Aloe Genus Plants: From Farm to Food Applications and Phytopharmacotherapy
Abstract
:1. Introduction
2. Habitat and Cultivation of Aloe Plants
3. Phytochemical Composition of Aloe Plants
3.1. General Reports on Aloe Species Phytochemicals
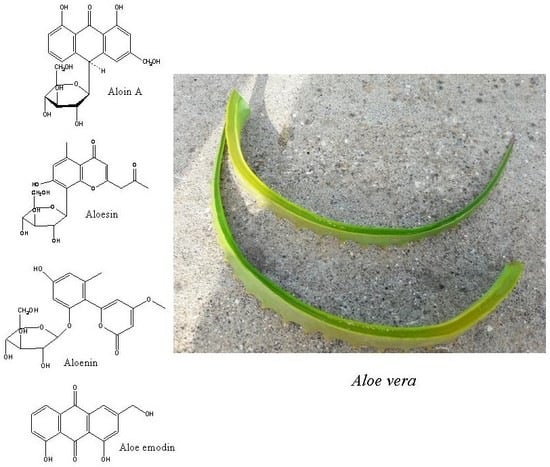
3.1.1. Anthraquinones of Aloe Species
3.1.2. Anthrones of Aloe Species
3.1.3. Chromones of Aloe Species
3.1.4. Coumarins, Pyrans, and Pyrones of Aloe Species
3.1.5. Alkaloids of Aloe Species
3.1.6. Benzene, Naphthalene, and Furan Derivatives of Aloe Species
3.1.7. Flavonoids of Aloe Species
3.1.8. Sterols of Aloe Species
3.1.9. Other Phenolic Constituent of Aloe Species
3.1.10. Non-Phenolic Components of Aloe Species
3.1.11. Vitamins of Aloe Species
3.1.12. Mineral Nutrients in Aloe species
3.2. Specific Reports on Aloe Phytoconstituents
Phytochemical Studies on Aloe Species
4. Traditional Medicine Use of Aloe Plants
5. Food Preservative Applications of Aloe Plants
6. Antimicrobial Activity
6.1. Antibacterial Activity
6.2. Antifungal Activity
7. In Vitro and In Vivo Biological Activities of Aloe Plants
7.1. Wound Healing and Cell Proliferation
7.2. Intestinal Absorption and Purgative Action
7.3. Anti-Inflammatory and Immunomodulatory Effects
7.4. Hepatoprotective Activity
7.5. Antioxidant Effect
7.6. Antibacterial, Antifungal, and Antiviral Activities
7.7. Antiplasmodial/Antimalarial Activity
7.8. Anthelmintic Activity
7.9. Anticancer Activity
7.10. Antidiabetic Activity
7.11. Antihyperlipidemic Activity
7.12. Effect on Estrogen Status
7.13. Antiulcer Activity
7.14. Treatment of Cardiovascular Disorders
7.15. Skin Use
7.16. Anti-Aging Effect
7.17. Antiallergic Activity
7.18. Effect on Central and Peripheral Nervous Systems
8. Clinical Efficacy of Aloe Plants in Humans
8.1. Wound Healing and Cell Proliferation
8.2. Anti-Inflammatory and Immunomodulatory Effects
8.3. Antidiabetic Activity
8.4. Antihyperlipidemic Activity
8.5. Treatment of Acquired Immune Deficiency Syndrome (AIDS)
8.6. Effect on Dental and Oral Diseases
9. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Klopper, R.R.; Smith, G.F. The genus ALOE (Asphodelaceae: Alooideae) in namaqualand, South Africa. Haseltonia 2007, 13, 38–51. [Google Scholar] [CrossRef]
- Surjushe, A.; Vasani, R.; Saple, D.G. Aloe vera: A short review. Indian J. Dermatol. 2008, 53, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, M.D.; Beland, F.A. An evaluation of the biological and toxicological properties of Aloe Barbadensis (miller), Aloe vera. J. Environ. Sci. Health C 2006, 24, 103–154. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.H.; Aldebasi, Y.H.; Srikar, S.; Khan, A.A.; Aly, S.M. Aloe vera: Potential candidate in health management via modulation of biological activities. Pharmacogn. Rev. 2015, 9, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Radha, M.H.; Laxmipriya, N.P. Evaluation of biological properties and clinical effectiveness of Aloe vera: A systematic review. J. Tradit. Complement. Med. 2015, 5, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Nejatzadeh-Barandozi, F. Antibacterial activities and antioxidant capacity of Aloe vera. Org. Med. Chem. Lett. 2013, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Rathod, N.; Nagi, R.; Sur, J.; Laheji, A.; Gupta, N.; Agrawal, P.; Prasad, S. Antibacterial effect of Aloe vera gel against oral pathogens: An in-vitro study. J. Clin. Diagn. Res. 2016, 10, ZC41–ZC44. [Google Scholar] [CrossRef] [PubMed]
- Athiban, P.P.; Borthakur, B.J.; Ganesan, S.; Swathika, B. Evaluation of antimicrobial efficacy of Aloe vera and its effectiveness in decontaminating gutta percha cones. J. Conservat. Dent. 2012, 15, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, M.D.; Mellick, P.W.; Olson, G.R.; Felton, R.P.; Thorn, B.T.; Beland, F.A. Clear evidence of carcinogenic activity by a whole-leaf extract of aloe barbadensis miller (Aloe vera) in F344/n rats. Toxicol. Sci. 2013, 131, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Grundmann, O. Aloe vera gel research review: An overview of its clinical uses and proposed mechanisms of action. Nat. Med. J. 2012, 4, 9. [Google Scholar]
- Thamlikitkul, V.; Bunyapraphatsara, N.; Riewpaiboon, W.; Theerapong, S.; Chantrakul, C.; Thanaveerasuwan, T.; Nimitnon, S.; Wongkonkatape, S.; Riewpaiboon, A.; Tenambergen, E.D. Clinical trial of Aloe vera linn. For treatment of minor burns. Siriraj Med. J. 2017, 43, 4. [Google Scholar]
- Hazrati, S.; Tahmasebi-Sarvestani, Z.; Mokhtassi-Bidgoli, A.; Modarres-Sanavy, S.A.M.; Mohammadi, H.; Nicola, S. Effects of zeolite and water stress on growth, yield and chemical compositions of Aloe vera L. Agric. Water Manag. 2017, 181, 66–72. [Google Scholar] [CrossRef]
- Datta, A.; Mandal, A.; Teixeira da Silva, J.; Saha, A.; Paul, R.; Sengupta, S.; Kumari Dubey, P.; Halder, S. An updated overview on Aloe vera (L.) Burm. F. Med. Aromat. Plant Sci. Biotechnol. 2012, 6, 1–11. [Google Scholar]
- Serrano, M.; Valverde, J.M.; Guillén, F.; Castillo, S.; Martínez-Romero, D.; Valero, D. Use of Aloe vera gel coating preserves the functional properties of table grapes. J. Agric. Food Chem. 2006, 54, 3882–3886. [Google Scholar] [CrossRef] [PubMed]
- Ahlawat, K.S.; Khatkar, B.S. Processing, food applications and safety of Aloe vera products: A review. J. Food Sci. Technol. 2011, 48, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Ernst van Jaarsveld, N. The genus aloe in south africa with special reference to Aloe hereroensis. Veld Flora 1989, 75, 73–76. [Google Scholar]
- Sachedina, H.; Bodeker, G. Wild aloe harvesting in South Africa. J. Altern. Complement. Med. 1998, 5, 121–123. [Google Scholar] [CrossRef]
- Jordan, J. The ecology of the aloes of zimbabwe. Excelsa 1996, 17, 101–110. [Google Scholar]
- Giddy, C. Aloes from seed. Veld Flora 1973, 59, 41. [Google Scholar]
- Cloete, E.; Plumstead, E. Toxic soils and Aloe colours. Veld Flora 2000, 86, 29. [Google Scholar]
- Lloyd, J.U. Aloe succotrina; The Western Druggist: Chicago, IL, USA, 1898. [Google Scholar]
- Grace, O.M.; Simmonds, M.S.; Smith, G.F.; van Wyk, A.E. Documented utility and biocultural value of Aloe L. (Asphodelaceae): A review. Econ. Bot. 2009, 63, 167–178. [Google Scholar] [CrossRef]
- King, E.G.; Stanton, M.L. Facilitative effects of aloe shrubs on grass establishment, growth, and reproduction in degraded kenyan rangelands: Implications for restoration. Restor. Ecol. 2008, 16, 464–474. [Google Scholar] [CrossRef]
- Smith, G.F.; de S Correia, R.I. Establishment of Aloe greatheadii var. Davyana from seed for use in reclamation trials. Landsc. Urban Plan. 1992, 23, 47–54. [Google Scholar] [CrossRef]
- Palermo, F.A.; Cocci, P.; Angeletti, M.; Felici, A.; Polzonetti-Magni, A.M.; Mosconi, G. Dietary Aloe vera components’ effects on cholesterol lowering and estrogenic responses in juvenile goldfish, Carassius auratus. Fish Physiol. Biochem. 2013, 39, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Cock, I. The genus aloe: Phytochemistry and therapeutic uses including treatments for gastrointestinal conditions and chronic inflammation. In Novel Natural Products: Therapeutic Effects in Pain, Arthritis and Gastro-Intestinal Diseases; Springer: Berlin, Germany, 2015; pp. 179–235. [Google Scholar]
- Reynolds, T. Aloes: The Genus Aloe; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Gutterman, Y.; Chauser-Volfson, E. The distribution of the phenolic metabolites barbaloin, aloeresin and aloenin as a peripheral defense strategy in the succulent leaf parts of aloe arborescens. Biochem. Syst. Ecol. 2000, 28, 825–838. [Google Scholar] [CrossRef]
- Dagne, E.; Bisrat, D.; Viljoen, A.; Van Wyk, B. Chemistry of aloe species. Curr. Org. Chem. 2000, 4, 1055–1078. [Google Scholar] [CrossRef]
- Hamman, J.H. Composition and applications of Aloe vera leaf gel. Molecules 2008, 13, 1599–1616. [Google Scholar] [CrossRef] [PubMed]
- Sharrif Moghaddasi, M.; Res, M. Aloe vera their chemicals composition and applications: A review. Int. J. Biol. Med. Res. 2011, 2, 466–471. [Google Scholar]
- Femenia, A.; Sánchez, E.S.; Simal, S.; Rosselló, C. Compositional features of polysaccharides from Aloe vera (Aloe barbadensis miller) plant tissues. Carbohydr. Polym. 1999, 39, 109–117. [Google Scholar] [CrossRef]
- Viljoen, A.M.; Van Wyk, B.-E.; Newton, L.E. The occurrence and taxonomic distribution of the anthrones aloin, aloinoside and microdontin in aloe. Biochem. Syst. Ecol. 2001, 29, 53–67. [Google Scholar] [CrossRef]
- Viljoen, A.M.; Van Wyk, B.-E. The chemotaxonomic significance of the phenyl pyrone aloenin in the genus aloe. Biochem. Syst. Ecol. 2000, 28, 1009–1017. [Google Scholar] [CrossRef]
- El Sayed, A.M.; Ezzat, S.M.; El Naggar, M.M.; El Hawary, S.S. In vivo diabetic wound healing effect and HPLC–DAD–ESI–MS/MS profiling of the methanol extracts of eight aloe species. Rev. Bras. Farmacogn. 2016, 26, 352–362. [Google Scholar] [CrossRef]
- Viljoen, A.M.; van Wyk, B.-E.; Newton, L.E. Plicataloside in aloe—A chemotaxonomic appraisal. Biochem. Syst. Ecol. 1999, 27, 507–517. [Google Scholar] [CrossRef]
- Lee, K.Y.; Weintraub, S.T.; Yu, B.P. Isolation and identification of a phenolic antioxidant from aloe barbadensis. Free Radic. Biol. Med. 2000, 28, 261–265. [Google Scholar] [CrossRef]
- Conner, J.M.; Gray, A.I.; Reynolds, T.; Waterman, P.G. Anthraquinone, anthrone and phenylpyrone components of Aloe nyeriensis var. Kedongensis leaf exudate. Phytochemistry 1987, 26, 2995–2997. [Google Scholar] [CrossRef]
- Van Heerden, F.R.; van Wyk, B.-E.; Viljoen, A.M. Aloeresins E and F, two chromone derivatives from Aloe peglerae. Phytochemistry 1996, 43, 867–869. [Google Scholar] [CrossRef]
- Rebecca, W.; Kayser, O.; Hagels, H.; Zessin, K.H.; Madundo, M.; Gamba, N. The phytochemical profile and identification of main phenolic compounds from the leaf exudate of aloe secundiflora by high-performance liquid chromatography-mass spectroscopy. Phytochem. Anal. Int. J. Plant Chem. Biochem. Techn. 2003, 14, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Ranghoo-Sanmukhiya, M.; Govinden-Soulange, J.; Lavergne, C.; Khoyratty, S.; Da Silva, D.; Frederich, M.; Kodja, H. Molecular biology, phytochemistry and bioactivity of three endemic Aloe species from Mauritius and Réunion islands. Phytochem. Anal. 2010, 21, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Dey, P.; Dutta, S.; Chowdhury, A.; Das, A.P.; Chaudhuri, T.K. Variation in phytochemical composition reveals distinct divergence of Aloe vera (L.) Burm. F. From other aloe species: Rationale behind selective preference of Aloe vera in nutritional and therapeutic use. J. Evid.-Based Complement. Altern. Med. 2017, 22, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Coopoosamy, R. Isolation of volatile compounds of Aloe excelsa (Berger). Afr. J. Biotechnol. 2010, 9, 7289–7294. [Google Scholar]
- Loots, D.T.; van der Westhuizen, F.H.; Botes, L. Aloe ferox leaf gel phytochemical content, antioxidant capacity, and possible health benefits. J. Agric. Food. Chem. 2007, 55, 6891–6896. [Google Scholar] [CrossRef] [PubMed]
- Bawankar, R.; Deepti, V.; Singh, P.; Subashkumar, R.; Vivekanandhan, G.; Babu, S. Evaluation of bioactive potential of an Aloe vera sterol extract. Phytother. Res. 2013, 27, 864–868. [Google Scholar] [CrossRef] [PubMed]
- Viljoen, A.; Van Wyk, B.-E.; Van Heerden, F. Distribution and chemotaxonomic significance of flavonoids inaloe (Asphodelaceae). Plant Syst. Evol. 1998, 211, 31–42. [Google Scholar] [CrossRef]
- Lobine, D.; Cummins, I.; Govinden-Soulange, J.; Ranghoo-Sanmukhiya, M.; Lindsey, K.; Chazot, P.; Ambler, C.; Grellscheid, S.; Sharples, G.; Lall, N. Medicinal mascarene aloes: An audit of their phytotherapeutic potential. Fitoterapia 2018, 124, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Chaithanya, K.K. Phytochemical screening and in vitro antioxidant activities of ethanolic gel extract of Aloe adigratana reynolds. J. Pharm. Res. 2018, 12, 13. [Google Scholar]
