Beeswax by-Products Efficiently Counteract the Oxidative Damage Induced by an Oxidant Agent in Human Dermal Fibroblasts
Abstract
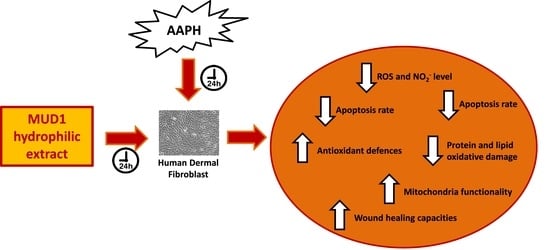
:1. Introduction
2. Results and Discussion
2.1. MUD1 Treatments Reduced AAPH-Mediated ROS Production and NO2− Accumulation
2.2. Regulation of Apoptosis Level by MUD1
2.3. MUD1 Treatment Reduced Protein and Lipid Biomarkers of Oxidative Stress
2.4. MUD1 Treatment Improved the Endogenous Antioxidant Defence System
2.5. Effect of MUD1 Treatment on OCR and ECAR
2.6. MUD1 Promotes Tissue Repair by Fibroblast Migration and Wound Closure
3. Materials and Methods
3.1. Sample Collection and Preparation
3.2. Cell Culture and Treatments
3.3. TALI® ROS Concentration Assay
3.4. Determination of Nitrite Production
3.5. Apoptosis Detection
3.6. Measurements of the Protein and Lipid Oxidative Damage
3.7. Antioxidant Enzyme Activities
3.8. Determination of Mitochondrial Respiration and Extracellular Acidification Rate in Cells
3.9. In Vitro Skin Fibroblast Migration and Proliferation Assay
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| 2,4 DNP | 2,4-Dinitrophenol |
| 2-DG | 2-deoxyglucose |
| AAPH | 2,2′-azobis(2-amidinopropane) dihydrochloride |
| ABB | annexin binding buffer |
| DMEM | Dulbecco’s Modified Eagle Medium |
| ECAR | extracellular acidification rate |
| HDF | human dermal fibroblast |
| HepG2 | human hepatocellular carcinoma |
| GPx | glutathione peroxidase |
| GR | glutathione reductase |
| GSH | glutathione |
| GST | glutathione trasferase |
| NO | nitric oxide |
| NO2− | nitrite |
| OCR | oxygen consumption rate |
| PBS | phosphate-buffered saline solution |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
| TBARS | thiobarbituric acid-reactive substances |
References
- Baiano, A. Recovery of biomolecules from food wastes—A review. Molecules 2014, 19, 14821–14842. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, F.; Quiles, J.L.; Orantes-Bermejo, F.J.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Sánchez-González, C.; Llopis, J.; Rivas-García, L.; Afrin, S.; Varela-López, A.; et al. Are by-products from beeswax recycling process a new promising source of bioactive compounds with biomedical properties? Food Chem. Toxicol. 2018, 112, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Crowder, L. Top-Bar Beekeeping: Organic Practices for Honeybee Health; Chelsea Green Publishing: White River Junction, VT, USA, 2012; ISBN 1603584617. [Google Scholar]
- Fratini, F.; Cili, G.; Turchi, B.; Felicioli, A. Beeswax: A minireview of its antimicrobial activity and its application in medicine. Asian Pac. J. Trop. Med. 2016, 9, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Scientific Opinion of the Panel on Food additives, Flavourings, Processing aids and Materials in Contact with Food (AFC) on a request from the Commission on the safety in use of beeswax. EFSA J. 2007, 615, 1–28.
