOXO3 via AMPK. Stem Cells Transl. Med. 2013, 1, 811–824. [Google Scholar] [CrossRef] [PubMed]
- Sesen, J.; Dahan, P.; Scotland, S.J.; Saland, E.; Dang, V.T.; Lemarie, A.; Tyler, B.M.; Brem, H.; Toulas, C.; Cohen-Jonathan Moyal, E.; et al. Metformin inhibits growth of human glioblastoma cells and enhances therapeutic response. PLoS ONE 2015, 10, e0123721. [Google Scholar] [CrossRef] [PubMed]
- Wurth, R.; Pattarozzi, A.; Gatti, M.; Bajetto, A.; Corsaro, A.; Parodi, A.; Sirito, R.; Massollo, M.; Marini, C.; Zona, G.; et al. Metformin selectively affects human glioblastoma tumor-initiating cell viability: A role for metformin-induced inhibition of akt. Cell Cycle 2013, 12, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Gritti, M.; Wurth, R.; Angelini, M.; Barbieri, F.; Peretti, M.; Pizzi, E.; Pattarozzi, A.; Carra, E.; Sirito, R.; Daga, A.; et al. Metformin repositioning as antitumoral agent: Selective antiproliferative effects in human glioblastoma stem cells, via inhibition of clic1-mediated ion current. Oncotarget 2014, 5, 11252–11268. [Google Scholar] [CrossRef] [PubMed]
- Seliger, C.; Meyer, A.L.; Renner, K.; Leidgens, V.; Moeckel, S.; Jachnik, B.; Dettmer, K.; Tischler, U.; Gerthofer, V.; Rauer, L.; et al. Metformin inhibits proliferation and migration of glioblastoma cells independently of tgf-beta2. Cell Cycle 2016, 15, 1755–1766. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Zhao, G.; Xie, G.; Zhao, L.; Chen, Y.; Yu, H.; Zhang, Z.; Li, C.; Li, Y. Metformin and temozolomide act synergistically to inhibit growth of glioma cells and glioma stem cells in vitro and in vivo. Oncotarget 2015, 6, 32930–32943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, L.B.; Tian, S.; Gao, H.H.; Xu, Y.Y. Metformin inhibits glioma cell u251 invasion by downregulation of fibulin-3. Neuroreport 2013, 24, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Leidgens, V.; Proske, J.; Rauer, L.; Moeckel, S.; Renner, K.; Bogdahn, U.; Riemenschneider, M.J.; Proescholdt, M.; Vollmann-Zwerenz, A.; Hau, P.; et al. Stattic and metformin inhibit brain tumor initiating cells by reducing stat3-phosphorylation. Oncotarget 2016, 8, 8250–8263. [Google Scholar] [CrossRef] [PubMed]
- Seliger, C.; Ricci, C.; Meier, C.R.; Bodmer, M.; Jick, S.S.; Bogdahn, U.; Hau, P.; Leitzmann, M.F. Diabetes, use of antidiabetic drugs, and the risk of glioma. Neuro-Oncology 2015, 18, 340–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labuzek, K.; Suchy, D.; Gabryel, B.; Bielecka, A.; Liber, S.; Okopien, B. Quantification of metformin by the hplc method in brain regions, cerebrospinal fluid and plasma of rats treated with lipopolysaccharide. Pharmacol. Rep. 2010, 62, 956–965. [Google Scholar] [CrossRef]
- Welch, M.R.; Grommes, C. Retrospective analysis of the effects of steroid therapy and antidiabetic medication on survival in diabetic glioblastoma patients. CNS Oncol. 2013, 2, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Seliger, C.; Luber, C.; Gerken, M.; Schaertl, J.; Proescholdt, M.; Riemenschneider, M.J.; Meier, C.R.; Bogdahn, U.; Leitzmann, M.F.; Klinkhammer-Schalke, M.; et al. Use of metformin and survival of patients with high-grade glioma. Int. J. Cancer 2018. [Google Scholar] [CrossRef] [PubMed]
- Cuyas, E.; Fernandez-Arroyo, S.; Corominas-Faja, B.; Rodriguez-Gallego, E.; Bosch-Barrera, J.; Martin-Castillo, B.; De Llorens, R.; Joven, J.; Menendez, J.A. Oncometabolic mutation idh1 r132h confers a metformin-hypersensitive phenotype. Oncotarget 2015, 6, 12279–12296. [Google Scholar] [CrossRef] [PubMed]