- Lucini, L.; Pellizzoni, M.; Pellegrino, R.; Molinari, G.P.; Colla, G. Phytochemical constituents and in vitro radical scavenging activity of different aloe species. Food Chem. 2015, 170, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Bisi-Johnson, M.A.; Obi, C.L.; Samuel, B.B.; Eloff, J.N.; Okoh, A.I. Antibacterial activity of crude extracts of some South African medicinal plants against multidrug resistant etiological agents of diarrhoea. BMC Complement. Altern. Med. 2017, 17, 321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Do, S.-G.; Kim, S.Y.; Kim, J.; Jin, Y.; Lee, C.H. Mass spectrometry-based metabolite profiling and antioxidant activity of Aloe vera (Aloe barbadensis miller) in different growth stages. J. Agric. Food. Chem. 2012, 60, 11222–11228. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Huang, Y.; Ding, W.; Wu, X.; Wan, J.; Luo, H. Chemical constituents of Aloe barbadensis miller and their inhibitory effects on phosphodiesterase-4D. Fitoterapia 2013, 91, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Ding, W.; Zhong, J.; Wan, J.; Xie, Z. Simultaneous qualitative and quantitative determination of phenolic compounds in Aloe barbadensis mill by liquid chromatography–mass spectrometry-ion trap-time-of-flight and high performance liquid chromatography-diode array detector. J. Pharm. Biomed. Anal. 2013, 80, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Gauniyal, P.; Teotia, U.V.S. Phytochemical screening and antimicrobial activity of some medicinal plants against oral flora. Asian Pac. J. Health Sci. 2014, 1, 255–263. [Google Scholar]
- Abeje, F.; Bisrat, D.; Hailu, A.; Asres, K. Phytochemistry and antileishmanial activity of the leaf latex of aloe calidophila reynolds. Phytother. Res. 2014, 28, 1801–1805. [Google Scholar] [CrossRef] [PubMed]
- Kambizi, L.; Sultana, N.; Afolayan, A. Bioactive compounds isolated from Aloe ferox.: A plant traditionally used for the treatment of sexually transmitted infections in the eastern cape, South Africa. Pharm. Biol. 2005, 42, 636–639. [Google Scholar] [CrossRef]
- Kametani, S.; Kojima-Yuasa, A.; Kikuzaki, H.; Kennedy, D.O.; Honzawa, M.; Matsui-Yuasa, I. Chemical constituents of cape aloe and their synergistic growth-inhibiting effect on ehrlich ascites tumor cells. Biosci. Biotechnol. Biochem. 2007, 71, 1220–1229. [Google Scholar] [CrossRef] [PubMed]
- Fawole, O.; Amoo, S.; Ndhlala, A.; Light, M.; Finnie, J.; Van Staden, J. Anti-inflammatory, anticholinesterase, antioxidant and phytochemical properties of medicinal plants used for pain-related ailments in South Africa. J. Ethnopharmacol. 2010, 127, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Wintola, O.A.; Afolayan, A.J. Phytochemical constituents and antioxidant activities of the whole leaf extract of Aloe ferox mill. Pharmacogn. Mag. 2011, 7, 325–333. [Google Scholar] [PubMed]
- Sun, Y.N.; Li, L.Y.; Li, W.; Kang, J.S.; Hwang, I.; Kim, Y.H. Chemical components from Aloe and their inhibition of indoleamine 2,3-dioxygenase. Pharmacogn. Mag. 2017, 13, 58–63. [Google Scholar] [PubMed]
- Bisrat, D.; Dagne, E.; van Wyk, B.-E.; Viljoen, A. Chromones and anthrones from Aloe marlothii and Aloe rupestris. Phytochemistry 2000, 55, 949–952. [Google Scholar] [CrossRef]
- Yagi, A.; Harada, N.; Yamada, H.; Iwadare, S.; Nishioka, I. Antibradykinin active material in aloe saponaria. J. Pharm. Sci. 1982, 71, 1172–1174. [Google Scholar] [CrossRef] [PubMed]
- Oumer, A.; Bisrat, D.; Mazumder, A.; Asres, K. A new antimicrobial anthrone from the leaf latex of Aloe trichosantha. Nat. Prod. Commun. 2014, 9, 949–952. [Google Scholar] [PubMed]
- Kammoun, M.; Miladi, S.; Ali, Y.B.; Damak, M.; Gargouri, Y.; Bezzine, S. In vitro study of the PLA2 inhibition and antioxidant activities of Aloe vera leaf skin extracts. Lipids Health Dis. 2011, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Yadav, A.; Yadav, M.; Yadav, J.P. Effect of climate change on phytochemical diversity, total phenolic content and in vitro antioxidant activity of Aloe vera (L.) Burm. F. BMC Res. Notes 2017, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Chen, D.; Si, J.; Tu, G.; Ma, L. The chemical constituents of Aloe vera L. Acta Pharm. Sin. 2000, 35, 120–123. [Google Scholar]
- Lawrence, R.; Tripathi, P.; Jeyakumar, E. Isolation, purification and evaluation of antibacterial agents from Aloe vera. Braz. J. Microbiol. 2009, 40, 906–915. [Google Scholar] [CrossRef] [PubMed]
- Abdissa, D.; Geleta, G.; Bacha, K.; Abdissa, N. Phytochemical investigation of aloe pulcherrima roots and evaluation for its antibacterial and antiplasmodial activities. PLoS ONE 2017, 12, e0173882. [Google Scholar] [CrossRef] [PubMed]
- Abdissa, N.; Gohlke, S.; Frese, M.; Sewald, N. Cytotoxic compounds from aloe megalacantha. Molecules 2017, 22, 1136. [Google Scholar] [CrossRef] [PubMed]
- Muthii, R.Z.; Mucunu, M.J.; Peter, M.M.; Gitahi, K.S. Phytochemistry and toxicity studies of aqueous and methanol extract of naturally growing and cultivated Aloe turkanensis. J. Pharmacogn. Phytochem. 2015, 3, 144–147. [Google Scholar]
- Ndhlala, A.; Amoo, S.; Stafford, G.; Finnie, J.; Van Staden, J. Antimicrobial, anti-inflammatory and mutagenic investigation of the south african tree aloe (Aloe barberae). J. Ethnopharmacol. 2009, 124, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Hirata, T.; Suga, T. Biologically active constituents of leaves and roots of Aloe arborescens var. Natalensis. Z. Naturforsch. C 1977, 32, 731–734. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Misawa, E.; Ito, Y.; Habara, N.; Nomaguchi, K.; Yamada, M.; Toida, T.; Hayasawa, H.; Takase, M.; Inagaki, M. Identification of five phytosterols from Aloe vera gel as anti-diabetic compounds. Biol. Pharm. Bull. 2006, 29, 1418–1422. [Google Scholar] [CrossRef] [PubMed]
- Misawa, E.; Tanaka, M.; Nomaguchi, K.; Nabeshima, K.; Yamada, M.; Toida, T.; Iwatsuki, K. Oral ingestion of Aloe vera phytosterols alters hepatic gene expression profiles and ameliorates obesity-associated metabolic disorders in zucker diabetic fatty rats. J. Agric. Food. Chem. 2012, 60, 2799–2806. [Google Scholar] [CrossRef] [PubMed]
- Pugh, N.; Ross, S.A.; ElSohly, M.A.; Pasco, D.S. Characterization of aloeride, a new high-molecular-weight polysaccharide from Aloe vera with potent immunostimulatory activity. J. Agric. Food. Chem. 2001, 49, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, B.; Avila, G.; Segura, D.; Escalante, B. Antiinflammatory activity of extracts from Aloe vera gel. J. Ethnopharmacol. 1996, 55, 69–75. [Google Scholar] [CrossRef]
- Van Heerden, F.R.; Viljoen, A.M.; van Wyk, B.-E. 6′-O-coumaroylaloesin from Aloe castanea—A taxonomic marker for aloe section anguialoe. Phytochemistry 2000, 55, 117–120. [Google Scholar] [CrossRef]
- Dagne, E.; Bisrat, D.; Van Wyk, B.-E.; Viljoen, A. 10-hydroxyaloin B 6′-O-acetate, an oxanthrone from Aloe claviflora. J. Nat. Prod. 1998, 61, 256–257. [Google Scholar] [CrossRef] [PubMed]
- Coopoosamy, R.; Magwa, M. Antibacterial activity of aloe emodin and aloin a isolated from Aloe excelsa. Afr. J. Biotechnol. 2006, 5, 1092–1094. [Google Scholar]
- Coopoosamy, R. In-vitro studies on lectin derivatives of Aloe excelsa (Berger). J. Med. Plants Res. 2010, 4, 1738–1742. [Google Scholar]
- Speranza, G.; Manitto, P.; Monti, D.; Lianza, F. Feroxidin, a novel 1-methyltetralin derivative isolated from Cape Aloe. Tetrahedron Lett. 1990, 31, 3077–3080. [Google Scholar] [CrossRef]
- Speranza, G.; Dadà, G.; Lunazzi, L.; Gramatica, P.; Manitto, P. A c-glucosylated 5-methylchromone from kenya Aloe. Phytochemistry 1986, 25, 2219–2222. [Google Scholar] [CrossRef]
- Speranza, G.; Manitto, P.; Monti, D. Feralolide, a dihydroisocoumarin from cape aloe. Phytochemistry 1993, 33, 175–178. [Google Scholar] [CrossRef]
- Koyama, J.; Ogura, T.; Tagahara, K. Naphtho [2,3-c]furan-4,9-dione and its derivatives from Aloe ferox. Phytochemistry 1994, 37, 1147–1148. [Google Scholar] [CrossRef]
- Dagne, E.; Bisrat, D.; Van Wyk, B.-E.; Viljoen, A.; Hellwig, V.; Steglich, W. Anthrones from Aloe microstigma. Phytochemistry 1997, 44, 1271–1274. [Google Scholar] [CrossRef]
- Conner, J.M.; Gray, A.I.; Reynolds, T.; Waterman, P.G. Anthracene and chromone derivatives in the exudate of Aloe rabaiensis. Phytochemistry 1989, 28, 3551–3553. [Google Scholar] [CrossRef]
- Blitzke, T.; Masaoud, M.; Schmidt, J. Constituents of Aloe rubroviolacea. Fitoterapia 2001, 72, 78–79. [Google Scholar] [CrossRef]
- Blitzke, T.; Porzel, A.; Masaoud, M.; Schmidt, J. A chlorinated amide and piperidine alkaloids from Aloe sabaea. Phytochemistry 2000, 55, 979–982. [Google Scholar] [CrossRef]
- Kedarnath, N.; Surekha, R.S.; Mahantesh, S.; Patil, C. Phytochemical screening and antimicrobial activity of Aloe vera. World Res. J. Med. Aromat. Plants 2012, 1, 11–13. [Google Scholar]
- Dagne, E.; Casser, I.; Steglich, W. Aloechrysone, a dihydroanthracenone from Aloe berhana. Phytochemistry 1992, 31, 1791–1793. [Google Scholar] [CrossRef]
- Yagi, A.; Makino, K.; Nishioka, I. Studies on the constituents of Aloe sapnaria haw. I. The structures of tetrahydroanthracene derivatives and the related anthraquinones. Chem. Pharm. Bull. 1974, 22, 1159–1166. [Google Scholar] [CrossRef]
- Al-Oqail, M.M.; El-Shaibany, A.; Al-Jassas, E.; Al-Sheddi, E.S.; Al-Massarani, S.M.; Farshori, N.N. In vitro anti-proliferative activities of Aloe perryi flowers extract on human liver, colon, breast, lung, prostate and epithelial cancer cell lines. Pak. J. Pharm. Sci. 2016, 29, 723–729. [Google Scholar] [PubMed]
- Abd-Alla, H.I.; Shaaban, M.; Shaaban, K.A.; Abu-Gabal, N.S.; Shalaby, N.M.; Laatsch, H. New bioactive compounds from Aloe hijazensis. Nat. Prod. Res. 2009, 23, 1035–1049. [Google Scholar] [CrossRef] [PubMed]
- Beppu, H.; Koike, T.; Shimpo, K.; Chihara, T.; Hoshino, M.; Ida, C.; Kuzuya, H. Radical-scavenging effects of Aloe arborescens miller on prevention of pancreatic islet B-cell destruction in rats. J. Ethnopharmacol. 2003, 89, 37–45. [Google Scholar] [CrossRef]
- Akaberi, M.; Sobhani, Z.; Javadi, B.; Sahebkar, A.; Emami, S.A. Therapeutic effects of Aloe spp. In traditional and modern medicine: A review. Biomed. Pharm. 2016, 84, 759–772. [Google Scholar] [CrossRef] [PubMed]
- Atreya, K.; Pyakurel, D.; Thagunna, K.S.; Bhatta, L.D.; Uprety, Y.; Chaudhary, R.P.; Oli, B.N.; Rimal, S.K. Factors contributing to the decline of traditional practices in communities from the gwallek–kedar area, kailash sacred landscape, Nepal. Environ. Manag. 2018, 61, 741–755. [Google Scholar] [CrossRef] [PubMed]
- Gautam, T.P. Indigenous uses of some medicinal plants in panchthar district, Nepal. Nepalese J. Biosci. 2011, 1, 125–130. [Google Scholar] [CrossRef]
- Limbu, K.; Basanta Kumar Rai, D. Ethno-medicinal practices among the Limbu community in Limbuwan, Eastern Nepal. Global J. Hum. Soc. Sci. Res. 2013, 13, 2. [Google Scholar]
- Acharya, R. Ethnobotanical study of medicinal plants of resunga hill used by magar community of badagaun VDC, gulmi district, Nepal. Sci. World 2012, 10, 54–65. [Google Scholar] [CrossRef]
- Siwakoti, M.; Siwakoti, S. Ethnomedicinal uses of plants among the satar tribe of Nepal. J. Econ. Taxon. Bot. 2000, 24, 323–333. [Google Scholar]
- Pradhan, S. Antihyperglycemic effect of various medicinal plants of sikkim himalayas—A review. Int. J. Res. Phytochem. Pharmacol. 2011, 1, 124–130. [Google Scholar]
- Sushen, U.; Unnithan, C.; Rajan, S.; Chouhan, R.; Chouhan, S.; Uddin, F.; Kowsalya, R. Aloe vera: A potential herb used as traditional medicine by tribal people of kondagatu and purudu of karimnagar district, telangana state, India. And their preparative methods. Eur. J. Pharm. Med. Res. 2017, 4, 820–831. [Google Scholar]
- Parajuli, R. Ethnomedicinal use of plants in rai community of maimajuwa and puwamajuwa VDCS of Ilam District, Eastern Nepal. Bull. Dept. Plant Res. 2012, 34, 65–73. [Google Scholar]
- Parajuli, R.R. Indigenous knowledge on medicinal plants: Maipokhari, Maimajhuwa and mabu VDCS of Ilam District, Eastern Nepal. J. Dept. Plant Resour. Nepal. 2013, 35, 50–58. [Google Scholar]
- Rai, S. Medicinal plants used by meche people of Jhapa District, Eastern Nepal. Our Nat. 2004, 2, 27–32. [Google Scholar] [CrossRef]
- Hussain, S.; Hore, D. Collection and conservation of major medicinal pants of Darjeeling and Sikkim Himalayas. Indian J. Tradit. Knowl. 2007, 6, 352–357. [Google Scholar]
- Badola, H.K.; Pradhan, B.K. Plants used in healthcare practices by limboo tribe in south–west of khangchendzonga biosphere reserve, Sikkim, India. Indian J. Tradit. Knowl. 2013, 12, 355–369. [Google Scholar]
- Acharya, K.; Chaudhary, R.; Vetaas, O. Medicinal plants of nepal: Distribution pattern along an elevational gradient and effectiveness of existing protected areas for their conservation. Banko Janakari 2009, 19, 16–22. [Google Scholar] [CrossRef]
- Thapa, L.B.; Dhakal, T.M.; Chaudhary, R.; Thapa, H. Medicinal plants used by raji ethnic tribe of Nepal in treatment of gastrointestinal disorders. Our Nat. 2013, 11, 177–186. [Google Scholar] [CrossRef]
