Mackerel-Derived Fermented Fish Oil Promotes Hair Growth by Anagen-Stimulating Pathways
Abstract
:1. Introduction
2. Results
2.1. FFO Extract Increased the Hair-Fiber Length of Rat Vibrissa Follicles
2.2. FFO Extract Stimulated the Telogen-to-Anagen Transition of the Hair-Cycle in Mice
2.3. FFO Extract Increased the Proliferation of DPC through the Progression of Cell Cycle
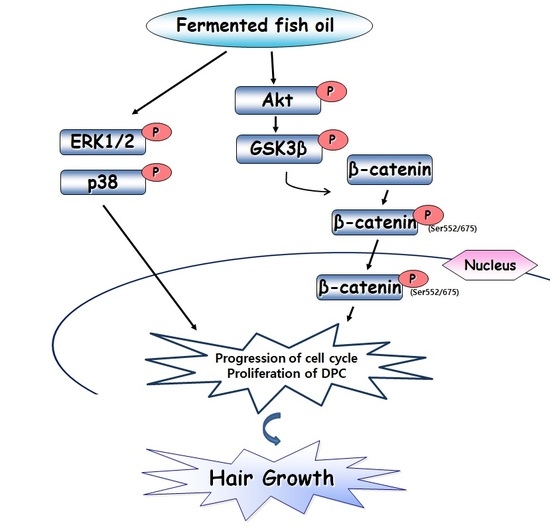
2.4. FFO Extract Activated the Akt and MAPK Signaling in DPC
2.5. FFO Extract Activated Wnt/β-catenin Signaling in DPC
2.6. DHA Increased the Proliferation of DPC
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Preparation of Fermented Fish Oil (FFO) Extract
4.3. Animals
4.4. Isolation and Culture of Rat Vibrissa Follicles
4.5. Hair Growth Activity In Vivo
4.6. Cell Viability Assay
4.7. Cell Cycle Analysis
4.8. Western Blot Analysis
4.9. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maurer, M.; Fischer, E.; Handjiski, B.; Von Stebut, E.; Algermissen, B.; Bavandi, A.; Paus, R. Activated skin mast cells are involved in murine hair follicle regression (catagen). Lab. Investig. 1997, 77, 319–332. [Google Scholar] [PubMed]
- Cotsarelis, G.; Millar, S.E. Towards a molecular understanding of hair loss and its treatment. Trends Mol. Med. 2001, 7, 293–301. [Google Scholar] [CrossRef]
- Daniells, S.; Hardy, G. Hair loss in long-term or home parenteral nutrition: Are micronutrient deficiencies to blame? Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Paus, R.; Haslam, I.S.; Sharov, A.A.; Botchkarev, V.A. Pathobiology of chemotherapy-induced hair loss. Lancet Oncol. 2013, 14, e50–e59. [Google Scholar] [CrossRef] [Green Version]
- Avram, M.R.; Finney, R.; Rogers, N. Hair transplantation controversies. Dermatol. Surg. 2017, 43 (Suppl. 2), S158–S162. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Pino, A.; Martinez, N.; Orive, G.; Berridi, D. The effect of plasma rich in growth factors on pattern hair loss: A pilot study. Dermatol. Surg. 2017, 43, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Burton, J.L.; Marshall, A. Hypertrichosis due to minoxidil. Br. J. Dermatol. 1979, 101, 593–595. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, K.D.; Olsen, E.A.; Whiting, D.; Savin, R.; DeVillez, R.; Bergfeld, W.; Price, V.H.; Van Neste, D.; Roberts, J.L.; Hordinsky, M.; et al. Finasteride in the treatment of men with androgenetic alopecia. J. Am. Acad. Dermatol. 1998, 39, 578–589. [Google Scholar] [CrossRef]
- Dargie, H.J.; Dollery, C.T.; Daniel, J. Minoxidil in resistant hypertension. Lancet 1977, 2, 515–518. [Google Scholar] [CrossRef]
- Price, V.H. Treatment of hair loss. N. Engl. J. Med. 1999, 341, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Trueb, R.M. Molecular mechanisms of androgenetic alopecia. Exp. Gerontol. 2002, 37, 981–990. [Google Scholar] [CrossRef]
- Price, V.H.; Roberts, J.L.; Hordinsky, M.; Olsen, E.A.; Savin, R.; Bergfeld, W.; Fiedler, V.; Lucky, A.; Whiting, D.A.; Pappas, F.; et al. Lack of efficacy of finasteride in postmenopausal women with androgenetic alopecia. J. Am. Acad. Dermatol. 2000, 43, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Geyfman, M.; Plikus, M.V.; Treffeisen, E.; Andersen, B.; Paus, R. Resting no more: Re-defining telogen, the maintenance stage of the hair growth cycle. Biol. Rev. Camb. Philos. Soc. 2015, 90, 1179–1196. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.C.; Pasolli, H.A.; Fuchs, E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell 2011, 144, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Kwack, M.H.; Kang, B.M.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Minoxidil activates β-catenin pathway in human dermal papilla cells: A possible explanation for its anagen prolongation effect. J. Dermatol. Sci. 2011, 62, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, E. Epithelial skin biology: Three decades of developmental biology, a hundred questions answered and a thousand new ones to address. Curr. Top. Dev. Biol. 2016, 116, 357–374. [Google Scholar] [PubMed]