- Molenaar, R.J.; Coelen, R.J.S.; Khurshed, M.; Roos, E.; Caan, M.W.A.; van Linde, M.E.; Kouwenhoven, M.; Bramer, J.A.M.; Bovee, J.; Mathot, R.A.; et al. Study protocol of a phase ib/ii clinical trial of metformin and chloroquine in patients with idh1-mutated or idh2-mutated solid tumours. BMJ Open 2017, 7, e014961. [Google Scholar] [CrossRef] [PubMed]
- Kordes, S.; Pollak, M.N.; Zwinderman, A.H.; Mathot, R.A.; Weterman, M.J.; Beeker, A.; Punt, C.J.; Richel, D.J.; Wilmink, J.W. Metformin in patients with advanced pancreatic cancer: A double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol. 2015, 16, 839–847. [Google Scholar] [CrossRef]
- Minniti, G.; Scaringi, C.; Baldoni, A.; Lanzetta, G.; De Sanctis, V.; Esposito, V.; Enrici, R.M. Health-related quality of life in elderly patients with newly diagnosed glioblastoma treated with short-course radiation therapy plus concomitant and adjuvant temozolomide. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Sayed, R.; Saad, A.S.; El Wakeel, L.; Elkholy, E.; Badary, O. Metformin addition to chemotherapy in stage iv non-small cell lung cancer: An open label randomized controlled study. Asian Pac. J. Cancer Prev. 2015, 16, 6621–6626. [Google Scholar] [CrossRef] [PubMed]
- Reni, M.; Dugnani, E.; Cereda, S.; Belli, C.; Balzano, G.; Nicoletti, R.; Liberati, D.; Pasquale, V.; Scavini, M.; Maggiora, P.; et al. (Ir)relevance of metformin treatment in patients with metastatic pancreatic cancer: An open-label, randomized phase ii trial. Clin. Cancer Res. 2016, 22, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Parikh, A.B.; Kozuch, P.; Rohs, N.; Becker, D.J.; Levy, B.P. Metformin as a repurposed therapy in advanced non-small cell lung cancer (nsclc): Results of a phase ii trial. Investig. New Drugs 2017, 35, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Sonnenblick, A.; Agbor-Tarh, D.; Bradbury, I.; Di Cosimo, S.; Azim, H.A., Jr.; Fumagalli, D.; Sarp, S.; Wolff, A.C.; Andersson, M.; Kroep, J.; et al. Impact of diabetes, insulin, and metformin use on the outcome of patients with human epidermal growth factor receptor 2-positive primary breast cancer: Analysis from the altto phase iii randomized trial. J. Clin. Oncol. 2017, 35, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Marrone, K.A.; Zhou, X.; Forde, P.M.; Purtell, M.; Brahmer, J.R.; Hann, C.L.; Kelly, R.J.; Coleman, B.; Gabrielson, E.; Rosner, G.L.; et al. A randomized phase ii study of metformin plus paclitaxel/carboplatin/bevacizumab in patients with chemotherapy-naive advanced or metastatic nonsquamous non-small cell lung cancer. Oncologist 2018, 23, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Afshordel, S.; Kern, B.; Clasohm, J.; Konig, H.; Priester, M.; Weissenberger, J.; Kogel, D.; Eckert, G.P. Lovastatin and perillyl alcohol inhibit glioma cell invasion, migration, and proliferation—impact of ras-/rho-prenylation. Pharmacol. Res. 2015, 91, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Yanae, M.; Tsubaki, M.; Satou, T.; Itoh, T.; Imano, M.; Yamazoe, Y.; Nishida, S. Statin-induced apoptosis via the suppression of erk1/2 and akt activation by inhibition of the geranylgeranyl-pyrophosphate biosynthesis in glioblastoma. J. Exp. Clin. Cancer Res. 2011, 30, 74. [Google Scholar] [CrossRef] [PubMed]