- Ghimire, K.; Bastakoti, R.R. Ethnomedicinal knowledge and healthcare practices among the tharus of nawalparasi district in central Nepal. For. Ecol. Manag. 2009, 257, 2066–2072. [Google Scholar] [CrossRef]
- Manandhar, N. Plants and People of Nepal; Timber Press: Oregon, OR, USA, 2002; ISBN 0-88192-527-6. [Google Scholar]
- Bhattarai, N. Folk herbal remedies for diarrhoea and dysentery in central Nepal. Fitoterapia Milano 1993, 64, 243. [Google Scholar]
- Singh, A.G.; Kumar, A.; Tewari, D.D. An ethnobotanical survey of medicinal plants used in terai forest of western Nepal. J. Ethnobiol. Ethnomed. 2012, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Acharya, E.; Pokhrel, B. Ethno-medicinal plants used by bantar of Bhaudaha, Morang, Nepal. Our Nat. 2006, 4, 96–103. [Google Scholar]
- Grover, J.; Yadav, S.; Vats, V. Medicinal plants of india with anti-diabetic potential. J. Ethnopharmacol. 2002, 81, 81–100. [Google Scholar] [CrossRef]
- Keter, L.K.; Mutiso, P.C. Ethnobotanical studies of medicinal plants used by traditional health practitioners in the management of diabetes in lower eastern province, Kenya. J. Ethnopharmacol. 2012, 139, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Tahraoui, A.; El-Hilaly, J.; Israili, Z.; Lyoussi, B. Ethnopharmacological survey of plants used in the traditional treatment of hypertension and diabetes in South-Eastern Morocco (Errachidia province). J. Ethnopharmacol. 2007, 110, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Frei, B.; Baltisberger, M.; Sticher, O.; Heinrich, M. Medical ethnobotany of the zapotecs of the isthmus-sierra (Oaxaca, Mexico): Documentation and assessment of indigenous uses. J. Ethnopharmacol. 1998, 62, 149–165. [Google Scholar] [CrossRef]
- Babb, D.A.; Pemba, L.; Seatlanyane, P.; Charalambous, S.; Churchyard, G.J.; Grant, A.D. Use of traditional medicine by HIV-infected individuals in south africa in the era of antiretroviral therapy. Psychol. Health Med. 2007, 12, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Anil Kumar, N.V.; Şener, B.; Sharifi-Rad, M.; Kılıç, M.; Mahady, G.B.; Vlaisavljevic, S.; Iriti, M.; Kobarfard, F.; Setzer, W.N.; et al. Medicinal plants used in the treatment of human immunodeficiency virus. Int. J. Mol. Sci. 2018, 19, 1459. [Google Scholar] [CrossRef] [PubMed]
- Scherrer, A.M.; Motti, R.; Weckerle, C.S. Traditional plant use in the areas of monte vesole and ascea, cilento national park (Campania, Southern Italy). J. Ethnopharmacol. 2005, 97, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Cheikhyoussef, A.; Shapi, M.; Matengu, K.; Ashekele, H.M. Ethnobotanical study of indigenous knowledge on medicinal plant use by traditional healers in oshikoto region, Namibia. J. Ethnobiol. Ethnomed. 2011, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Gemedo-Dalle, T.; Maass, B.L.; Isselstein, J. Plant biodiversity and ethnobotany of borana pastoralists in Southern Oromia, Ethiopia. Econ. Bot. 2005, 59, 43–65. [Google Scholar] [CrossRef]
- Abdolshahi, A.; Naybandi-Atashi, S.; Heydari-Majd, M.; Salehi, B.; Kobarfard, F.; Ayatollahi, S.A.; Ata, A.; Tabanelli, G.; Sharifi-Rad, M.; Montanari, C.; et al. Antibacterial activity of some Lamiaceae species against Staphylococcus aureus in yoghurt-based drink (Doogh). Cell. Mol. Biol. (Noisy-le-Grand, France) 2018, 64, 71–77. [Google Scholar] [CrossRef]
- Mishra, A.P.; Sharifi-Rad, M.; Shariati, M.A.; Mabkhot, Y.N.; Al-Showiman, S.S.; Rauf, A.; Salehi, B.; Župunski, M.; Sharifi-Rad, M.; Gusain, P.; et al. Bioactive compounds and health benefits of edible Rumex species—A review. Cell. Mol. Biol. (Noisy-le-Grand, France) 2018, 64, 27–34. [Google Scholar] [CrossRef]
- Marvdashti, L.M.; Abdolshahi, A.; Hedayati, S.; Sharifi-Rad, M.; Iriti, M.; Salehi, B.; Sharifi-Rad, J. Pullulan gum production from low-quality fig syrup using Aureobasidium pullulans. Cell. Mol. Biol. 2018, 64, 22–26. [Google Scholar] [CrossRef]
- Mishra, A.P.; Saklani, S.; Salehi, B.; Parcha, V.; Sharifi-Rad, M.; Milella, L.; Iriti, M.; Sharifi-Rad, J.; Srivastava, M. Satyrium nepalense, a high altitude medicinal orchid of indian himalayan region: Chemical profile and biological activities of tuber extracts. Cell. Mol. Biol. (Noisy-le-Grand, France) 2018, 64, 35–43. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Tayeboon, G.S.; Niknam, F.; Sharifi-Rad, M.; Mohajeri, M.; Salehi, B.; Iriti, M.; Sharifi-Rad, M. Veronica persica Poir. Extract—Antibacterial, antifungal and scolicidal activities, and inhibitory potential on Acetylcholinesterase, Tyrosinase, Lipoxygenase and Xanthine oxidase. Cell. Mol. Biol. (Noisy-le-Grand, France) 2018, 64, 50–56. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Fokou, P.; Sharopov, F.; Martorell, M.; Ademiluyi, A.; Rajkovic, J.; Salehi, B.; Martins, N.; Iriti, M.; Sharifi-Rad, J. Antiulcer agents: From plant extracts to phytochemicals in healing promotion. Molecules 2018, 23, 1751. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Varoni, E.M.; Salehi, B.; Sharifi-Rad, J.; Matthews, K.R.; Ayatollahi, S.A.; Kobarfard, F.; Ibrahim, S.A.; Mnayer, D.; Zakaria, Z.A. Plants of the genus Zingiber as a source of bioactive phytochemicals: From tradition to pharmacy. Molecules 2017, 22, 2145. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Ozcelik, B.; Altın, G.; Daşkaya-Dikmen, C.; Martorell, M.; Ramírez-Alarcón, K.; Alarcón-Zapata, P.; Morais-Braga, M.F.B.; Carneiro, J.N.P.; Alves Borges Leal, A.L.; et al. Salvia spp. Plants-from farm to food applications and phytopharmacotherapy. Trends Food Sci. Technol. 2018, 80, 242–263. [Google Scholar] [CrossRef]
- Salehi, B.; Valussi, M.; Jugran, A.K.; Martorell, M.; Ramírez-Alarcón, K.; Stojanović-Radić, Z.Z.; Antolak, H.; Kręgiel, D.; Mileski, K.S.; Sharifi-Rad, M.; et al. Nepeta species: From farm to food applications and phytotherapy. Trends Food Sci. Technol. 2018, 80, 104–122. [Google Scholar] [CrossRef]
- Sánchez-Machado, D.I.; López-Cervantes, J.; Sendón, R.; Sanches-Silva, A. Aloe vera: Ancient knowledge with new frontiers. Trends Food Sci. Technol. 2017, 61, 94–102. [Google Scholar] [CrossRef]
- Mulaudzi, R.; Ndhlala, A.; Kulkarni, M.; Finnie, J.; Van Staden, J. Antimicrobial properties and phenolic contents of medicinal plants used by the venda people for conditions related to venereal diseases. J. Ethnopharmacol. 2011, 135, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Baruah, A.; Bordoloi, M.; Baruah, H.P.D. Aloe vera: A multipurpose industrial crop. Ind. Crops Prod. 2016, 94, 951–963. [Google Scholar] [CrossRef]
- Ferro, V.A.; Bradbury, F.; Cameron, P.; Shakir, E.; Rahman, S.R.; Stimson, W.H. In vitro susceptibilities of shigella flexneri and streptococcus pyogenes to inner gel of Aloe barbadensis miller. Antimicrob. Agents Chemother. 2003, 47, 1137–1139. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Shringi, B.; Patidar, D.K.; Chalichem, N.S.S.; Javvadi, A.K. Screening of antimicrobial activity of alcoholic & aqueous extract of some indigenous plants. Indo-Global J. Pharm. Sci. 2011, 1, 186–193. [Google Scholar]
- Luiz, C.; da Rocha Neto, A.C.; Franco, P.O.; Di Piero, R.M. Emulsions of essential oils and Aloe polysaccharides: Antimicrobial activity and resistance inducer potential against Xanthomonas fragariae. Trop. Plant Pathol. 2017, 42, 370–381. [Google Scholar] [CrossRef]
- Alemdar, S.; Agaoglu, S. Investigation of in vitro antimicrobial activity of Aloe vera juice. J. Anim. Vet. Adv. 2009, 8, 99–102. [Google Scholar]
- Dharajiya, D.; Pagi, N.; Jasani, H.; Patel, P. Antimicrobial activity and phytochemical screening of Aloe vera (Aloe barbadensis Miller). Int. J. Curr. Microbiol. App. Sci. 2017, 6, 2152–2162. [Google Scholar]
- Chen, W.; Van Wyk, B.-E.; Vermaak, I.; Viljoen, A.M. Cape aloes—A review of the phytochemistry, pharmacology and commercialisation of Aloe ferox. Phytochem. Lett. 2012, 5, 1–12. [Google Scholar] [CrossRef]
- Abakar, H.O.M.; Bakhiet, S.E.; Abadi, R.S.M. Antimicrobial activity and minimum inhibitory concentration of Aloe vera sap and leaves using different extracts. J. Pharmacogn. Phytochem. 2017, 6, 298–303. [Google Scholar]
- Jain, I.; Jain, P.; Bisht, D.; Sharma, A.; Srivastava, B.; Gupta, N. Comparative evaluation of antibacterial efficacy of six indian plant extracts against streptococcus mutans. J. Clin. Diagn. Res. 2015, 9, ZC50. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Romero, D.; Alburquerque, N.; Valverde, J.; Guillén, F.; Castillo, S.; Valero, D.; Serrano, M. Postharvest sweet cherry quality and safety maintenance by Aloe vera treatment: A new edible coating. Postharvest Biol. Technol. 2006, 39, 93–100. [Google Scholar] [CrossRef]
- Wang, H.H.; Chung, J.G.; Ho, C.C.; Wu, L.T.; Chang, S.H. Aloe-emodin effects on arylamine n-acetyltransferase activity in the bacterium helicobacter pylori. Planta Med. 1998, 64, 176–178. [Google Scholar] [CrossRef] [PubMed]
- Cellini, L.; Di Bartolomeo, S.; Di Campli, E.; Genovese, S.; Locatelli, M.; Di Giulio, M. In vitro activity of a loe vera inner gel against h elicobacter pylori strains. Lett. Appl. Microbiol. 2014, 59, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Kargaran, M.; Moradabadi, A.R.; Arjomandzadegan, M.; Hosseini, H.; Habibi, G.; Tayeboon, M.; Karami, H.; Akbari, A. Effects of the aqueous extract of Aloe vera on the morphological and physiological properties of E. coli. Iran. Red Crescent Med. J. 2017, 19. [Google Scholar] [CrossRef]
- Das, S.; Mishra, B.; Gill, K.; Ashraf, M.S.; Singh, A.K.; Sinha, M.; Sharma, S.; Xess, I.; Dalal, K.; Singh, T.P. Isolation and characterization of novel protein with anti-fungal and anti-inflammatory properties from Aloe vera leaf gel. Int. J. Biol. Macromol. 2011, 48, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Radi, M.; Firouzi, E.; Akhavan, H.; Amiri, S. Effect of gelatin-based edible coatings incorporated with Aloe vera and black and green tea extracts on the shelf life of fresh-cut oranges. J. Food Qual. 2017, 2017, 10. [Google Scholar] [CrossRef]
- Chen, C.P.; Wang, B.J.; Weng, Y.M. Physiochemical and antimicrobial properties of edible aloe/gelatin composite films. Int. J. Food Sci. Technol. 2010, 45, 1050–1055. [Google Scholar] [CrossRef]
- Benítez, S.; Achaerandio, I.; Sepulcre, F.; Pujolà, M. Aloe vera based edible coatings improve the quality of minimally processed ‘hayward’kiwifruit. Postharvest Biol. Technol. 2013, 81, 29–36. [Google Scholar] [CrossRef]
- Benítez, S.; Achaerandio, I.; Pujolà, M.; Sepulcre, F. Aloe vera as an alternative to traditional edible coatings used in fresh-cut fruits: A case of study with kiwifruit slices. LWT-Food Sci. Technol. 2015, 61, 184–193. [Google Scholar] [CrossRef]
- Sogvar, O.B.; Saba, M.K.; Emamifar, A. Aloe vera and ascorbic acid coatings maintain postharvest quality and reduce microbial load of strawberry fruit. Postharvest Biol. Technol. 2016, 114, 29–35. [Google Scholar] [CrossRef]
- Saks, Y.; Barkai-Golan, R. Aloe vera gel activity against plant pathogenic fungi. Postharvest Biol. Technol. 1995, 6, 159–165. [Google Scholar] [CrossRef]
- De Rodrýguez, D.J.; Hernández-Castillo, D.; Rodrýguez-Garcýa, R.; Angulo-Sánchez, J. Antifungal activity in vitro of Aloe vera pulp and liquid fraction against plant pathogenic fungi. Ind. Crops Prod. 2005, 21, 81–87. [Google Scholar] [CrossRef]
- Castillo, S.; Navarro, D.; Zapata, P.; Guillén, F.; Valero, D.; Serrano, M.; Martínez-Romero, D. Antifungal efficacy of Aloe vera in vitro and its use as a preharvest treatment to maintain postharvest table grape quality. Postharvest Biol. Technol. 2010, 57, 183–188. [Google Scholar] [CrossRef]
- Ortega-Toro, R.; Collazo-Bigliardi, S.; Roselló, J.; Santamarina, P.; Chiralt, A. Antifungal starch-based edible films containing Aloe vera. Food Hydrocoll. 2017, 72, 1–10. [Google Scholar] [CrossRef]
- Navarro, D.; Díaz-Mula, H.M.; Guillén, F.; Zapata, P.J.; Castillo, S.; Serrano, M.; Valero, D.; Martínez-Romero, D. Reduction of nectarine decay caused by rhizopus stolonifer, botrytis cinerea and penicillium digitatum with Aloe vera gel alone or with the addition of thymol. Int. J. Food Microbiol. 2011, 151, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Vieira, J.M.; Flores-López, M.L.; de Rodríguez, D.J.; Sousa, M.C.; Vicente, A.A.; Martins, J.T. Effect of chitosan—Aloe vera coating on postharvest quality of blueberry (Vaccinium corymbosum) fruit. Postharvest Biol. Technol. 2016, 116, 88–97. [Google Scholar] [CrossRef] [Green Version]