- Huelsken, J.; Vogel, R.; Erdmann, B.; Cotsarelis, G.; Birchmeier, W. β-catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 2001, 105, 533–545. [Google Scholar] [CrossRef]
- Whittaker, S.R.; Mallinger, A.; Workman, P.; Clarke, P.A. Inhibitors of cyclin-dependent kinases as cancer therapeutics. Pharmacol. Ther. 2017, 173, 83–105. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Kwon, O.S.; Chung, J.H.; Cho, K.H.; Eun, H.C.; Kim, K.H. Effect of minoxidil on proliferation and apoptosis in dermal papilla cells of human hair follicle. J. Dermatol. Sci. 2004, 34, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.I.; Kim, M.K.; Lee, J.H.; Jeon, Y.J.; Hwang, E.K.; Koh, Y.S.; Hyun, J.W.; Kwon, S.Y.; Yoo, E.S.; Kang, H.K. Undariopsis peterseniana promotes hair growth by the activation of Wnt/β-catenin and ERK pathways. Mar. Drugs 2017, 15, 130. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.A.; Hwang, Y.L.; Lee, M.H.; Kim, N.R.; Roh, S.S.; Lee, Y.; Kim, C.D.; Lee, J.H.; Choi, K.C. Adenosine stimulates growth of dermal papilla and lengthens the anagen phase by increasing the cysteine level via fibroblast growth factors 2 and 7 in an organ culture of mouse vibrissae hair follicles. Int. J. Mol. Med. 2012, 29, 195–201. [Google Scholar] [PubMed]
- Li, W.; Man, X.Y.; Li, C.M.; Chen, J.Q.; Zhou, J.; Cai, S.Q.; Lu, Z.F.; Zheng, M. VEGF induces proliferation of human hair follicle dermal papilla cells through VEGFR-2-mediated activation of ERK. Exp. Cell Res. 2012, 318, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Recio, J.A.; Merlino, G. Hepatocyte growth factor/scatter factor activates proliferation in melanoma cells through p38 MAPK, ATF-2 and cyclin D1. Oncogene 2002, 21, 1000–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, S.; Hozumi, Y.; Kondo, S. Influence of prostaglandin F2α and its analogues on hair regrowth and follicular melanogenesis in a murine model. Exp. Dermatol. 2005, 14, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Khidhir, K.G.; Woodward, D.F.; Farjo, N.P.; Farjo, B.K.; Tang, E.S.; Wang, J.W.; Picksley, S.M.; Randall, V.A. The prostamide-related glaucoma therapy, bimatoprost, offers a novel approach for treating scalp alopecias. FASEB J. 2013, 27, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Isobe, N.; Suzuki, M.; Oda, M.; Tanabe, S. Enzyme-modified cheese exerts inhibitory effects on allergen permeation in rats suffering from indomethacin-induced intestinal inflammation. Biosci. Biotechnol. Biochem. 2008, 72, 1740–1745. [Google Scholar] [CrossRef] [PubMed]
- Uchida, M.; Shimizu, K.; Kurakazu, K. Yogurt containing Lactobacillus gasseri OLL 2716 (LG21 yogurt) accelerated the healing of acetic acid-induced gastric ulcer in rats. Biosci. Biotechnol. Biochem. 2010, 74, 1891–1894. [Google Scholar] [CrossRef] [PubMed]
- Han, S.C.; Kang, G.J.; Ko, Y.J.; Kang, H.K.; Moon, S.W.; Ann, Y.S.; Yoo, E.S. Fermented fish oil suppresses t helper 1/2 cell response in a mouse model of atopic dermatitis via generation of CD4+CD25+Foxp3+ T cells. BMC Immunol. 2012, 13, 44. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Hyun, Y.J.; Piao, M.J.; Kang, K.A.; Ryu, Y.S.; Shilnikova, K.; Zhen, A.X.; Ahn, M.J.; Ahn, Y.S.; Koh, Y.S.; et al. Mackerel-derived fermented fish oil protects skin against UVB-induced cellular damage by inhibiting oxidative stress. J. Funct. Foods 2018, 46, 147–158. [Google Scholar] [CrossRef]
- Han, S.C.; Koo, D.H.; Kang, N.J.; Yoon, W.J.; Kang, G.J.; Kang, H.K.; Yoo, E.S. Docosahexaenoic acid alleviates atopic dermatitis by generating Tregs and IL-10/TGF-β-modified macrophages via a TGF-β-dependent mechanism. J. Investig. Dermatol. 2015, 135, 1556–1564. [Google Scholar] [CrossRef] [PubMed]