- Ferris, J.S.; McCoy, L.; Neugut, A.I.; Wrensch, M.; Lai, R. Hmg coa reductase inhibitors, nsaids and risk of glioma. Int. J. Cancer 2012, 131, E1031–E1037. [Google Scholar] [CrossRef] [PubMed]
- Gaist, D.; Andersen, L.; Hallas, J.; Sorensen, H.T.; Schroder, H.D.; Friis, S. Use of statins and risk of glioma: A nationwide case-control study in denmark. Br. J. Cancer 2013, 108, 715–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seliger, C.; Meier, C.R.; Becker, C.; Jick, S.S.; Bogdahn, U.; Hau, P.; Leitzmann, M.F. Statin use and risk of glioma: Population-based case-control analysis. Eur. J. Epidemiol. 2016, 31, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Bhavsar, S.; Hagan, K.; Arunkumar, R.; Potylchansky, Y.; Grasu, R.; Dang, A.; Carlson, R.; Cowels, C.; Arnold, B.; Rahlfs, T.F.; et al. Preoperative statin use is not associated with improvement in survival after glioblastoma surgery. J. Clin. Neurosci. 2016, 31, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Gaist, D.; Hallas, J.; Friis, S.; Hansen, S.; Sorensen, H.T. Statin use and survival following glioblastoma multiforme. Cancer Epidemiol. 2014, 38, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Seckl, M.J.; Ottensmeier, C.H.; Cullen, M.; Schmid, P.; Ngai, Y.; Muthukumar, D.; Thompson, J.; Harden, S.; Middleton, G.; Fife, K.M.; et al. Multicenter, phase iii, randomized, double-blind, placebo-controlled trial of pravastatin added to first-line standard chemotherapy in small-cell lung cancer (lungstar). J. Clin. Oncol. 2017, 35, 1506–1514. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Kim, T.W.; Hong, Y.S.; Han, S.W.; Lee, K.H.; Kang, H.J.; Hwang, I.G.; Lee, J.Y.; Kim, H.S.; Kim, S.T.; et al. A randomised, double-blind, placebo-controlled multi-centre phase iii trial of xeliri/folfiri plus simvastatin for patients with metastatic colorectal cancer. Br. J. Cancer 2015, 113, 1421–1426. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, K.H.; Lee, G.K.; Lee, S.H.; Lim, K.Y.; Joo, J.; Go, Y.J.; Lee, J.S.; Han, J.Y. Randomized phase ii study of afatinib plus simvastatin versus afatinib alone in previously treated patients with advanced nonadenocarcinomatous non-small cell lung cancer. Cancer Res. Treat. 2017, 49, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Eberhart, K.; Renner, K.; Ritter, I.; Kastenberger, M.; Singer, K.; Hellerbrand, C.; Kreutz, M.; Kofler, R.; Oefner, P.J. Low doses of 2-deoxy-glucose sensitize acute lymphoblastic leukemia cells to glucocorticoid-induced apoptosis. Leukemia 2009, 23, 2167–2170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goss, G.D.; Jonker, D.J.; Laurie, S.A.; Weberpals, J.I.; Oza, A.M.; Spaans, J.N.; la Porte, C.; Dimitroulakos, J. A phase i study of high-dose rosuvastatin with standard dose erlotinib in patients with advanced solid malignancies. J. Transl. Med. 2016, 14, 83. [Google Scholar] [CrossRef] [PubMed]
- Wongrakpanich, S.; Wongrakpanich, A.; Melhado, K.; Rangaswami, J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. 2018, 9, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Wang, L.; Shu, H.K. Cox-2 overexpression increases malignant potential of human glioma cells through id1. Oncotarget 2014, 5, 1241–1252. [Google Scholar] [CrossRef] [PubMed]
- Gottfried, E.; Lang, S.A.; Renner, K.; Bosserhoff, A.; Gronwald, W.; Rehli, M.; Einhell, S.; Gedig, I.; Singer, K.; Seilbeck, A.; et al. New aspects of an old drug—diclofenac targets myc and glucose metabolism in tumor cells. PLoS ONE 2013, 8, e66987. [Google Scholar] [CrossRef] [PubMed]