- Zapata, P.; Navarro, D.; Guillén, F.; Castillo, S.; Martínez-Romero, D.; Valero, D.; Serrano, M. Characterisation of gels from different aloe spp. As antifungal treatment: Potential crops for industrial applications. Ind. Crops Prod. 2013, 42, 223–230. [Google Scholar] [CrossRef]
- Nidiry, E.S.J.; Ganeshan, G.; Lokesha, A. Antifungal activity of some extractives and constituents of Aloe vera. Res. J. Med. Plant 2011, 5, 196–200. [Google Scholar]
- Martínez-Romero, D.; Castillo, S.; Guillén, F.; Díaz-Mula, H.M.; Zapata, P.J.; Valero, D.; Serrano, M. Aloe vera gel coating maintains quality and safety of ready-to-eat pomegranate arils. Postharvest Biol. Technol. 2013, 86, 107–112. [Google Scholar] [CrossRef]
- Bill, M.; Sivakumar, D.; Korsten, L.; Thompson, A.K. The efficacy of combined application of edible coatings and thyme oil in inducing resistance components in Avocado (Persea americana mill.) against anthracnose during post-harvest storage. Crop Prot. 2014, 64, 159–167. [Google Scholar] [CrossRef]
- Sitara, U.; Hassan, N.; Naseem, J. Antifungal activity of Aloe vera gel against plant pathogenic fungi. Pak. J. Bot. 2011, 43, 2231–2233. [Google Scholar]
- Hassanpour, H. Effect of Aloe vera gel coating on antioxidant capacity, antioxidant enzyme activities and decay in Raspberry fruit. LWT-Food Sci. Technol. 2015, 60, 495–501. [Google Scholar] [CrossRef]
- Marpudi, S.L.; Abirami, L.; Srividya, N. Enhancement of storage life and quality maintenance of papaya fruits using Aloe vera based antimicrobial coating. Indian J. Biotechnol. 2011, 10, 83–89. [Google Scholar]
- Valverde, J.M.; Valero, D.; Martínez-Romero, D.; Guillén, F.; Castillo, S.; Serrano, M. Novel edible coating based on Aloe vera gel to maintain table grape quality and safety. J. Agric. Food. Chem. 2005, 53, 7807–7813. [Google Scholar] [CrossRef] [PubMed]
- Adetunji, C.; Fawole, O.; Arowora, K.; Nwaubani, S.; Ajayi, E.; Oloke, J.; Majolagbe, O.; Ogundele, B.; Aina, J.; Adetunji, J. Effects of edible coatings from Aloe vera gel on quality and postharvest physiology of Ananas comosus L. Fruit during ambient storage. Global J. Sci. Front. Res. Biotech Gene. 2012, 12, 39–43. [Google Scholar]
- Chauhan, O.; Nanjappa, C.; Ashok, N.; Ravi, N.; Roopa, N.; Raju, P. Shellac and Aloe vera gel based surface coating for shelf life extension of tomatoes. J. Food Sci. Technol. 2015, 52, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Afolayan, A.; Grierson, D.; Kambizi, L.; Madamombe, I.; Masika, P.; Jäger, A. In vitro antifungal activity of some South African medicinal plants. S. Afr. J. Bot. 2002, 68, 72–76. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, S.; Kumar, D.S.; Arulselvan, P.; Senthilkumar, G. In vitro antibacterial and antifungal activities of ethanolic extract of Aloe vera leaf gel. J. Plant Sci. 2006, 1, 348–355. [Google Scholar]
- Rosca-Casian, O.; Parvu, M.; Vlase, L.; Tamas, M. Antifungal activity of Aloe vera leaves. Fitoterapia 2007, 78, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Olaleye, M.; Bello-Michael, C. Comparative antimicrobial activities of Aloe vera gel and leaf. Afr. J. Biotechnol. 2005, 4, 1413–1414. [Google Scholar]
- Hashemi, S.A.; Madani, S.A.; Abediankenari, S. The review on properties of Aloe vera in healing of cutaneous wounds. BioMed. Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Tarameshloo, M.; Norouzian, M.; Zarein-Dolab, S.; Dadpay, M.; Mohsenifar, J.; Gazor, R. Aloe vera gel and thyroid hormone cream may improve wound healing in wistar rats. Anat. Cell Biol. 2012, 45, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Leung, M.; Koon, J.; Zhu, L.; Hui, Y.; Yu, B.; Fung, K. Macrophage activation by polysaccharide biological response modifier isolated from Aloe vera L. Var. Chinensis (HAW.) berg. Int. Immunopharmacol. 2006, 6, 1634–1641. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.H. Biological activity of Aloe vera. Sofw J. 1993, 119, 646. [Google Scholar]
- Tizard, I.; Carpenter, R.; McAnalley, B.; Kemp, M. The biological activities of mannans and related complex carbohydrates. Mol. Biother. 1989, 1, 290–296. [Google Scholar] [PubMed]
- Subramanian, S.; Kumar, D.S.; Arulselvan, P. Wound healing potential of Aloe vera leaf gel studied in experimental rabbits. Asian J. Biochem. 2006, 1, 178–185. [Google Scholar]
- Liu, L.; Chen, X.; Wu, B.; Jiang, Q. Influence of aloe polysaccharide on proliferation and hyaluronic acid and hydroxyproline secretion of human fibroblasts in vitro. J. Chin. Integr. Med. 2010, 8, 256–262. [Google Scholar] [CrossRef]
- Feily, A.; Namazi, M. Aloe vera in dermatology: A brief review. Giornale Italiano di Dermatologia e Venereologia: Organo Ufficiale, Societa Italiana di Dermatologia e Sifilografia 2009, 144, 85–91. [Google Scholar]
- Tizard, I.; Busbee, D.; Maxwell, B.; Kemp, M. Effects of acemannan, a complex carbohydrate, on wound-healing in young and aged rats. Wounds-A Compend. Clin. Res. Pract. 1994, 6, 201–209. [Google Scholar]
- Reynolds, T.; Dweck, A. Aloe vera leaf gel: A review update. J. Ethnopharmacol. 1999, 68, 3–37. [Google Scholar] [CrossRef]
- Choi, S.W.; Son, B.W.; Son, Y.S.; Park, Y.I.; Lee, S.K.; Chung, M.H. The wound-healing effect of a glycoprotein fraction isolated from Aloe vera. Brit. J. Dermatol. 2001, 145, 535–545. [Google Scholar] [CrossRef]
- Yao, H.; Chen, Y.; Li, S.; Huang, L.; Chen, W.; Lin, X. Promotion proliferation effect of a polysaccharide from aloe barbadensis miller on human fibroblasts in vitro. Int. J. Biol. Macromol. 2009, 45, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Rajput, S.S.; Soni, K.K.; Saxena, R. Pharmacology and phytochemistry of saponin isolated from Aloe vera for wound healing activity. Asian J. Chem. 2009, 21, 1029–1032. [Google Scholar]
- Ray, A.; Aswatha, S.M. An analysis of the influence of growth periods on physical appearance, and acemannan and elemental distribution of Aloe vera L. Gel. Ind. Crops Prod. 2013, 48, 36–42. [Google Scholar] [CrossRef]
- Carien, B.; Alvaro, V.; Josias, H. Modulation of drug efflux by aloe materials: An in vitro investigation across rat intestinal tissue. Pharmacogn. Mag. 2013, 9, S44. [Google Scholar] [PubMed]
- Ishii, Y.; Tanizawa, H.; Takino, Y. Studies of aloe. V. Mechanism of cathartic effect. (4). Biol. Pharm. Bull. 1994, 17, 651–653. [Google Scholar] [CrossRef] [PubMed]
- Joseph, B.; Raj, S.J. Pharmacognostic and phytochemical properties of Aloe vera linn an overview. Int. J. Pharm. Sci. Rev. Res. 2010, 4, 106–110. [Google Scholar]
- Yagi, T.; Yamauchi, K. Synergistic effects of anthraquinones of the purgative activity of rhein anthrone in mice. J. Pharm. Pharmacol. 1999, 51, 93–95. [Google Scholar] [PubMed]
- Sahu, P.K.; Giri, D.D.; Singh, R.; Pandey, P.; Gupta, S.; Shrivastava, A.K.; Kumar, A.; Pandey, K.D. Therapeutic and medicinal uses of Aloe vera: A review. Pharmacol. Pharm. 2013, 4, 599. [Google Scholar] [CrossRef]
- Steenkamp, V.; Stewart, M. Medicinal applications and toxicological activities of aloe. Products. Pharm. Biol. 2007, 45, 411–420. [Google Scholar] [CrossRef]
- Wintola, O.A.; Sunmonu, T.O.; Afolayan, A.J. The effect of aloe ferox mill. In the treatment of loperamide-induced constipation in wistar rats. BMC Gastroenterol. 2010, 10, 95. [Google Scholar] [CrossRef] [PubMed]
- Chihara, T.; Shimpo, K.; Beppu, H.; Yamamoto, N.; Kaneko, T.; Wakamatsu, K.; Sonoda, S. Effects of aloe-emodin and emodin on proliferation of the MKN45 human gastric cancer cell line. Asian Pac. J. Cancer Prev. 2015, 16, 3887–3891. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Zhu, M.; Du, T.; She, Q.; Yang, J.; Zhu, X.; Wei, L.; Zhang, J. Effects of aloe-emodin on proliferation and migration of human gastric cancer cell line BGC-823. Acta Anat. Sin. 2010, 41, 909–911. [Google Scholar]
- Steinmeyer, J. Pharmacological basis for the therapy of pain and inflammation with nonsteroidal anti-inflammatory drugs. Arthritis Res. Ther. 2000, 2, 379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amoo, S.O.; Aremu, A.O.; Van Staden, J. Unraveling the medicinal potential of South African aloe species. J. Ethnopharmacol. 2014, 153, 19–41. [Google Scholar] [CrossRef] [PubMed]
- Li, R.W.; Lin, G.D.; Leach, D.N.; Waterman, P.G.; Myers, S.P. Inhibition of coxs and 5-LOX and activation of ppars by australian clematis species (Ranunculaceae). J. Ethnopharmacol. 2006, 104, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Park, M.-Y.; Kwon, H.-J.; Sung, M.-K. Evaluation of aloin and aloe-emodin as anti-inflammatory agents in aloe by using murine macrophages. Biosci. Biotechnol. Biochem. 2009, 73, 828–832. [Google Scholar] [CrossRef] [PubMed]
- Im, S.-A.; Oh, S.-T.; Song, S.; Kim, M.-R.; Kim, D.-S.; Woo, S.-S.; Jo, T.H.; Park, Y.I.; Lee, C.-K. Identification of optimal molecular size of modified Aloe polysaccharides with maximum immunomodulatory activity. Int. Immunopharmacol. 2005, 5, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, K.; Jäger, A.; Viljoen, A.; van Wyk, B.-E. Cyclooxygenase inhibitory activity of aloe species. S. Afr. J. Bot. 2002, 68, 47–50. [Google Scholar] [CrossRef]
- Arosio, B.; Gagliano, N.; Fusaro, L.M.P.; Parmeggiani, L.; Tagliabue, J.; Galetti, P.; De Castri, D.; Moscheni, C.; Annoni, G. Aloe-emodin quinone pretreatment reduces acute liver injury induced by carbon tetrachloride. Pharmacol. Toxicol. 2000, 87, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Duansak, D.; Somboonwong, J.; Patumraj, S. Effects of Aloe vera on leukocyte adhesion and TNF-α and IL-6 levels in burn wounded rats. Clin. Hemorheol. Microcirc. 2003, 29, 239–246. [Google Scholar] [PubMed]
- Liu, Z.; Ge, X.; Lu, Y.; Dong, S.; Zhao, Y.; Zeng, M. Effects of chitosan molecular weight and degree of deacetylation on the properties of gelatine-based films. Food Hydrocoll. 2012, 26, 311–317. [Google Scholar] [CrossRef]
- Esua, M.F.; Rauwald, J.-W. Novel bioactive maloyl glucans from Aloe vera gel: Isolation, structure elucidation and in vitro bioassays. Carbohydr. Res. 2006, 341, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Che, Q.-M.; Akao, T.; Hattori, M.; Kobashi, K.; Namba, T. Isolation of a human intestinal bacterium capable of transforming barbaloin to aloe-emodin anthrone1. Planta Med. 1991, 57, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Hanley, D.; Solomon, W.; Saffran, B.; Davis, R. The evaluation of natural substances in the treatment of adjuvant arthritis. J. Am. Podiat. Assoc. 1982, 72, 275. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Rakesh, S.; Nagpal, R.; Hemalatha, R.; Ramakrishna, A.; Sudarshan, V.; Ramagoni, R.; Shujauddin, M.; Verma, V.; Kumar, A. Probiotic lactobacillus rhamnosus gg and Aloe vera gel improve lipid profiles in hypercholesterolemic rats. Nutrition 2013, 29, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Etim, O.E.; Farombi, E.O.; Usoh, I.F.; Akpan, E.J. The protective effect of Aloe vera juice on lindane induced hepatotoxicity and genotoxicity. Pak. J. Pharm. Sci. 2006, 19, 333–337. [Google Scholar]
- Singab, A.N.B.; El-Hefnawy, H.M.; Esmat, A.; Gad, H.A.; Nazeam, J.A. A systemic review on aloe arborescens pharmacological profile: Biological activities and pilot clinical trials. Phytother. Res. 2015, 29, 1858–1867. [Google Scholar] [CrossRef] [PubMed]
- Saini, D.K.; Saini, M.R. Evaluation of radioprotective efficacy and possible mechanism of action of aloe gel. Environ. Toxicol. Pharmacol. 2011, 31, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.; Hughes, J.; Hong, M.; Jia, Q.; Orndorff, S. Modulation of melanogenesis by aloesin: A competitive inhibitor of tyrosinase. Pigm. Cell Res. 2002, 15, 335–340. [Google Scholar] [CrossRef]
- Jia, Q.; Farrow, T.M. 7-Hydroxy Chromones as Potent Antioxidants. U.S. Patent 6,884,783, 26 April 2005. [Google Scholar]
- Frum, Y.; Viljoen, A. In vitro 5-lipoxygenase and anti-oxidant activities of south african medicinal plants commonly used topically for skin diseases. Skin Pharmacol. Physiol. 2006, 19, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Sazhina, N.; Lapshin, P.; Zagoskina, N.; Misin, V. Comparative study of antioxidant properties of extracts of various aloe species. Russ. J. Bioorganic Chem. 2016, 42, 735–740. [Google Scholar] [CrossRef]