- Yassine, H.N.; Braskie, M.N.; Mack, W.J.; Castor, K.J.; Fonteh, A.N.; Schneider, L.S.; Harrington, M.G.; Chui, H.C. Association of docosahexaenoic acid supplementation with Alzheimer disease stage in apolipoprotein E ε4 carriers: A review. JAMA Neurol. 2017, 74, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Philpott, M.P.; Kealey, T. Cyclical changes in rat vibrissa follicles maintained in vitro. J. Investig. Dermatol. 2000, 115, 1152–1155. [Google Scholar] [CrossRef] [PubMed]
- Everts, H.B.; Silva, K.A.; Montgomery, S.; Suo, L.; Menser, M.; Valet, A.S.; King, L.E.; Ong, D.E.; Sundberg, J.P. Retinoid metabolism is altered in human and mouse cicatricial alopecia. J. Investig. Dermatol. 2013, 133, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.; Wu, X.J.; Li, Y.L.; Cai, S.Q.; Zheng, M.; Lu, Z.F. Expression of decorin throughout the murine hair follicle cycle: Hair cycle dependence and anagen phase prolongation. Exp. Dermatol. 2014, 23, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Muller-Rover, S.; Handjiski, B.; van der Veen, C.; Eichmuller, S.; Foitzik, K.; McKay, I.A.; Stenn, K.S.; Paus, R. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J. Investig. Dermatol. 2001, 117, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Sherr, C.J. Cancer cell cycles. Science 1996, 274, 1672–1677. [Google Scholar] [CrossRef] [PubMed]
- Langemann, D.; Trochimiuk, M.; Appl, B.; Hundsdoerfer, P.; Reinshagen, K.; Eschenburg, G. Sensitization of neuroblastoma for vincristine-induced apoptosis by smac mimetic LCL161 is attended by G2 cell cycle arrest but is independent of NFκB, RIP1 and TNF-α. Oncotarget 2017, 8, 87763–87772. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tang, N.; Hadden, T.J.; Rishi, A.K. Akt, Foxo and regulation of apoptosis. Biochim. Biophys. Acta 2011, 1813, 1978–1986. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, M.; Yang, Z.; Andl, T.; Cui, C.; Kim, N.; Millar, S.E.; Cotsarelis, G. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature 2007, 447, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, K.; Kurosaka, A. Inhibition of glycogen synthase kinase-3 enhances the expression of alkaline phosphatase and insulin-like growth factor-1 in human primary dermal papilla cell culture and maintains mouse hair bulbs in organ culture. Arch. Dermatol. Res. 2009, 301, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Paus, R. Exploring the “brain-skin connection”: Leads and lessons from the hair follicle. Curr. Res. Transl. Med. 2016, 64, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Purba, T.S.; Peake, M.; Farjo, B.; Farjo, N.; Bhogal, R.K.; Jenkins, G.; Paus, R. Divergent proliferation patterns of distinct human hair follicle epithelial progenitor niches in situ and their differential responsiveness to prostaglandin D2. Sci. Rep. 2017, 7, 15197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, J.I.; Yoo, E.S.; Hyun, J.W.; Koh, Y.S.; Lee, N.H.; Ko, M.H.; Ko, C.S.; Kang, H.K. Promotion effect of apo-9′-fucoxanthinone from sargassum muticum on hair growth via the activation of Wnt/β-catenin and VEGF-R2. Biol. Pharm. Bull. 2016, 39, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Greco, V.; Chen, T.; Rendl, M.; Schober, M.; Pasolli, H.A.; Stokes, N.; Dela Cruz-Racelis, J.; Fuchs, E. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell 2009, 4, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, E.; Tsuchiya, A.; Imoto, M. Functions of cyclin D1 as an oncogene and regulation of cyclin D1 expression. Cancer Sci. 2007, 98, 629–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Datta, S.R.; Dudek, H.; Tao, X.; Masters, S.; Fu, H.; Gotoh, Y.; Greenberg, M.E. Akt phosphorylation of bad couples survival signals to the cell-intrinsic death machinery. Cell 1997, 91, 231–241. [Google Scholar] [CrossRef]