- Leidgens, V.; Seliger, C.; Jachnik, B.; Welz, T.; Leukel, P.; Vollmann-Zwerenz, A.; Bogdahn, U.; Kreutz, M.; Grauer, O.M.; Hau, P. Ibuprofen and diclofenac restrict migration and proliferation of human glioma cells by distinct molecular mechanisms. PLoS ONE 2015, 10, e0140613. [Google Scholar] [CrossRef] [PubMed]
- Maiti, R. Metronomic chemotherapy. J. Pharmacol. Pharmacother. 2014, 5, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Kerschbaumer, J.; Schmidt, F.A.; Grams, A.E.; Nowosielski, M.; Pinggera, D.; Brawanski, K.R.; Petr, O.; Thome, C.; Tuettenberg, J.; Seiz, M.; et al. Dual anti-angiogenic chemotherapy with temozolomide and celecoxib in selected patients with malignant glioma not eligible for standard treatment. Anticancer Res. 2015, 35, 4955–4960. [Google Scholar] [PubMed]
- Porkholm, M.; Valanne, L.; Lonnqvist, T.; Holm, S.; Lannering, B.; Riikonen, P.; Wojcik, D.; Sehested, A.; Clausen, N.; Harila-Saari, A.; et al. Radiation therapy and concurrent topotecan followed by maintenance triple anti-angiogenic therapy with thalidomide, etoposide, and celecoxib for pediatric diffuse intrinsic pontine glioma. Pediatr. Blood Cancer 2014, 61, 1603–1609. [Google Scholar] [CrossRef] [PubMed]
- Welzel, G.; Gehweiler, J.; Brehmer, S.; Appelt, J.U.; von Deimling, A.; Seiz-Rosenhagen, M.; Schmiedek, P.; Wenz, F.; Giordano, F.A. Metronomic chemotherapy with daily low-dose temozolomide and celecoxib in elderly patients with newly diagnosed glioblastoma multiforme: A retrospective analysis. J. Neuro-Oncol. 2015, 124, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Reardon, D.A.; Quinn, J.A.; Vredenburgh, J.; Rich, J.N.; Gururangan, S.; Badruddoja, M.; Herndon, J.E., 2nd; Dowell, J.M.; Friedman, A.H.; Friedman, H.S. Phase II trial of irinotecan plus celecoxib in adults with recurrent malignant glioma. Cancer 2005, 103, 329–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stockhammer, F.; Misch, M.; Koch, A.; Czabanka, M.; Plotkin, M.; Blechschmidt, C.; Tuettenberg, J.; Vajkoczy, P. Continuous low-dose temozolomide and celecoxib in recurrent glioblastoma. J. Neuro-Oncol. 2010, 100, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Penas-Prado, M.; Hess, K.R.; Fisch, M.J.; Lagrone, L.W.; Groves, M.D.; Levin, V.A.; De Groot, J.F.; Puduvalli, V.K.; Colman, H.; Volas-Redd, G.; et al. Randomized phase ii adjuvant factorial study of dose-dense temozolomide alone and in combination with isotretinoin, celecoxib, and/or thalidomide for glioblastoma. Neuro-Oncology 2015, 17, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Kesari, S.; Schiff, D.; Henson, J.W.; Muzikansky, A.; Gigas, D.C.; Doherty, L.; Batchelor, T.T.; Longtine, J.A.; Ligon, K.L.; Weaver, S.; et al. Phase II study of temozolomide, thalidomide, and celecoxib for newly diagnosed glioblastoma in adults. Neuro-Oncology 2008, 10, 300–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kesari, S.; Schiff, D.; Doherty, L.; Gigas, D.C.; Batchelor, T.T.; Muzikansky, A.; O’Neill, A.; Drappatz, J.; Chen-Plotkin, A.S.; Ramakrishna, N.; et al. Phase II study of metronomic chemotherapy for recurrent malignant gliomas in adults. Neuro-Oncology 2007, 9, 354–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seliger, C.; Meier, C.R.; Becker, C.; Jick, S.S.; Bogdahn, U.; Hau, P.; Leitzmann, M.F. Use of selective cyclooxygenase-2 inhibitors, other analgesics, and risk of glioma. PLoS ONE 2016, 11, e0149293. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, S.E.; Moore, S.C.; Pfeiffer, R.M.; Inskip, P.D.; Park, Y.; Hollenbeck, A.; Rajaraman, P. Nonsteroidal anti-inflammatory drugs and glioma in the nih-aarp diet and health study cohort. Cancer Prev. Res. 2011, 4, 2027–2034. [Google Scholar] [CrossRef] [PubMed]