- Mayne, S.T. Antioxidant nutrients and chronic disease: Use of biomarkers of exposure and oxidative stress status in epidemiologic research. J. Nutr. 2003, 133, S933–S940. [Google Scholar] [CrossRef] [PubMed]
- Gasparrini, M.; Forbes-Hernandez, T.Y.; Giampieri, F.; Afrin, S.; Mezzetti, B.; Quiles, J.L.; Bompadre, S.; Battino, M. Protective Effect of Strawberry Extract against Inflammatory Stress Induced in Human Dermal Fibroblasts. Molecules 2017, 22, 164. [Google Scholar] [CrossRef] [PubMed]
- Gasparrini, M.; Giampieri, F.; Forbes-Hernandez, T.Y.; Afrin, S.; Cianciosi, D.; Reboredo-Rodriguez, P.; Varela-Lopez, A.; Zhang, J.; Quiles, J.L.; Mezzetti, B.; et al. Strawberry extracts efficiently counteract inflammatory stress induced by the endotoxin lipopolysaccharide in Human Dermal Fibroblast. Food Chem. Toxicol. 2018, 114, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Pistollato, F.; Battino, M. Role of plant-based diets in the prevention and regression of metabolic syndrome and neurodegenerative diseases. Trends Food Sci. Technol. 2014, 40, 62–81. [Google Scholar] [CrossRef]
- Afrin, S.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Bompadre, S.; Quiles, J.L.; Sanna, G.; Spano, N.; Giampieri, F.; Battino, M. Strawberry-tree honey induces growth inhibition of human colon cancer cells and increases ROS generation: A comparison with Manuka honey. Int. J. Mol. Sci. 2017, 18, 613. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.M.; Giampieri, F.; Cordero, M.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Mazzoni, L.; Afrin, S.; Beltran-Ayala, P.; Gonzalez-Paramas, A.M.; Santos-Buelga, C.; et al. Activation of AMPK/Nrf2 signalling by Manuka honey protects human dermal fibroblasts against oxidative damage by improving antioxidant response and mitochondrial function promoting wound healing. J. Funct. Foods 2016, 25, 38–49. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Ghahary, A.; Shen, Y.J.; Scott, P.G.; Tredget, E.E. Human dermal fibroblasts produce nitric oxide and express both constitutive and inducible nitric oxide synthase isoforms. J. Investig. Dermatol. 1996, 106, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Park, C.M.; Park, J.Y.; Noh, K.H.; Shin, J.H.; Song, Y.S. Taraxacum officinale Weber extracts inhibit LPS-induced oxidative stress and nitric oxide production via the NF-kB modulation in RAW264.7 cells. J. Ethnopharmacol. 2011, 133, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, F.; Alvarez-Suarez, J.M.; Mazzoni, L.; Forbes-Hernandez, T.Y.; Gasparrini, M.; González-Paramás, A.M.; Santos-Buelga, C.; Quiles, J.L.; Bompadre, S.; Mezzetti, B.; et al. Polyphenol-Rich Strawberry Extract Protects Human Dermal Fibroblasts against Hydrogen Peroxide Oxidative Damage and Improves Mitochondrial Functionality. Molecules 2014, 19, 7798–7816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasparrini, M.; Forbes-Hernandez, T.Y.; Afrin, S.; Reboredo-Rodríguez, P.; Cianciosi, D.; Mezzetti, B.; Quiles, J.L.; Bompadre, S.; Battino, M.; Giampieri, F. Strawberry-Based Cosmetic Formulations Protect Human Dermal Fibroblasts against UVA-Induced. Nutrients 2017, 9, 605. [Google Scholar] [CrossRef] [PubMed]
- Forbes-Hernández, T.Y.; Giampieri, F.; Gasparrini, M.; Mazzoni, L.; Quiles, J.L.; Alvarez-Suarez, J.M.; Battino, M. The effects of bioactive compounds from plant foodson mitochondrial function: A focus on apoptotic mechanisms. Food Chem. Toxicol. 2014, 68, 154–182. [Google Scholar] [CrossRef] [PubMed]
- Babujanarthanam, R.; Kavitha, P.; Mahadeva Rao, U.S.; Pandian, M.R. Quercitrin a bioflavonoid improves the antioxidant status in streptozotocin: Induced diabetic rat tissues. Mol. Cell. Biochem. 2011, 358, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Dalle-Donne, I.; Rossi, R.; Giustarini, D.; Milzani, A.; Colombo, R. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta 2003, 329, 23–38. [Google Scholar] [CrossRef]