- Kang, M.-C.; Kim, S.Y.; Kim, Y.T.; Kim, E.-A.; Lee, S.-H.; Ko, S.-C.; Wijesinghe, W.; Samarakoon, K.W.; Kim, Y.-S.; Cho, J.H. In vitro and in vivo antioxidant activities of polysaccharide purified from Aloe vera (Aloe barbadensis) gel. Carbohydr. Polym. 2014, 99, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Nwajo, H. Antioxidant activity of the exudate from aloe barbadensis leaves in diabetic rats. Biokemistri 2006, 18, 2. [Google Scholar] [CrossRef]
- Habeeb, F.; Shakir, E.; Bradbury, F.; Cameron, P.; Taravati, M.R.; Drummond, A.J.; Gray, A.I.; Ferro, V.A. Screening methods used to determine the anti-microbial properties of Aloe vera inner gel. Methods 2007, 42, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Grace, O.; Simmonds, M.; Smith, G.; Van Wyk, A. Therapeutic uses of Aloe L. (Asphodelaceae) in Southern Africa. J. Ethnopharmacol. 2008, 119, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Kambiz, L.; Afolayan, A. Extracts from aloe ferox and withania somnifera inhibit candida albicans and neisseria gonorrhoea. Afr. J. Biotechnol. 2008, 7. [Google Scholar] [CrossRef]
- Bisi-Johnson, M.A.; Obi, C.L.; Hattori, T.; Oshima, Y.; Li, S.; Kambizi, L.; Eloff, J.N.; Vasaikar, S.D. Evaluation of the antibacterial and anticancer activities of some South African medicinal plants. BMC Complement. Altern. Med. 2011, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Luseba, D.; Elgorashi, E.; Ntloedibe, D.; Van Staden, J. Antibacterial, anti-inflammatory and mutagenic effects of some medicinal plants used in south africa for the treatment of wounds and retained placenta in livestock. S. Afr. J. Bot. 2007, 73, 378–383. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [PubMed]
- El-Shemy, H.; Aboul-Soud, M.; Nassr-Allah, A.; Aboul-Enein, K.; Kabash, A.; Yagi, A. Antitumor properties and modulation of antioxidant enzymes’ activity by Aloe vera leaf active principles isolated via supercritical carbon dioxide extraction. Curr. Med. Chem. 2010, 17, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Salehi, B.; Stojanović-Radić, Z.Z.; Fokou, P.V.T.; Sharifi-Rad, M.; Mahady, G.B.; Sharifi-Rad, M.; Masjedi, M.R.; Lawal, T.O.; Ayatollahi, S.A.; et al. Medicinal plants used in the treatment of tuberculosis—Ethnobotanical and ethnopharmacological approaches. Biotechnol. Adv. 2017. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Li, F.; Xing, J. Separation and purification of aloe anthraquinones using PEG/salt aqueous two-phase system. Sep. Sci. Technol. 2011, 46, 1503–1510. [Google Scholar] [CrossRef]
- Sharma, A.; GAUTAM, S. An overview on medicinal properties of Aloe vera: Antibacterial & antifungal aspects. Int. J. Pharma Bio Sci. 2013, 4, 694–705. [Google Scholar]
- Schmidt, J.M.; Greenspoon, J.S. Aloe vera dermal wound gel is associated with a delay in wound healing. Obstet. Gynecol. 1991, 78, 115–117. [Google Scholar] [PubMed]
- Soeda, M. Studies on anti-bacterial and anti-fungal activities of cape aloe. Nippon Saikingaku Zasshi 1966, 21, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-W.; Yang, T.-C.; Lai, C.-C.; Huang, S.-H.; Liao, J.-M.; Wan, L.; Lin, Y.-J.; Lin, C.-W. Antiviral activity of aloe-emodin against influenza a virus via galectin-3 up-regulation. Eur. J. Pharmacol. 2014, 738, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Olatunya, O.S.; Olatunya, A.M.; Anyabolu, H.C.; Adejuyigbe, E.A.; Oyelami, O.A. Preliminary trial of Aloe vera gruel on hiv infection. J. Altern. Complement. Med. 2012, 18, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Sydiskis, R.; Owen, D.; Lohr, J.; Rosler, K.; Blomster, R. Inactivation of enveloped viruses by anthraquinones extracted from plants. Antimicrob. Agents Chemother. 1991, 35, 2463–2466. [Google Scholar] [CrossRef] [PubMed]
- Hamiza, O.; Rehman, M.; Khan, R.; Tahir, M.; Khan, A.; Lateef, A.; Sultana, S. Chemopreventive effects of aloin against 1,2-dimethylhydrazine-induced preneoplastic lesions in the colon of wistar rats. Hum. Exp. Toxicol. 2014, 33, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Pan, H.; Lou, H.; Xu, Y.; Tian, L. Inhibition of the angiogenesis and growth of aloin in human colorectal cancer in vitro and in vivo. Cancer Cell Int. 2013, 13, 69. [Google Scholar] [CrossRef] [PubMed]
- Masaldan, S.; Iyer, V.V. Exploration of effects of emodin in selected cancer cell lines: Enhanced growth inhibition by ascorbic acid and regulation of LRP1 and AR under hypoxia-like conditions. J. Appl. Toxicol. 2014, 34, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.L.; Lu, Y.C.; Su, H.L.; Lin, H.T.; Lee, C.C.; Kang, S.E.; Lai, T.C.; Chung, J.G.; Chen, S.S. Destabilization of carp mRNAs by aloe-emodin contributes to caspase-8-mediated p53-independent apoptosis of human carcinoma cells. J. Cell. Biochem. 2011, 112, 1176–1191. [Google Scholar] [CrossRef] [PubMed]
- Muto, A.; Hori, M.; Sasaki, Y.; Saitoh, A.; Yasuda, I.; Maekawa, T.; Uchida, T.; Asakura, K.; Nakazato, T.; Kaneda, T. Emodin has a cytotoxic activity against human multiple myeloma as a janus-activated kinase 2 inhibitor. Mol. Cancer Therapeut. 2007, 6, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-Y.; Lai, W.-W.; Ho, C.-C.; Yu, F.-S.; Chen, G.-W.; Yang, J.-S.; Liu, K.-C.; Lin, M.-L.; Wu, P.-P.; Fan, M.-J. Emodin induces apoptosis of human tongue squamous cancer SCC-4 cells through reactive oxygen species and mitochondria-dependent pathways. Anticancer Res. 2009, 29, 327–335. [Google Scholar] [PubMed]
- LIN, C.-C.; KAO, S.-T.; CHEN, G.-W.; CHUNG, J.-G. Berberine decreased n-acetylation of 2-aminofluorene through inhibition of n-acetyltransferase gene expression in human leukemia Hl-60 cells. Anticancer Res. 2005, 25, 4149–4155. [Google Scholar] [PubMed]
- Lin, J.-G.; Chen, G.-W.; Li, T.-M.; Chouh, S.-T.; Tan, T.-W.; Chung, J.-G. Aloe-emodin induces apoptosis in T24 human bladder cancer cells through the p53 dependent apoptotic pathway. J. Urol. 2006, 175, 343–347. [Google Scholar] [CrossRef]
- Jackson, T.; Verrier, J.; Kochanek, P. Anthraquinone-2-sulfonic acid (AQ2S) is a Novel neurotherapeutic agent. Cell Death Dis. 2014, 4, e451. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Sharifi-Rad, M.; Salehi, B.; Iriti, M.; Roointan, A.; Mnayer, D.; Soltani-Nejad, A.; Afshari, A. In vitro and in vivo assessment of free radical scavenging and antioxidant activities of Veronica persica Poir. Cell. Mol. Biol. (Noisy-le-Grand, France) 2018, 64, 57–64. [Google Scholar] [CrossRef]
- Yagi, A.; Kabash, A.; Mizuno, K.; Moustafa, S.; Khalifa, T.; Tsuji, H. Radical scavenging glycoprotein inhibiting cyclooxygenase-2 and thromboxane A2 synthase from Aloe vera gel. Planta Med. 2003, 69, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Lee, B.M. Inhibition of benzo[a]pyrene-DNA adduct formation by Aloe barbadensis miller. Carcinogenesis 1997, 18, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kacew, S.; Lee, B.M. In vitro chemopreventive effects of plant polysaccharides (Aloe barbadensis miller, Lentinus edodes, Ganoderma lucidum and Coriolus versicolor). Carcinogenesis 1999, 20, 1637–1640. [Google Scholar] [CrossRef] [PubMed]
- Buenz, E.J. Aloin induces apoptosis in jurkat cells. Toxicol. Vitro 2008, 22, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Xu, A.; Bi, Z. Effect of aloin on inducible nitric oxide synthase and nuclear factor Kappa B synthesis of hacat cells irradiated by Ultraviolet B. Chin. J. Dermatol. 2005, 38, 565–568. [Google Scholar]
- Grimaudo, S.; Tolomeo, M.; Gancitano, R.; Dalessandro, N.; Aiello, E. Effects of highly purified anthraquinoid compounds from Aloe vera on sensitive and multidrug resistant leukemia cells. Oncol. Rep. 1997, 4, 341–343. [Google Scholar] [CrossRef] [PubMed]
- Kabbash, A.; El-Soud, K.; Zalat, E.; Shoeib, N.; Yagi, A. Supercritical carbon dioxide extraction of aloe emodin and barbaloin from Aloe vera L. Leaves and their in-vitro cytotoxic activity. Saudi Pharm. J. 2008, 16, 75–81. [Google Scholar]
- Lee, K.H.; Hong, H.S.; Lee, C.H.; Kim, C.H. Induction of apoptosis in human leukaemic cell lines k562, hl60 and u937 by diethylhexylphthalate isolated from Aloe vera linne. J. Pharm. Pharmacol. 2000, 52, 1037–1041. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Kim, J.H.; Lim, D.S.; Kim, C.H. Anti-leukaemic and anti-mutagenic effects of di (2-ethylhexyl) phthalate isolated from Aloe vera linne. J. Pharm. Pharmacol. 2000, 52, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Li, T.-D.; Gu, J.-Y.; Luo, Y.; Li, J.-G. The antitumor active components from Aloe vera var. Chinesis Berg. Lishizhen Med. Mater. Med. Res. 2009, 10, 7. [Google Scholar]
- Womble, D.; Helderman, J.H. Enhancement of allo-resposiveness of human lymphocytes by acemannan (Carrisyntm). Int. J. Immunopharmacol. 1988, 10, 967–974. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, B.; Tomar, N.R.; Roy, P.; Gupta, A.K.; Kumar, A. In vivo evalution of hypoglycemic activity of Aloe spp. And identification of its mode of action on glut-4 gene expression in vitro. Appl. Biochem. Biotechnol. 2011, 164, 1246–1256. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, S.; Sivagnanam, K.; Subramanian, S. Modulatory effects of Aloe vera leaf gel extract on oxidative stress in rats treated with streptozotocin. J. Pharm. Pharmacol. 2005, 57, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Ghannam, N.; Kingston, M.; Al-Meshaal, I.A.; Tariq, M.; Parman, N.S.; Woodhouse, N. The antidiabetic activity of aloes: Preliminary clinical and experimental observations. Horm. Res. Paediatr. 1986, 24, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Franz, G. Polysaccharides in pharmacy: Current applications and future concepts. Planta Med. 1989, 55, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Muthusamy, V.; Sujatha, S.; Sangeetha, K.; Raja, R.B.; Sudhagar, S.; Devi, N.P.; Lakshmi, B. Aloe emodin glycosides stimulates glucose transport and glycogen storage through PI3K dependent mechanism in l6 myotubes and inhibits adipocyte differentiation in 3T3L1 adipocytes. FEBS Lett. 2010, 584, 3170–3178. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Vijayaraghavan, R.; Pant, S.C.; Lomash, V.; Ali, M. Aloe vera gel alleviates cardiotoxicity in streptozocin-induced diabetes in rats. J. Pharm. Pharmacol. 2010, 62, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Dana, N.; Javanmard, S.H.; Asgary, S.; Asnaashari, H.; Abdian, N. The effect of Aloe vera leaf gel on fatty streak formation in hypercholesterolemic rabbits. J. Res. Med. Sci. 2012, 17, 439. [Google Scholar] [PubMed]
- Dhingra, D.; Lamba, D.; Kumar, R.; Nath, P.; Gauttam, S. Antihyperlipidemic activity of Aloe succotrina in rats: Possibly mediated by inhibition of hmg-coa reductase. ISRN Pharmacol. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, R.; Nagar, P.S.; Nampoothiri, L. Effect of Aloe barbadensis mill. Formulation on letrozole induced polycystic ovarian syndrome rat model. J. Ayurveda Integr. Med. 2010, 1, 273. [Google Scholar] [PubMed]
- Desai, B.N.; Maharjan, R.H.; Nampoothiri, L.P. Aloe barbadensis mill. Formulation restores lipid profile to normal in a letrozole-induced polycystic ovarian syndrome rat model. Pharmacogn. Res. 2012, 4, 109. [Google Scholar]
- Bhalla, A.; Chauhan, U. Identification of antihyperlipidemic components in Aloe vera through reverse phase HPlC. J. Biol. Sci. Med. 2015, 1, 21–27. [Google Scholar]
- Babaee, N.; Zabihi, E.; Mohseni, S.; Moghadamnia, A.A. Evaluation of the therapeutic effects of Aloe vera gel on minor recurrent aphthous stomatitis. Dental Res. J. 2012, 9, 381. [Google Scholar]
- Suvitayavat, W.; Sumrongkit, C.; Thirawarapan, S.; Bunyapraphatsara, N. Effects of aloe preparation on the histamine-induced gastric secretion in rats. J. Ethnopharmacol. 2004, 90, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Agunu, A.; Diana, M. The effect of Aloe vera A. Berger (Liliaceae) on gastric acid secretion and acute gastric mucosal injury in rats. J. Ethnopharmacol. 2004, 93, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Eamlamnam, K.; Patumraj, S.; Visedopas, N.; Thong-Ngam, D. Effects of Aloe vera and sucralfate on gastric microcirculatory changes, cytokine levels and gastric ulcer healing in rats. World J. Gastroenterol. 2006, 12, 2034. [Google Scholar] [CrossRef] [PubMed]
- Ernst, E. Adverse effects of herbal drugs in dermatology. Brit. J. Dermatol. 2000, 143, 923–929. [Google Scholar] [CrossRef]
- Sorrell, J.M.; Caplan, A.I. Fibroblasts—A diverse population at the center of it all. Int. Rev. Cell Mol. Biol. 2009, 276, 161–214. [Google Scholar] [PubMed]