- Gaist, D.; Garcia-Rodriguez, L.A.; Sorensen, H.T.; Hallas, J.; Friis, S. Use of low-dose aspirin and non-aspirin nonsteroidal anti-inflammatory drugs and risk of glioma: A case-control study. Br. J. Cancer 2013, 108, 1189–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.; Yang, X.; Liu, P.; Zhou, J.; Luo, J.; Wang, H.; Li, A.; Zhou, Y. Association between nonsteroidal anti-inflammatory drugs use and risk of central nervous system tumors: A dose-response meta analysis. Oncotarget 2017, 8, 102486–102498. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, P.M.; Fowkes, F.G.; Belch, J.F.; Ogawa, H.; Warlow, C.P.; Meade, T.W. Effect of daily aspirin on long-term risk of death due to cancer: Analysis of individual patient data from randomised trials. Lancet 2011, 377, 31–41. [Google Scholar] [CrossRef]
- Tolloczko-Iwaniuk, N.; Dziemianczyk-Pakiela, D.; Nowaszewska, B.K.; Celinska-Janowicz, K.; Miltyk, W. Celecoxib in cancer therapy and prevention-review. Curr. Drug Targets 2018. [Google Scholar] [CrossRef]
- Skinner, M.D.; Lahmek, P.; Pham, H.; Aubin, H.J. Disulfiram efficacy in the treatment of alcohol dependence: A meta-analysis. PLoS ONE 2014, 9, e87366. [Google Scholar] [CrossRef] [PubMed]
- Triscott, J.; Rose Pambid, M.; Dunn, S.E. Concise review: Bullseye: Targeting cancer stem cells to improve the treatment of gliomas by repurposing disulfiram. Stem Cells 2015, 33, 1042–1046. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.L.; Liu, S.; Cui, W.; Shi, Y.; Liu, Q.; Duan, J.J.; Yu, S.C.; Zhang, X.; Cui, Y.H.; Kung, H.F.; et al. Aldehyde dehydrogenase 1a1 circumscribes high invasive glioma cells and predicts poor prognosis. Am. J. Cancer Res. 2015, 5, 1471–1483. [Google Scholar] [PubMed]
- Lun, X.; Wells, J.C.; Grinshtein, N.; King, J.C.; Hao, X.; Dang, N.H.; Wang, X.; Aman, A.; Uehling, D.; Datti, A.; et al. Disulfiram when combined with copper enhances the therapeutic effects of temozolomide for the treatment of glioblastoma. Clin. Cancer Res. 2016, 22, 3860–3875. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Brown, S.; Goktug, T.; Channathodiyil, P.; Kannappan, V.; Hugnot, J.P.; Guichet, P.O.; Bian, X.; Armesilla, A.L.; Darling, J.L.; et al. Cytotoxic effect of disulfiram/copper on human glioblastoma cell lines and aldh-positive cancer-stem-like cells. Br. J. Cancer 2012, 107, 1488–1497. [Google Scholar] [CrossRef] [PubMed]
- Mashimo, T.; Pichumani, K.; Vemireddy, V.; Hatanpaa, K.J.; Singh, D.K.; Sirasanagandla, S.; Nannepaga, S.; Piccirillo, S.G.; Kovacs, Z.; Foong, C.; et al. Acetate is a bioenergetic substrate for human glioblastoma and brain metastases. Cell 2014, 159, 1603–1614. [Google Scholar] [CrossRef] [PubMed]