- DeLeve, L.D.; Kaplowitz, N. Importance and regulation of hepatic glutathione. Semin. Liver Dis. 1990, 10, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, F.; Alvarez-Suarez, J.M.; Mazzoni, L.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Gonzàlez-Paramàs, A.M.; Santos-Buelga, C.; Quiles, J.L.; Bompadre, S.; Mezzetti, B.; et al. An anthocyanin-rich strawberry extract protects against oxidative stress damage and improves mitochondrial functionality in human dermal fibroblasts exposed to an oxidizing agent. Food Funct. 2014, 5, 1939–1948. [Google Scholar] [CrossRef] [PubMed]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Poljsak, B.; Šuput, D.; Milisav, I. Achieving the balance between ROS and antioxidants: When to use the synthetic antioxidants. Oxid. Med. Cell Longev. 2013, 2013, 956792. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Giampieri, F.; Janjusevic, M.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Mazzoni, L.; Greco, S.; Giannubilo, S.R.; Ciavattini, A.; Mezzetti, B.; et al. An anthocyanin rich strawberry extract induces apoptosis and ROS while decreases glycolysis and fibrosis in human uterine leiomyoma cells. Oncotarget 2017, 8, 23575–23587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasparrini, M.; Forbes-Hernandez, T.Y.; Giampieri, F.; Afrin, S.; Alvarez-Suarez, J.M.; Mazzoni, L.; Mezzetti, B.; Quiles, J.L.; Battino, M. Anti-inflammatory effect of strawberry extract against LPS-induced stress in RAW 264.7 macrophages. Food Chem. Toxicol. 2017, 102, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, F.; Alvarez-Suarez, J.M.; Cordero, M.D.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Afrin, S.; Santos-Buelga, C.; González-Paramás, A.M.; Astolfi, P.; Rubini, C.; et al. Strawberry consumption improves aging-associated impairments, mitochondrial biogenesis and functionality through the AMP-activated protein kinase signaling cascade. Food Chem. 2017, 234, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Afrin, S.; Giampieri, F.; Gasparrini, M.; Forbes-Hernández, T.Y.; Cianciosi, D.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Quiles, J.L.; Battino, M. The inhibitory effect of Manuka honey on human colon cancer HCT-116 and LoVo cell growth. Part 2: Induction of oxidative stress, alteration of mitochondrial respiration and glycolysis, and suppression of metastatic ability. Food Funct. 2018, 9, 2158–2170. [Google Scholar] [CrossRef] [PubMed]
- Afrin, S.; Gasparrini, M.; Forbes-Hernández, T.Y.; Cianciosi, D.; Reboredo-Rodriguez, P.; Manna, P.P.; Battino, M.; Giampieri, F. Protective effects of Manuka honey on LPS-treated RAW 264.7 macrophages. Part 1: Enhancement of cellular viability, regulation of cellular apoptosis and improvement of mitochondrial functionality. Food Chem. Toxicol. 2018, 121, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Bischofberger, A.S.; Tsang, A.S.; Horadagoda, N.; Dart, C.M.; Perkins, N.R.; Jeffcott, L.B.; Jackson, C.J.; Dart, A.J. Effect of activated protein C in second intention healing of equine distal limb wounds: A preliminary study. Aust. Vet. J. 2015, 93, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Bischofberger, A.S.; Dart, C.M.; Horadagoda, N.; Perkins, N.R.; Jeffcott, L.B.; Little, C.B.; Dart, A.J. Effect of Manuka honey gel on the transforming growth factor β1 and β3 concentrations, bacterial counts and histomorphology of contaminated full-thickness skin wounds in equine distal limbs. Aust. Vet. J. 2016, 94, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Sell, S.A.; Wolfe, P.S.; Spence, A.J.; Rodriguez, I.A.; McCool, J.M.; Petrella, R.L.; Garg, K.; Ericksen, J.J.; Bowlin, G.L. A preliminary study on the potential of manuka honey and platelet-rich plasma in wound healing. Int. J. Biomater. 2012, 2012, 313781. [Google Scholar] [CrossRef] [PubMed]
- Molan, P.C. The evidence and the rationale for the use of honey as a wound dressing. Wound Pract. Res. 2011, 19, 204–220. [Google Scholar]
- Molan, P.; Rhodes, T. Honey: A biologic wound dressing. Wounds 2015, 27, 141–151. [Google Scholar] [PubMed]
- Alvarez-Suarez, J.M.; Tulipani, S.; Díaz, D.; Estevez, Y.; Romandini, S.; Giampieri, F.; Damiani, E.; Astolfi, P.; Bompadre, S.; Battino, M. Antioxidant and antimicrobial capacity of several monofloral Cuban honeys and their correlation with color, polyphenol content and other chemical compounds. Food Chem. Toxicol. 2010, 48, 2490–2499. [Google Scholar] [CrossRef] [PubMed]