- Sorrell, J.M.; Caplan, A.I. Fibroblast heterogeneity: More than skin deep. J. Cell Sci. 2004, 117, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, H.; Byrne, M.; Krane, S.; Jaenisch, R. Type iii collagen is crucial for collagen I fibrillogenesis and for normal cardiovascular development. Proc. Natl. Acad. Sci. USA 1997, 94, 1852–1856. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.A.; Galgut, J.M.; Choudhary, R.K. On the novel action of melanolysis by a leaf extract of Aloe vera and its active ingredient aloin, potent skin depigmenting agents. Planta Med. 2012, 78, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yin, S.; Zhong, J.; Ding, W.; Wan, J.; Xie, Z. Mushroom tyrosinase inhibitors from Aloe barbadensis miller. Fitoterapia 2012, 83, 1706–1711. [Google Scholar] [CrossRef] [PubMed]
- Ro, J.Y.; Lee, B.C.; Kim, J.Y.; Chung, Y.J.; Chung, M.H.; Lee, S.K.; Jo, T.H.; Kim, K.H.; Park, Y.I. Inhibitory mechanism of aloe single component (Alprogen) on mediator release in guinea pig lung mast cells activated with specific antigen-antibody reactions. J. Pharmacol. Exp. Ther. 2000, 292, 114–121. [Google Scholar] [PubMed]
- Sharifi-Rad, J.; Ayatollahi, S.A.; Varoni, E.M.; Salehi, B.; Kobarfard, F.; Sharifi-Rad, M.; Iriti, M.; Sharifi-Rad, M. Chemical composition and functional properties of essential oils from Nepeta schiraziana boiss. Farmacia 2017, 65, 802–812. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M. Free Radicals in Biology and Medicine; Oxford University Press: New York, NY, USA, 2015. [Google Scholar]
- Zhang, X.-F.; Xie, L.; Liu, Y.; Xiang, J.-F.; Tang, Y.-L. Binding of the bioactive component Aloe dihydroisocoumarin with human serum albumin. J. Mol. Struct. 2008, 891, 87–92. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Wang, H.-M.; Song, Y.-L.; Nie, L.-H.; Wang, L.-F.; Liu, B.; Shen, P.-P.; Liu, Y. Isolation, structure elucidation, antioxidative and immunomodulatory properties of two novel dihydrocoumarins from Aloe vera. Bioorg. Med. Chem. Lett. 2006, 16, 949–953. [Google Scholar] [CrossRef] [PubMed]
- López, A.; de Tangil, M.S.; Vega-Orellana, O.; Ramírez, A.S.; Rico, M. Phenolic constituents, antioxidant and preliminary antimycoplasmic activities of leaf skin and flowers of Aloe vera (L.) Burm. f. (syn. A. Barbadensis Mill.) from the Canary islands (Spain). Molecules 2013, 18, 4942–4954. [Google Scholar] [PubMed]
- Botes, L.; Van der Westhuizen, F.H.; Loots, D.T. Phytochemical contents and antioxidant capacities of two aloe greatheadii var. Davyana extracts. Molecules 2008, 13, 2169–2180. [Google Scholar] [CrossRef] [PubMed]
- Asamenew, G.; Bisrat, D.; Mazumder, A.; Asres, K. In vitro antimicrobial and antioxidant activities of anthrone and chromone from the latex of aloe harlana reynolds. Phytother. Res. 2011, 25, 1756–1760. [Google Scholar] [CrossRef] [PubMed]
- Yoo, E.A.; Kim, S.D.; Lee, W.M.; Park, H.J.; Kim, S.K.; Cho, J.Y.; Min, W.; Rhee, M.H. Evaluation of antioxidant, antinociceptive, and anti-inflammatory activities of ethanol extracts from aloe saponaria haw. Phytother. Res. 2008, 22, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Cardarelli, M.; Rouphael, Y.; Pellizzoni, M.; Colla, G.; Lucini, L. Profile of bioactive secondary metabolites and antioxidant capacity of leaf exudates from eighteen aloe species. Ind. Crops Prod. 2017, 108, 44–51. [Google Scholar] [CrossRef]
- Silva, M.A.; Trevisan, G.; Hoffmeister, C.; Rossato, M.F.; Boligon, A.A.; Walker, C.I.B.; Klafke, J.Z.; Oliveira, S.M.; Silva, C.R.; Athayde, M.L. Anti-inflammatory and antioxidant effects of aloe saponaria haw in a model of uvb-induced paw sunburn in rats. J. Photochem. Photobiol. B Biol. 2014, 133, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Zandi, K.; Zadeh, M.A.; Sartavi, K.; Rastian, Z. Antiviral activity of Aloe vera against herpes simplex virus type 2: An in vitro study. Afr. J. Biotechnol. 2007, 6, 1770–1773. [Google Scholar]
- Visuthikosol, V.; Chowchuen, B.; Sukwanarat, Y.; Sriurairatana, S.; Boonpucknavig, V. Effect of Aloe vera gel to healing of burn wound a clinical and histologic study. J. Med. Assoc. Thai. 1995, 78, 403–409. [Google Scholar] [PubMed]
- Kemp, M.; Kahlon, J.; Chinnah, A.; Carpenter, R.; McAnalley, B.; McDaniel, H.; Shannon, W. In-vitro evaluation of the antiviral effects of acemannan on the replication and pathogenesis of HIV-1 and other enveloped viruses: Modification of the processing of glycoprotein precursors. Antivir. Res. 1990, 13, 83. [Google Scholar]
- Azghani, A.O.; Williams, I.; Holiday, D.B.; Johnson, A.R. A beta-linked mannan inhibits adherence of pseudomonas aeruginosa to human lung epithelial cells. Glycobiology 1995, 5, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Waihenya, R.; Mtambo, M.; Nkwengulila, G. Evaluation of the efficacy of the crude extract of aloe secundiflora in chickens experimentally infected with newcastle disease virus. J. Ethnopharmacol. 2002, 79, 299–304. [Google Scholar] [CrossRef]
- Kambizi, L.; Goosen, B.; Taylor, M.; Afolayan, A. Anti-viral effects of aqueous extracts of aloe ferox and withania somnifera on herpes simplex virus type 1 in cell culture. S. Afr. J. Sci. 2007, 103, 359–360. [Google Scholar]
- Zofou, D.; Kuete, V.; Titanji, V.P. Antimalarial and other antiprotozoal products from african medicinal plants. In Medicinal Plant Research in Africa; Elsevier: Amsterdam, The Netherlands, 2013; pp. 661–709. [Google Scholar]
- Van Zyl, R.; Viljoen, A.; Jäger, A. In vitro activity of aloe extracts against plasmodium falciparum. S. Afr. J. Bot. 2002, 68, 106–110. [Google Scholar] [CrossRef]
- Clarkson, C.; Maharaj, V.J.; Crouch, N.R.; Grace, O.M.; Pillay, P.; Matsabisa, M.G.; Bhagwandin, N.; Smith, P.J.; Folb, P.I. In vitro antiplasmodial activity of medicinal plants native to or naturalised in South Africa. J. Ethnopharmacol. 2004, 92, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Maphosa, V.; Masika, P.J.; Bizimenyera, E.S.; Eloff, J. In-vitro anthelminthic activity of crude aqueous extracts of Aloe ferox, leonotis leonurus and elephantorrhiza elephantina against haemonchus contortus. Trop. Anim. Health Prod. 2010, 42, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Nićiforović, A.; Adžić, M.; Zarić, B.; Radojčić, M. Adjuvant antiproliferative and cytotoxic effect of aloin in irradiated HELAS3 cells. Russ. J. Phys. Chem. A 2007, 81, 1463–1466. [Google Scholar] [CrossRef]
- Niciforovic, A.; Adzic, M.; Spasic, S.D.; Radojcic, M.B. Antitumor effects of a natural anthracycline analog (Aloin) involve altered activity of antioxidant enzymes in HELAS3 cells. Cancer Biol. Ther. 2007, 6, 1211–1216. [Google Scholar] [CrossRef]
- Van Wyk, B.-E. The potential of south african plants in the development of new medicinal products. S. Afr. J. Bot. 2011, 77, 812–829. [Google Scholar] [CrossRef]
- Pecere, T.; Gazzola, M.V.; Mucignat, C.; Parolin, C.; Dalla Vecchia, F.; Cavaggioni, A.; Basso, G.; Diaspro, A.; Salvato, B.; Carli, M. Aloe-emodin is a new type of anticancer agent with selective activity against neuroectodermal tumors. Cancer Res. 2000, 60, 2800–2804. [Google Scholar] [PubMed]
- Capasso, F.; Borrelli, F.; Capasso, R.; Carlo, G.D.; Izzo, A.; Pinto, L.; Mascolo, N.; Castaldo, S.; Longo, R. Aloe and its therapeutic use. Phytother. Res. 1998, 12, S124–S127. [Google Scholar] [CrossRef]
- Pecere, T.; Sarinella, F.; Salata, C.; Gatto, B.; Bet, A.; Dalla Vecchia, F.; Diaspro, A.; Carli, M.; Palumbo, M.; Palù, G. Involvement of p53 in specific anti-neuroectodermal tumor activity of aloe-emodin. Int. J. Cancer 2003, 106, 836–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.R.; Kang, C.G.; Roh, Y.S.; Son, B.W.; Choi, H.D.; Park, Y.I.; Lee, S.K.; Choi, S.W.; Chung, M.K. The glycopeptide, a promoter of thymidine uptake, from Aloe vera. Nat. Prod. Sci. 1998, 4, 62–67. [Google Scholar]
- Clementi, M.E.; Tringali, G.; Triggiani, D.; Giardina, B. Aloe arborescens extract protects IMR-32 cells against alzheimer amyloid beta peptide via inhibition of radical peroxide production. Nat. Prod. Commun. 2015, 10, 1993–1995. [Google Scholar] [PubMed]
- Tao, L.; Xie, J.; Wang, Y.; Wang, S.; Wu, S.; Wang, Q.; Ding, H. Protective effects of aloe-emodin on scopolamine-induced memory impairment in mice and H2O2-induced cytotoxicity in PC12 cells. Bioorg. Med. Chem. Lett. 2014, 24, 5385–5389. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.; Mehta, A.K.; Mediratta, P.K. Aloe vera improves memory and reduces depression in mice. Nutr. Neurosci. 2013, 16, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cao, L.; Du, G. Protective effects of Aloe vera extract on mitochondria of neuronal cells and rat brain. China J. Chin. Mater. Med. 2010, 35, 364–368. [Google Scholar]
- Bagewadi, H.G.; Rathor, N. Effect of Aloe vera on animal models of parkinson disease in mice. Int. J. Pharm. Bio. Sci. 2014, 5, 549–559. [Google Scholar]
- Rathor, N.; Arora, T.; Manocha, S.; Patil, A.N.; Mediratta, P.K.; Sharma, K.K. Anticonvulsant activity of a loe vera leaf extract in acute and chronic models of epilepsy in mice. J. Pharm. Pharmacol. 2014, 66, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.H.; Lee, S.J.; Chung, M.H.; Park, J.H.; Park, Y.I.; Cho, T.H.; Lee, S.K. Aloesin and arbutin inhibit tyrosinase activity in a synergistic manner via a different action mechanism. Arch. Pharm. Res. 1999, 22, 232. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Park, Y.I.; Lee, S.K.; Kim, J.E.; Chung, M.H. Aloesin inhibits hyperpigmentation induced by UV radiation. Clin. Exp. Dermatol. 2002, 27, 513–515. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.H.; Rosenthal, K.Y.; Cesario, L.R.; Rouw, G.A. Processed Aloe vera administered topically inhibits inflammation. J. Am. Podiat. Med. Assoc. 1989, 79, 395–397. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zhao, G.; Jia, J. Preliminary evaluation: The effects of Aloe ferox Miller and Aloe arborescens miller on wound healing. J. Ethnopharmacol. 2008, 120, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, M.N.; Ahmed, N. Effectiveness of Aloe vera gel compared with 1% silver sulphadiazine cream as burn wound dressing in second degree burns. J. Pak. Med. Assoc. 2013, 63, 225–230. [Google Scholar] [PubMed]
- Khorasani, G.; Hosseinimehr, S.J.; Azadbakht, M.; Zamani, A.; Mahdavi, M.R. Aloe versus silver sulfadiazine creams for second-degree burns: A randomized controlled study. Surg. Today 2009, 39, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Tabandeh, M.R.; Oryan, A.; Mohammadalipour, A. Polysaccharides of Aloe vera induce MMP-3 and TIMP-2 gene expression during the skin wound repair of rat. Int. J. Biol. Macromol. 2014, 65, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Lakhanpal, G.; Bhalerao, S.; Sharma, S.; Patil, H. To study the efficacy of different formulations of Aloe vera (spp. Aloe barbadensis) on wound healing in rats. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 432–440. [Google Scholar]
- Woźniak, A.; Paduch, R. Aloe vera extract activity on human corneal cells. Pharm. Biol. 2012, 50, 147–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Budai, M.M.; Varga, A.; Milesz, S.; Tőzsér, J.; Benkő, S. Aloe vera downregulates LPS-induced inflammatory cytokine production and expression of NLRP3 inflammasome in human macrophages. Mol. Immunol. 2013, 56, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Shida, T.; Yagi, A.; Nishimura, H.; Nishioka, I. Effect of aloe extract on peripheral phagocytosis in adult bronchial asthma. Planta Med. 1985, 51, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Karaca, K.; Sharma, J.; Nordgren, R. Nitric oxide production by chicken macrophages activated by acemannan, a complex carbohydrate extracted from Aloe Vera. Int. J. Immunopharmacol. 1995, 17, 183–188. [Google Scholar] [CrossRef]
- Devaraj, S.; Jialal, R.; Jialal, I.; Rockwood, J. A pilot randomized placebo controlled trial of 2 Aloe vera supplements in patients with pre-diabetes/metabolic syndrome. Planta Med. 2008, 74, SL77. [Google Scholar] [CrossRef]
- Huseini, H.F.; Kianbakht, S.; Hajiaghaee, R.; Dabaghian, F.H. Anti-hyperglycemic and anti-hypercholesterolemic effects of Aloe vera leaf gel in hyperlipidemic type 2 diabetic patients: A randomized double-blind placebo-controlled clinical trial. Planta Med. 2012, 78, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Urch, D. Aloe vera Nature’s Gift; Blackdown Publications: Bristol, UK, 1999; pp. 7–13. [Google Scholar]
- Oral ulcers remedy gets fda clearance. J. Am. Dent. Assoc. 1994, 125, 1308–1310.