- Alao, J.P. The regulation of cyclin D1 degradation: Roles in cancer development and the potential for therapeutic invention. Mol. Cancer 2007, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Endo, H.; Nito, C.; Kamada, H.; Nishi, T.; Chan, P.H. Activation of the Akt/GSK3β signaling pathway mediates survival of vulnerable hippocampal neurons after transient global cerebral ischemia in rats. J. Cereb. Blood Flow Metab. 2006, 26, 1479–1489. [Google Scholar] [CrossRef] [PubMed]
- Ouji, Y.; Yoshikawa, M.; Moriya, K.; Nishiofuku, M.; Matsuda, R.; Ishizaka, S. Wnt-10b, uniquely among wnts, promotes epithelial differentiation and shaft growth. Biochem. Biophys. Res. Commun. 2008, 367, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Hamazaki, T.S.; Ohnuma, K.; Tamaki, K.; Asashima, M.; Okochi, H. Isolation of murine hair-inducing cells using the cell surface marker prominin-1/CD133. J. Investig. Dermatol. 2007, 127, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xu, M.; Yang, Y.; Yang, K.; Wickett, R.R.; Andl, T.; Millar, S.E.; Zhang, Y. Activation of β-catenin signaling in CD133-positive dermal papilla cells drives postnatal hair growth. PLoS ONE 2016, 11, e0160425. [Google Scholar] [CrossRef] [PubMed]
- Ohnemus, U.; Uenalan, M.; Conrad, F.; Handjiski, B.; Mecklenburg, L.; Nakamura, M.; Inzunza, J.; Gustafsson, J.A.; Paus, R. Hair cycle control by estrogens: Catagen induction via estrogen receptor (ER)-α is checked by ERβ signaling. Endocrinology 2005, 146, 1214–1225. [Google Scholar] [CrossRef] [PubMed]






| Antibodies | Supplier | Species | dilution |
|---|---|---|---|
| phospho(Ser473)-Akt | Cell Signaling | Rabbit | 1:1000 |
| Akt | Cell Signaling | Rabbit | 1:1000 |
| phosho(Thr202/Tyr204)-ERK1/2 | Cell Signaling | Rabbit | 1:1000 |
| ERK1/2 | Cell Signaling | Rabbit | 1:1000 |
| phospho(Thr183/Tyr185)-JNK | Cell Signaling | Mouse | 1:1000 |
| JNK | Cell Signaling | Rabbit | 1:1000 |
| phospho(Thr180/Tyr182)-p38 | Cell Signaling | Rabbit | 1:1000 |
| p38 | Cell Signaling | Rabbit | 1:1000 |
| β-catenin | Santa Cruz | Rabbit | 1:1000 |
| phospho(Ser9)-GSK3β | Cell Signaling | Rabbit | 1:1000 |
| GSK3β | Cell Signaling | Rabbit | 1:1000 |
| Lamin B1 | Abcam | Rabbit | 1:2000 |
| α-Tubulin | Santa Cruz | Mouse | 1:250 |
| Cyclin D1 | BD Biosciences | Mouse | 1:1000 |
| Cdc2 p34 | Santa Cruz | Mouse | 1:1000 |
| Cyclin A | Santa Cruz | Rabbit | 1:1000 |
| β-actin | Sigma-Aldrich | Mouse | 1:5000 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, J.-I.; Yoon, H.-S.; Kim, S.M.; Park, J.E.; Hyun, Y.J.; Ko, A.; Ahn, Y.-S.; Koh, Y.S.; Hyun, J.W.; Yoo, E.-S.; et al. Mackerel-Derived Fermented Fish Oil Promotes Hair Growth by Anagen-Stimulating Pathways. Int. J. Mol. Sci. 2018, 19, 2770. https://doi.org/10.3390/ijms19092770
Kang J-I, Yoon H-S, Kim SM, Park JE, Hyun YJ, Ko A, Ahn Y-S, Koh YS, Hyun JW, Yoo E-S, et al. Mackerel-Derived Fermented Fish Oil Promotes Hair Growth by Anagen-Stimulating Pathways. International Journal of Molecular Sciences. 2018; 19(9):2770. https://doi.org/10.3390/ijms19092770
Chicago/Turabian StyleKang, Jung-Il, Hoon-Seok Yoon, Sung Min Kim, Jeong Eon Park, Yu Jae Hyun, Ara Ko, Yong-Seok Ahn, Young Sang Koh, Jin Won Hyun, Eun-Sook Yoo, and et al. 2018. "Mackerel-Derived Fermented Fish Oil Promotes Hair Growth by Anagen-Stimulating Pathways" International Journal of Molecular Sciences 19, no. 9: 2770. https://doi.org/10.3390/ijms19092770
APA StyleKang, J. -I., Yoon, H. -S., Kim, S. M., Park, J. E., Hyun, Y. J., Ko, A., Ahn, Y. -S., Koh, Y. S., Hyun, J. W., Yoo, E. -S., & Kang, H. -K. (2018). Mackerel-Derived Fermented Fish Oil Promotes Hair Growth by Anagen-Stimulating Pathways. International Journal of Molecular Sciences, 19(9), 2770. https://doi.org/10.3390/ijms19092770