- Pekarova, M.; Kralova, J.; Kubala, L.; Ciz, M.; Papezikova, I.; Macickova, T.; Pecivova, J.; Nosal, R.; Lojek, A. Carvedilol and adrenergic agonists suppress the lipopolysaccharide-induced NO production in RAW 264.7 macrophages via the adrenergic receptors. J. Physiol. Pharmacol. 2009, 60, 143–150. [Google Scholar] [PubMed]
- Doktorovová, S.; Santos, D.L.; Costa, I.; Andreani, T.; Souto, E.B.; Silva, A.M. Cationic solid lipid nanoparticles interfere with the activity of antioxidant enzymes in hepatocellular carcinoma cells. Int. J. Pharm. 2014, 471, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Giampieri, F.; Alvarez-Suarez, J.M.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Afrin, S.; Bompadre, S.; Rubini, C.; Zizzi, A.; Astolfi, P.; Santos-Buelga, C.; et al. Strawberry consumption alleviates doxorubicin-induced toxicity by suppressing oxidative stress. Food Chem. Toxicol. 2016, 94, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Esposito, D.; Munafo, J.P., Jr.; Lucibello, T.; Baldeon, M.; Komarnytsky, S.; Gianfagna, T.J. Steroidal glycosides from the bulbs of Easter lily (Lilium longiflorum Thunb.) promote dermal fibroblast migration in vitro. J. Ethnopharmacol. 2013, 148, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.N.; Kwon, Y.; Jang, H.D. Protective mechanism of quercetin and rutin on 2,2’-azobis(2-amidinopropane)dihydrochloride or Cu2+-induced oxidative stress in HepG2 cells. Toxicol. In Vitro 2011, 25, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.N.; Jang, H.D. Flavonol content in the water extract of the mulberry (Morus alba L.) leaf and their antioxidant capacities. J. Food Sci. 2011, 76, C869–C873. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Tai, B.H.; Cuong, N.M.; Kim, Y.-K.; Jang, H.D. Antioxidative and anti-inflammatory effect of quercetin and its glycosides isolated from mampat (Cratoxylum formosum). Food Sci. Biotechnol. 2012, 21, 587. [Google Scholar] [CrossRef]
- Gasparrini, M.; Afrin, S.; Forbes-Hernández, T.Y.; Cianciosi, D.; Reboredo-Rodriguez, P.; Amici, A.; Battino, M.; Giampieri, F. Protective effects of Manuka honey on LPS-treated RAW 264.7 macrophages. Part 2: Control of oxidative stress induced damage, increase of antioxidant enzyme activities and attenuation of inflammation. Food Chem. Toxicol. 2018, 120, 578–587. [Google Scholar] [CrossRef] [PubMed]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giampieri, F.; Gasparrini, M.; Forbes-Hernández, T.Y.; Manna, P.P.; Zhang, J.; Reboredo-Rodríguez, P.; Cianciosi, D.; Quiles, J.L.; Torres Fernández-Piñar, C.; Orantes-Bermejo, F.J.; et al. Beeswax by-Products Efficiently Counteract the Oxidative Damage Induced by an Oxidant Agent in Human Dermal Fibroblasts. Int. J. Mol. Sci. 2018, 19, 2842. https://doi.org/10.3390/ijms19092842
Giampieri F, Gasparrini M, Forbes-Hernández TY, Manna PP, Zhang J, Reboredo-Rodríguez P, Cianciosi D, Quiles JL, Torres Fernández-Piñar C, Orantes-Bermejo FJ, et al. Beeswax by-Products Efficiently Counteract the Oxidative Damage Induced by an Oxidant Agent in Human Dermal Fibroblasts. International Journal of Molecular Sciences. 2018; 19(9):2842. https://doi.org/10.3390/ijms19092842
Chicago/Turabian StyleGiampieri, Francesca, Massimiliano Gasparrini, Tamara Y. Forbes-Hernández, Piera Pia Manna, Jiaojiao Zhang, Patricia Reboredo-Rodríguez, Danila Cianciosi, Jose L. Quiles, Cristina Torres Fernández-Piñar, Francisco Josè Orantes-Bermejo, and et al. 2018. "Beeswax by-Products Efficiently Counteract the Oxidative Damage Induced by an Oxidant Agent in Human Dermal Fibroblasts" International Journal of Molecular Sciences 19, no. 9: 2842. https://doi.org/10.3390/ijms19092842
APA StyleGiampieri, F., Gasparrini, M., Forbes-Hernández, T. Y., Manna, P. P., Zhang, J., Reboredo-Rodríguez, P., Cianciosi, D., Quiles, J. L., Torres Fernández-Piñar, C., Orantes-Bermejo, F. J., Bompadre, S., Afrin, S., & Battino, M. (2018). Beeswax by-Products Efficiently Counteract the Oxidative Damage Induced by an Oxidant Agent in Human Dermal Fibroblasts. International Journal of Molecular Sciences, 19(9), 2842. https://doi.org/10.3390/ijms19092842