- Chandrahas, B.; Jayakumar, A.; Naveen, A.; Butchibabu, K.; Reddy, P.K.; Muralikrishna, T. A randomized, double-blind clinical study to assess the antiplaque and antigingivitis efficacy of Aloe vera mouth rinse. J. Indian Soc. Periodontol. 2012, 16, 543. [Google Scholar] [PubMed]
- Grindlay, D.; Reynolds, T. The Aloe vera phenomenon: A review of the properties and modern uses of the leaf parenchyma gel. J. Ethnopharmacol. 1986, 16, 117–151. [Google Scholar] [CrossRef]

| Aloe Species | Phytochemicals | Reference |
|---|---|---|
| Leaves | ||
| A. africana | Aloesin D, aloin A, aloin B, aloinoside A, and aloinoside B | [33] |
| A. arborescens1 | Aloenin, a phenyl pyrone, aloesin, aloeresin, aloin B, aloin A | [34] |
| Aloe emodin-diglucoside, lucenion II, 6′-O-caffeoyl-5-hydroxyaloin A, vicenin II, trans-p-coumaric derivatives, 3-O-(E)-caffeoyl-4-O-feruloylquinic acid, luteolin-O-xylosylglucoside malonylated, aloeresin C isomer, isorhamnetin-3-O-deoxyhexosyl(1-6) hexoside, 7-O-methyl kaempferol dimmer, caffeoyl quinic acid hexoside, kaempferol-3-O-hexosyl-O-pentoside, orientin, 3-O-caffeoyl-5-O-coumaroylquinic acid, 4-succinyl-3,4-dicaffeoylquinic acid, 6′-malonylnataloin, cholestenol, isoquercetrin, aloinoside A/B, 3,4-di-O-(E)-p-coumaroylquinic acid, 2′-O-feruloylaloesin, 7-O-methylaloesin-penta acetate, malonyl-4,5-O-dicaffeoylquinic acid, nataloin, veracylglucan A, aloenin B, wighteone-O-diglucoside malonylate, aloin A, caffeoylester of aloesin, aloeresin E, barbaloin (10R)/isobarbaloin (10S), quercetin-7-O-hexoside-3-O-malonylhexoside, aloe-emodin-8-O-glucoside, chrysophanol-8-O-(6′-O-galloyl-) glucoside, aloeresin H, and pentahydroxyflavonol-O-hexosyl rhamnoside | [35] | |
| A. archeri | Plicataloside | [36] |
| A. babatiensis | Plicataloside | [36] |
| A. barbadensis | 8-C-b-d-[2-O-(E)-coumaroyl]glucopyranosyl[2-[2-hydroxy]propyl-7-methoxy-5-methylchromone, aloeresin D, C-glucosyl chromone, and alcohol | [37] |
| A. boscawenii | Aloin A, aloin B, aloinoside A, aloinoside B, microdontin A, and microdontin B | [33] |
| A. brachystachys1 | Aloesin, aloenin, aloin B, and aloin A | [34] |
| A. brandhamii1 | Aloesin, aloenin, aloin B, and aloin A | [34] |
| A. brevifolia | cis-p-Coumaric acid derivatives, 5-O-caffeolyquinic acid, vicenin II, luteolin-O-xylosylglucoside malonylated, aloeresin C isomer, epi-catechin digalloyl rhamnoside, isorhamnetin-3-O-deoxyhexosyl(1-6) hexoside, caffeoyl quinic acid hexoside, 4-succinyl-3,4-dicaffeoylquinic acid, nataloin, cholestenol, 2’-O-feruloylaloesin, isoaloeresin D, and aloeresin | [35] |
| A. brunneostriata | Dihydroisocoumaringlucoside, aloin A, aloin B, aloinoside A, aloinoside B, microdontin A, and microdontin B | [33] |
| A. buchlohii | Aloin A, aloin B, microdontin A, and microdontin B | [33] |
| A. bussei1 | aloesin, aloenin, aloin B, and aloin A | [34] |
| A. calidophila | Dihydroisocoumaringlucoside, aloin A, aloin B, aloinoside A, aloinoside B, microdontin A, and microdontin B | [33] |
| A. cameronii | Aloesin, aloeresin A, aloeresin D, aloin A, aloin B, aloinoside A, aloinoside B, microdontin A, and microdontin B | [33] |
| A. camperi | Dihydroisocoumaringlucoside, aloin A, aloin B, aloinoside A, aloinoside B, microdontin A, and microdontin B | [33] |
| A. canarina | Aloesin, aloeresin D, aloin A, aloin B, aloinoside A, aloinoside B, microdontin A, and microdontin B | [33] |
| A. chabaudii | Plicataloside | [36] |
| A. cheranganiensis1 | Aloesin, 7-O-methylaloesin, aloenin, aloeresin A, aloeresin D, aloin B, aloin A | [34] |
| A. chrysostachys | 7-O-Methylaloesin, aloeresin D, aloin A, aloin B, aloinoside A, aloinoside B, microdontin A, and microdontin B | [33] |
| A. classenii1 | Aloesin, aloenin, aloeresin D, aloin B, and aloin A | [34] |
| A. dawei1 | Aloesin, aloenin, aloeresin D, aloin B, and aloin A | [34] |
| A. deserti | Plicataloside | [36] |
| A. diolii | Aloin A, aloin B, aloinoside A, aloinoside B, microdontin A, and microdontin B | [33] |
| A. dorotheae1 | Aloesin, aloenin, aloin B, and aloin A | [34] |
| A. elegans | Dihydroisocoumaringlucoside, aloin A, aloin B, aloinoside A, aloinoside B, microdontin A, and microdontin B | [33] |
| A. eru | Vicenin II, 3-O-(E)-caffeoyl-4-O-feruloylquinic acid, iso pentyldihexose, apigenin-7-O-glycuronyl, aloenin, nataloin, cholestenol, isoquercetrin, aloinoside A/B, epi (afzelechin)—(epi) gallocatechin, 2′-O-feruloylaloesin, 7-methylether of 2′-feruloylaloesin, glucuronides, isoaloeresin D, aloeresin, kaempferol di deoxyhexosylhexoside, aloenin B, caffeoylester of aloesin, aloeresin E, apigenin-7-O-glycuronyl, aloe-emodin-8-O-glucoside, and 2′-p-methoxycoumaroylaloresin | [35] |
| A. ferox | cis-p-Coumaric acid derivatives, aloe emodin-diglucoside, lucenin II, vicenin II, orientin, 6’-malonylnataloin, kaempferol di deoxyhexosylhexoside, aloeresin E, quercetin pentosyl rutinoside, aloe-emodin-8-O-glucoside, and chrysophanol-8-O-(6′-O-galloyl-) glucoside | [35] |
| Aloin (A and B), aloinoside (A and B) and microdontin (A and B), aloesin, and aloeresin A | [33] | |
| A. fibrosa | Plicataloside | [36] |
| A. fleurentiniorum | Dihydroisocoumaringlucoside, aloin A, aloin B, aloinoside A, aloinoside B, microdontin A, and microdontin B | [33] |
| A. flexilifolia | Aloesin, 7-O-methylaloesin, aloeresin D, aloin A, aloin B, aloinoside A, aloinoside B, microdontin A, and microdontin B | [33] |
| A. francombei | Plicataloside | [36] |
| A. gilberti | Aloesin, 7-O-methylaloesin, aloin A, aloin B, aloinoside A, aloinoside B, microdontin A, and microdontin B | [33] |
| A. gossweileri1 | Aloesin, aloenin, aloin B, and aloin A | [34] |
| A. grandidentata | Aloesin, aloe emodin-diglucoside, caffeoyl ferulic acid derivatives, chrysoeriol-7-O-glycuronyl, lucenin II, 6′-O-caffeoyl-5-hydroxyaloin A, vicenin II, 3-O-(E)-caffeoyl-4-O-feruloylquinic acid, luteolin-O-xylosylglucoside malonylated, isorhamnetin-3-O-deoxyhexosyl(1-6) hexoside, kaempferol-3-O-hexosyl-O-pentoside, orientin, isoorientin, 5-hydroxyaloin A, nataloin, cholestenol, aloinoside A/B, 3,4-di-O-(E)-p-coumaroylquinic acid, epi(afzelechin)-(epi)gallocatechin, 2′-O-feruloylaloesin, 7-methylether of 2′-feruloylaloesin, isoaloeresin D, aloeresin, nataloin, aloenin B, aloin A, aloin B, hydroxy octadecenic acid, trihydroxycinnamic acid derivatives, aloeresin E, acetyl dicaffeoylquinic acid, and kaempferol-3-O-malonylhexoside | [35] |
| A. guillaumetii | 7-O-Methylaloesin, aloin A, aloin B, aloinoside A, and aloinoside B | [33] |
| A. harlana | Aloin A, aloin B, aloinoside A, aloinoside B, microdontin A, and microdontin B | [33] |
| A. hemmingii | Aloesin, 8-O-methyl-7-hydro-xyaloin, aloin A, aloin B, aloinoside A, and aloinoside B | [33] |
| A. kedongensis1 | Aloesin, 7-O-methylaloesin, aloenin, nataloin B, and nataloin A | [34] |
| A. labworana | Plicataloside | [36] |
| A. leachii1 | Aloesin, aloenin, aloeresin D, aloin B, and aloin A | [34] |
| A. lensayuensis | Aloin A, aloin B, aloinoside A, aloinoside B, microdontin A, and microdontin B | [33] |
| A. leptosiphon1 | Aloesin, aloenin, aloin B, and aloin A | [34] |
| A. mcloughlinii | Aloesin, dihydroisocoumaringlucoside, aloeresin D, aloin A, aloin B, aloinoside A, and aloinoside B | [33] |
| A. megalacantha | Dihydroisocoumaringlucoside, aloin A, aloin B, aloinoside A, aloinoside B, microdontin A, and microdontin B | [33] |
| A. microdonta | Aloin A, aloin B, aloinoside A, aloinoside B, microdontin A, and microdontin B | [33] |
| A. monticola1 | Aloesin, aloenin, aloeresin D, aloin B, and aloin A | [34] |
| A. morijensis | Plicataloside | [36] |
| A. multicolor | Plicataloside | [36] |
| A. murina | Plicataloside | [36] |
| A. ngongensis | Aloeresin D, aloin A, aloin B, aloinoside A, aloinoside B, microdontin A, and microdontin B | [33] |
| A. nyeriensis1 | Nataloe-emodin, nataloe-emodin-2-O-Glc, nataloin, aloenin, aloenin aglycone, and aloenin-2″-p-coumaroyl ester | [38] |
| A. otallensis | Plicataloside | [36] |
| A. palmiformis | Plicataloside | [36] |
| A. parvidens | Plicataloside | [36] |
| A. peckii | Aloesin, dihydroisocoumaringlucoside, aloeresin D, aloin A, aloin B, aloinoside A, and aloinoside B | [33] |
| A. peglerae | Aloesin, aloeresin E, aloeresin F, homonataloin A, and homonataloin B | [39] |
| A. penduliflora | 7-O-Methylaloesin, dihydroisocoumaringlucoside, aloin A, aloin B, aloinoside A, and aloinoside B | [33] |
| A. perfoliata | Aloesin, 1-hexanol-pentosylhexoside, 3-O-(E)-caffeoyl-4-O-feruloylquinic acid, luteolin-O-xylosylglucoside malonylated, aloeresin C isomer, epi-catechin digalloyl rhamnoside, 7-O-methyl kaempferol dimmer, orientin, 3-O-caffeoyl-5-O-coumaroylquinic acid, 4-succinyl-3,4-dicaffeoylquinic acid, 5-hydroxyaloin A, aloinoside A/B, epi (afzelechin)—(epi)gallocatechin, 7-O-Mmethylaloesin-penta acetate, glucuronides, isoaloeresin D, isovitexin, 6′-O-coumaroyl aloesin, aloeresin A isomer, caffeoylester of aloesin, aloeresin E, acetyl dicaffeoylquinic acid, quercetin-7-O-hexoside-3-O-malonylhexoside, aloe-emodin-8-O-glucoside, 2′-p-methoxycoumaroylaloresin, aloeresin H, pentahydroxyflavonol-O-hexosyl rhamnoside, and kaempferol-3-O-malonylhexoside | [35] |
| A. plicatilis | Plicataloside | [36] |
| A. pustuligemma | Plicataloside | [36] |
| A. rabaiensis | Aloeresin D, aloin A, aloin B, aloinoside A, and aloinoside B | [33] |
| A. rivae | Dihydroisocoumaringlucoside, aloin A, aloin B, aloinoside A, aloinoside B, microdontin A, and microdontin B | [33] |
| A. rugosifolia | Plicataloside | [36] |
| A. saponaria | cis-p-Coumaric acid derivatives, 3,4-di-O-(E)-caffeoylquinic acid, malonyl-3,4-O-dicaffeoyl quinic acid, lucenin II, luteolin-O-xylosylglucoside malonylated, isorhamnetin-3-O-deoxyhexosyl(1-6) hexoside, 4-succinyl-3,4-dicaffeoylquinic acid, 2′-O-feruloylaloesin, 7-O-methylaloesin-penta acetate, 7-methylether of 2′-feruloylaloesin, trihydroxy octadecenoic acid, quercetin-7-O-hexoside-3-O-malonylhexoside, aloe-emodin-8-O-glucoside, 2′-p-methoxycoumaroyl-aloresin, aloeresin H, and tetra-O-methyl ether | [35] |
| A. scabrifolia | Dihydroisocoumaringlucoside, aloin A, aloin B, aloinoside A, aloinoside B, microdontin A, and microdontin B | [33] |
| A. schelpei | 8-O-Methyl-7-hydroxyaloin, aloin A, aloin B, microdontin A, and microdontin B | [33] |
| A. schweinfurthii | Plicataloside | [36] |
| A. scobinifolia | Dihydroisocoumaringlucoside, aloin A, aloin B, aloinoside A, aloinoside B, microdontin A, and microdontin B | [33] |
| A. secundiflora | Aloenin, aloenin B, isobarbaloin (aloin B), barbaloin (aloin A), aloinside A, aloinside B, aloesin derivative, and an unidentified mixture of dimers | [40] |
| A. secundiflora1 | Aloenin, aloin B, and aloin A | [34] |
| A. sinkatana | 8-O-Methyl-7-hydroxyaloin, aloin A, aloin B, aloinoside A, and aloinoside B | [33] |
| A. somaliensis | Dihydroisocoumaringlucoside, aloin A, aloin B, aloinoside A, aloinoside B, microdontin A, and microdontin B | [33] |
| A. steudneri | Dihydroisocoumaringlucoside, 8-O-methyl-7-hydroxyaloin, aloin A, aloin B, microdontin A, and microdontin B | [33] |
| A. tewoldei | Dihydroisocoumaringlucoside, aloin A, aloin B, aloinoside A, aloinoside B, microdontin A, and microdontin B | [33] |
| A. tormentorii | Phenols, saponins, tannins, alkaloids, anthraquinones, terpenes, coumarins and flavonoids | [41] |
| A. tororoana1 | 7-O-Methylaloesin, aloenin, aloin B, and aloin A | [34] |
| A. tugenensis | Plicataloside | [36] |
| A. tweediae | Dihydroisocoumaringlucoside, aloeresin D, aloin A, aloin B, aloinoside A, and aloinoside B | [33] |
| A. ukambensis | Plicataloside | [36] |
| A. vera | cis-p-Coumaric acid derivatives, malonyl-3,4-O-dicaffeoyl quinic acid, lucenin II, 6′-O-caffeoyl-5-hydroxyaloin A, vicenin II, trans-p-coumaric derivatives, luteolin-O-xylosylglucoside malonylated, aloeresin C isomer, isorhamnetin-3-O-deoxyhexosyl(1-6) hexoside, 7-O-methyl kaempferol dimmer, caffeoyl quinic acid hexoside, orientin, isoorientin, 3-O-caffeoyl-5-O-coumaroylquinic acid, 6’-malonylnataloin, aloinoside A/B, 7-O-methylaloesin-penta acetate, malonyl-4,5-O-dicaffeoylquinic acid, nataloin, aloenin B, wighteone-O-diglucoside malonylate, aloin A, aloin B, aloeresin E, barbaloin (10R)/isobarbaloin (10S), quercetin-7-O-hexoside-3-O-malonylhexosidea, and aloe-emodin-8-O-glucoside | [35] |
| A. wredfordii | Plicataloside | [36] |
| Aloe Species | Phytochemicals | Reference |
|---|---|---|
| Leaves | ||
| A. albiflora | Trimethylsilyl ether 2-hexanol, benzene acetaldehyde, lactic acid, benzyldimethylsilyl ester hydrocinnamic acid, 2,4-dimethyl benzaldehyde, 2-ethyl phenol, trimethylsilyl ether 9-decen-1-ol, trimethylsilyl ester benzene acetic acid, trimethylsilyl ester nonanoic acid, 2-methoxy-3-(2-propenyl)-phenol, 3-(2-trimethylsilyloxyethyl)-phenol, methyleugenol, phenyl 1,2-ethanediol, 2,4-bis(1,1-dimethylethyl)-phenol, 2-methyl-1-hexadecanol, nonadecane, 1-methylethyl ester dodecanoic acid, lauric acid, β-bisabolol, 2,6,10-trimethyl-tetradecane, tert-hexadecanethiol, tetradecanoic acid, pentadecanoic acid, ethyl ester cholestenol acid, trimethylsilyl ester cis-9-hexadecenic acid, palmitic acid, cis-13-eisosenoic acid, heptadecanoic acid, ethyl 9,12,15-octadecatrienoate, linoleic acid, α-linolenic acid, octadecanoic acid, eicosanoic acid, squalene, and β-sitosterol | [42] |