- Paranjpe, A.; Zhang, R.; Ali-Osman, F.; Bobustuc, G.C.; Srivenugopal, K.S. Disulfiram is a direct and potent inhibitor of human o6-methylguanine-DNA methyltransferase (mgmt) in brain tumor cells and mouse brain and markedly increases the alkylating DNA damage. Carcinogenesis 2014, 35, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Campian, J.L.; Gujar, A.D.; Tran, D.D.; Lockhart, A.C.; DeWees, T.A.; Tsien, C.I.; Kim, A.H. A phase i study to repurpose disulfiram in combination with temozolomide to treat newly diagnosed glioblastoma after chemoradiotherapy. J. Neuro-Oncol. 2016, 128, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Kast, R.E.; Karpel-Massler, G.; Halatsch, M.E. Cusp9* treatment protocol for recurrent glioblastoma: Aprepitant, artesunate, auranofin, captopril, celecoxib, disulfiram, itraconazole, ritonavir, sertraline augmenting continuous low dose temozolomide. Oncotarget 2014, 5, 8052–8082. [Google Scholar] [CrossRef] [PubMed]
- Dalva-Aydemir, S.; Bajpai, R.; Martinez, M.; Adekola, K.U.; Kandela, I.; Wei, C.; Singhal, S.; Koblinski, J.E.; Raje, N.S.; Rosen, S.T.; et al. Targeting the metabolic plasticity of multiple myeloma with fda-approved ritonavir and metformin. Clin. Cancer Res. 2015, 21, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Adekola, K.U.; Dalva Aydemir, S.; Ma, S.; Zhou, Z.; Rosen, S.T.; Shanmugam, M. Investigating and targeting chronic lymphocytic leukemia metabolism with the human immunodeficiency virus protease inhibitor ritonavir and metformin. Leuk. Lymphoma 2015, 56, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Laurent, N.; de Bouard, S.; Guillamo, J.S.; Christov, C.; Zini, R.; Jouault, H.; Andre, P.; Lotteau, V.; Peschanski, M. Effects of the proteasome inhibitor ritonavir on glioma growth in vitro and in vivo. Mol. Cancer Ther. 2004, 3, 129–136. [Google Scholar] [PubMed]
- Azzalin, A.; Nato, G.; Parmigiani, E.; Garello, F.; Buffo, A.; Magrassi, L. Inhibitors of glut/slc2a enhance the action of bcnu and temozolomide against high-grade gliomas. Neoplasia 2017, 19, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Rappa, F.; Cappello, F.; Halatsch, M.E.; Scheuerle, A.; Kast, R.E. Aldehyde dehydrogenase and hsp90 co-localize in human glioblastoma biopsy cells. Biochimie 2013, 95, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Kast, R.E. The role of interleukin-18 in glioblastoma pathology implies therapeutic potential of two old drugs-disulfiram and ritonavir. Chin. J. Cancer 2015, 34, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Kast, R.E.; Ramiro, S.; Llado, S.; Toro, S.; Covenas, R.; Munoz, M. Antitumor action of temozolomide, ritonavir and aprepitant against human glioma cells. J. Neuro-Oncol. 2016, 126, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, M.S.; Patton, C.; Stevens, G.; Tekautz, T.; Angelov, L.; Vogelbaum, M.A.; Weil, R.J.; Chao, S.; Elson, P.; Suh, J.H.; et al. Phase II trial of ritonavir/lopinavir in patients with progressive or recurrent high-grade gliomas. J. Neuro-Oncol. 2011, 102, 317–321. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seliger, C.; Hau, P. Drug Repurposing of Metabolic Agents in Malignant Glioma. Int. J. Mol. Sci. 2018, 19, 2768. https://doi.org/10.3390/ijms19092768
Seliger C, Hau P. Drug Repurposing of Metabolic Agents in Malignant Glioma. International Journal of Molecular Sciences. 2018; 19(9):2768. https://doi.org/10.3390/ijms19092768
Chicago/Turabian StyleSeliger, Corinna, and Peter Hau. 2018. "Drug Repurposing of Metabolic Agents in Malignant Glioma" International Journal of Molecular Sciences 19, no. 9: 2768. https://doi.org/10.3390/ijms19092768
APA StyleSeliger, C., & Hau, P. (2018). Drug Repurposing of Metabolic Agents in Malignant Glioma. International Journal of Molecular Sciences, 19(9), 2768. https://doi.org/10.3390/ijms19092768