| A. aristata | Benzeneacetaldehyde, lactic acid, 2-methyl-2-indecanethiol, 2,5-dimethyl-benzaldehyde, 2-ethyl-phenol, trimethylsilyl ether 3-ethylphenol, p-ethylguaiacol, phosphoric acid, tridecane, (E)-3-eicosene, 2-methyl-1-hexadecanol, 2,6,11-trimethyl-dodecane, eugenol, vanillin, methyleugenol, hexadecane, 2,4-bis(1,1-dimethylethyl)-phenol, nonadecane, 1-methlethyl ester dodecanoic acid, lauric acid, 2,6,10-trimethyl tetradecane, 2,6,10,15-tetramethyl heptadecane, trimethylsilyl ester myristic acid, pentadecanoic acid, ethyl palmitate, trimethylsilyl ester cis-9-hexadecenoic acid, palmitic acid, trimethylsilyl ester cis-10-heptadecenoic acid, heptadecanoic acid, ethyl ester 9,12-octadecadienoic acid, ethyl 9,12,15-octadecatrienoate, linoleic acid, α-linolenic acid, octadecanoic acid, α-amyrin, squalene, ethyl iso-allocholate, and β-sitosterol | [42] |
| A. aspera | Trimethylsilyl ether 2-pentanol, m-pyrol, lactic acid, 3,5-dimethyl-benzaldehyde, benzoic acid, succinic acid, fumaric acid, nonanoic acid, tetradecane, trimethylsilyl ester decanoic acid, 2,4-bis(1,1-dimethylethyl)-phenol, 2,3,5,8-tetramethyl-decane, 2-methyl-1-hexadecanol, hexadecane, 1-methylethyl ester dodecanoic acid, lauric acid, nonadecane, 2,6,10,15-tetramethyl-heptadecane, 2,6,10-trimethyl-tetradecane, azelaic acid, isopropyl ester myristic acid, tetradecanoic acid pentadecanoic acid, trimethylsilyl ester cis-9-hexadecenoic acid, palmitic acid, heneicosane, heptadecanoic acid, linoleic acid, trimethylsilyl ester oleic acid, octadecanoic acid, eicosanoic acid, trimethylsilyl ester 1-docosanol, docosanoic acid, squalene, trimethylsilyl ester tetracosanoic acid, γ-tocopherol, ethyl iso-allocholate, 1-heptatriacotanol, campesterol, stigmasterol, β-sitosterol | [42] |
| A. excelsa | Limonene, carvone, and 2-phenylacetonitrile | [43] |
| A. ferox | Polyphenols/phenolic compounds (phenol, gentisic, cholestenol, homovanilic, O-hydroxycinnamic, protocatechuic, 3,4-dihydroxyphenylacetic, 5-methoxyprotocatechuic, syringic, sinapic, p-coumaric, caffeic, isoferulic, ferulic, 4-methoxycinnamic, aloe emodin, 4-phenyllactic, 4-ethylphenol, p-toluic, hydrocinnamic, p-salicylic, benzoic, mandelic, hydroxyphenylacetic, pyrocatechuic, hydro-p-coumaric, and 6,7-dihydroxycoumarin); organic acids (isovaleric, lactic, glycolic, furoic, 3-hydroxypropionic, 2-hydroxyvaleric, cyclohexanone-3-carboxylic, 3-hydroxyisovaleric, 3-methyl-1,3-hydroxybutanoic, 2-hydroxycaproic, 2-ketoisovaleric, succinic, 2-methylsuccinic, methylmalic, malic, 3,4,5-trihydroxypentanoic, d-ribonic, suberic, 3-hydroxypicolinic, and isonicotinic); fatty acids (lauric, myristic, cholestenol, palmitoleic, palmitic, stearic, linoleic, oleic, linolenic, erucic, cholestenol, arachidic, heneicosanoic, behenic, tricosanoic, lignoceric and pentacosanoic); alkaloids (hypoxanthine and xanthine); indoles (indole-5-acetic acid, and indole-3-acetic acid); pyrimidines (uracil and thymine); alkanes (1,3-dihydroxybutane); sterols (cholestenol, campestrol, â-sitosterol, and stigmasterol); dicarboxylic acids (azelaic and undecanedioic), and ketones (4,6-dimethyl-2-heptanone, acetophenone, and 2,4-dimethyl-4-heptanone) | [44] |
| A. jucunda | Benzaldehyde, lactic acid, 2-ethyl phenol, benzoic acid, ester octanoic acid, phenylacetic acid, 4-ethyl-1,2-benzene, dimethoxy-benzaldehyde, 4-vinylveratrole, eugenol, tetradecane, methyleugenol, 2-allyl-1,4-dimethoxy-3-methyl-benzene, nonadecane, 3,5-bis(1,1-dimethylethyl)-phenol, 1,2-dimethoxy-4-(2-methoxyethenyl)benzene, 1-dodecanol, estragole, dodecanoic acid, 1-methylethyl ester, 4-hydroxybenzoic acid lauric acid, β-bisabolol, 2,6,10-trimethyl-tetradecane, geranyl isovalerate, tetradecanoic acid, 3,5-bis(1,1-dimethylethyl)-4-benzoic acid, methyl ester hexadecenoic acid, 2,4,6-tris(1,1-dimethylethyl)-phenol, ethyl palmitate, trimethylsilyl ester palmitelaidic acid, palmitic acid, methyl ester linolenic acid, heptadecanoic acid, ethyl ester 9,12-octadecadienoic acid, ethyl 9,12,15-octadecatrienoate, ethyl ester stearic acid, linoleic acid, α-linolenic acid, octadecanoic acid, ester eicosanoic acid, bumetrizole, trimethylsilyl ester cis-13-docosenoic acid, ester docosanoic acid, squalene, heptacosane, ethyl iso-allocholate, and β-amyrin | [42] |
| A. vera | Debocane, 4-methyl, tricosane, 6-hydroxyhexane-3-1, 1-dodecanol, 1-octadecanol, cholestenol acid, 9-octadecenoic acid, octadecanoic acid, 1-(phenylthioxomethyl)piperidine, docosane, sitosterol, and stigmasterol | [45] |
| Phenolic acids or polyphenols (phenol, cholestenic acid, homovanilic acid, protocatechuic acid, 3,4-dihydroxyphenylacetic acid, 5-methoxyprotocatechuic acid, and syringic acid. Sinapic acid, p-coumaric acid, isoferulic acid, ferulic acid, aloe emodin, 4-phenyllactic acid, 4-ethylphenol, hydrocinnamic acid, p-salicylic acid, benzoic acid, and hydro-p-coumaric acid); alcohols (2-butanol, glycerol, and phenylethanol); aldehydes (benzaldehyde and m-tolualdehyde); organic acids (lactic acid, glycolic acid, pyruvic acid, furoic acid, phosphoric acid, succinic aid, 2-methylsuccinic acid, malicnaicd, tartaric acid, and isonicotinic acid); alkanes (1,3-dihydroxybutane); pyrimidines (uracil and thymine) fatty acids (lauric acid, myristic acid, palmitoleic acid, and linoleic acid) indoles (indole-3-acetic acid); alkaloids (hypoxanthine); ketones (acetophenone); sterols (cholestenol and β-sitosterol); dicarboxylic acids (azelaic acid and undecanedioic acid) | [6] | |
| Ethylene glycol, propylene glycol, 2,3-bis(trimethylsiloxy)-butane, glycolic acid, 6-methyl-octadecane, 4-ethylbenzaldehyde, 4-hydroxybutyric acid, benzoic acid, (±)-2-Hydroxyoctanoic acid, octanoic acid, succinate, methyl succinic acid, glyceric acid, fumaric aicd, nonanoic acid, 2-methoxy-3-(2-prophenyl)-phenol, teradecane, methyleugenol, glutarate, 4-allyl-2-methoxyphenoxy-i-dodecanol, β-copaene, malic acid, adipic acid, 2-methyl-1-hexadecanol, bis(trimethylsilyl-pyroglutamic acid, m-hydroxybenzoic acid, pimelic acid, 1-methylethyl ester dodecanoic acid, 4-hydroxybenzoic acid, lauric acid, 1-[(trimethylsilyl)oxy]-2-methylanthraquinone, suberic acid, geranyl isovalerate, 2,6,10-timethyl tetradecane, bis(trimethylsilyl) ester 1,3-benzenedicarboxylic acid, azelaic acid, protocatechuic acid, tetradecanoic acid, sebacic acid, pentadecanoic acid, 2,4,6-tris(1,1-dimethylethyl phenol, undecadioic acid, trimethylsilyl ester palmitelaidic acid, palmitic acid, phytol, (2E)-3,7,11,15-tetramethyl-2-hexadecenyl trimethylsilyl ether, dodecanedioic acid, linoleic acid, α-linolenic acid, octadecanoic acid, eicosanoic acid, 2-monopalmitoylglycerol trimethylsilyl ester, 1-monopalmitin trimethylsilyl ester, docosanoic acid, 9-octadecenoic acid, 1,3-bis-(OTMS)-2-propyl ester, squalene, trimethylsilyl ester tetracosanoic acid, heptacosane, O-trimethylsilyl-(+)-α-tocopherol, β-sitosterol and β-amyrin | [42] |
| Aloe Species | Phytochemicals | Reference |
|---|---|---|
| Leaves | ||
| A. bakeri | Dihydroisorhamnetin | [46] |
| A. bellatula | Flavonoids | [46] |
| A. boylei | Isovitexin | [46] |
| A. chortolirioides var. chortolirioides | Aloesin, nataloin A and B, and 7-hydroxyaloin | [46] |
| A. chortolirioides var. wooliana | Isovitexin, aloesin, aloin A and B, and 7-hydroxyaloin | [46] |
| A. christianii | Homonataloin A and B, aloeresin A, and nataloin A and B (anthrones) | [46] |
| A. ciliaris | Isovitexin and aloeresin A | [46] |
| A. commixta | Isovitexin | [46] |
| A. ecklonis | Isovitexin | [46] |
| A. glauca | Isovitexin, trace of dihydroisorhamnetin, and aloesin (a type of chromone) | [46] |
| A. hlangapies | Isovitexin | [46] |
| A. humilis | Isovitexin, dihydroisorhamnetin | [46] |
| A. inconspicua | Isovitexin | [46] |
| A. inyangensis | Isovitexin | [46] |
| A. kraussii | Isovitexin | [46] |
| A. linearifolia | Isovitexin | [46] |
| A. lineate | Dihydroisorhamnetin | [46] |
| A. macra | Phenols, saponins, tannins, alkaloids, anthraquinones, terpenes, coumarins, and flavonoids in traces amount in comparison to A | [41] |
| A. minima | Isovitexin | [46] |
| A. nubigena | Isovitexin | [46] |
| A. parviflora | Isovitexin | [46] |
| A. polyphylla | Isovitexin and nataloin A and B | [46] |
| A. pratensis | Aloesin | [46] |
| A. pretoriensis | Isovitexin and dihydroisorhamnetin | [46] |
| A. purpurea | 3-O-caffeoylquinic acid, aloesin, 4-O-p-coumaroylquinic acid, isoorientin pentoside, vitexin/isovitexin hexoside, vitexin/isovitexin pentoside, vitexin/isovitexin, aloin, 2″-O-trans-p-coumaroylaloenin, aloin B, aloeresin A, malonylnataloin, and aloeemodin dianthrone di-O-hexoside | [47] |
| A. saundersiae | Isovitexin | [46] |
| A. soutpansbergensis | Isovitexin | [46] |
| A. striatula | Aloeresin A and homonataloin A and B (chromones) | [46] |
| A. suprafoliata | Isovitexin, aloin A and B, and nataloin A and B | [46] |
| A. suzannae | Isovitexin, apigenin, and naringenin | [46] |
| A. tenuior | Isovitexin and homonataloin A and B | [46] |
| A. thompsoniae | Isovitexin | [46] |
| A. thorncroftii | Isovitexin and dihydroisorhamnetin | [46] |
| A. tidmarshii | Isovitexin | [46] |
| A. vaotsanda | Naringenin, dihydroisorhamnetin, aloesin, aloin A and B | [46] |
| A. verecunda | Isovitexin | [46] |
| A. vossii | Isovitexin | [46] |
| Aloe Species | Investigated Methods | Phytochemicals | Reference |
|---|---|---|---|
| Leaves | |||
| A. adigratana | Solvent increasing polarity-gel extraction | Alkaloids, flavonoids, tannins, polyphenolic, glycosides, terpenoids, steroids, carbohydrates, amino acids, and proteins | [48] |
| A. arborescens | TLC pre-coated plates | Barbaloin, aloeresin, and aloenin | [28] |
| Colorimetric assay, triple quadrupole and time-of-flight mass spectrometry, UPLC/Q-ToF high resolution mass spectrometry | Chromones (aloesin, aloesone, 8-C-glucosyl-noreugenin, aloeresin E, and 7-hydroxy-2,5-dimethylchromone); anthrones (aloin, aloe-barbendol, and aloesaponarin II); phenolic naphthalene (feroxidin); phenolic dimer (feralolide); flavonoids (naringenin, isovitexin, isorhamnetin, daidzenin, and genistein), and hydroxycinnamic acids (feruloylquinic acid, sinapic acid, chlorogenic acid, ferulic acid, and caffeic acid) | [49] | |
| Phytochemical screening | Flavonoids, terpeneoids, and aromatic compounds | [50] | |
| A. barbadensis | Colorimetric assay | Glucose, galactose, mannose, and arabinose | |
| GC-IT-MS; UPLC-Q-ToF-MS | Alanine, valine, succinic acid, arabitol, malic acid, pyroglutamic acid, aspartic acid, γ-aminobutyric acid, arabinose, fructose, glucose, glucuronic acid, sucrose, aloesin, homonataloside, 7-hydroxy-8-O-methylaloin, 7-hydroxyaloin B, 7-hydroxyaloin A, nataoemodin, aloeresin A, aloin B, isoaloeresin D, 7-O-methylaloeresin A, aloin A, 6′-malonylnataloin B, and 6′-malonylnataloin A | [51] | |
| Recrystallization, semi-preparative HPLC, or column chromatography | Chrysophanol, aloe-emodin, 7-hydroxy-2,5-dimethylchromone, 5-(hydroxymethyl)-7- methoxy-2-methyl chromone, saiko-chromone A, 5-((4E)-2′-oxopentenyl)-2-hydroxymethylchromone, 7-hydroxy-5-(hydroxymethyl)-2-methylchromone, aloenin aglycone, 5-((S)-2′-oxo-4′-hydroxypentyl)-2-hydroxymethylchromone, aloenin-2′-p-coumaroyl ester, 10-hydroxyaloin B, 10-hydroxyaloin A, isoaloeresin D, aloin B and A, aloesin, 8-C-glucosyl-I-aloesol, 8-C-glucosyl-7-O-methyl-(S)-aloesol, 10-O-β-d-glucopyranosyl aloenin, 5-((S)-2′-oxo-4′-hydroxypentyl)-2-(β-glucopyranosyl-oxy-methyl)-chromone, and aloenin B | [52] | |
| LCMS-IT-TOF; HPLC-DAD | Chromones (aloesin, 8-C-glucosyl-I-aloesol, 8-C-glucosyl-7-O-methyl-(S)-aloesol, isoaloeresin, 5-((S)-2′-oxo-4′-hydroxypentyl)-2-(β-glucopyranosyl-oxy-methyl) chromone, and 5-((S)-2′-oxo-4′-hydroxypentyl)-2-methoxychromone); phenyl pyrones (10-O-β-d-glucopyranosyl aloenin and aloenin-2′-p-coumaroyl ester); anthrones (aloin A and aloe-emodin), and naphthalene derivative (aloveroside B), aloesin, 8-C-glucosyl-I-aloesol, 8-C-glucosyl-7-O-(S)-methyl-aloesol, 10-O-β-d-clucopyranosyl aloenin, 5-((S)-2′-oxo-4′- hydroxypentyl-2(β-glucopyranosyl-oxy-methyl)chromone, 5-((S)-2′-oxo-4′-hydroxypentyl)-2-methoxy chromone, aloenin, 10-hydroxyaloin B, 10-hydroxyaloin A, aloveroside B, aloenin B, isoaloeresin D, aloin B, aloin A, aloenin-2′-p-coumaroyl ester, (E)-2-acetonyl-8-[(2″-O-cinnamoyl)-β-d-glucopyranosyl-7-methoxy-5-methylchromone, aloinoside B, aloinoside A, (E)-2-((S)-2-hydroxypropyl)-8-(2′-O-OCH3-cinnamoyl)-β-d-glucopyranosyl-7-methoxy-5-methyl-chromone, and aloe-emodin | [53] | |
| Phytochemical screening | Alkaloids, terpenoids, steroids, flavonoids, tannins, and reducing sugars | [54] | |
| Colorimetric assay, triple quadrupole and time-of-flight mass spectrometry, UPLC/Q-ToF high-resolution mass spectrometry | Chromones (aloesin, aloesone, 8-C-glucosyl-noreugenin, and aloeresin E); anthrones (aloin, aloe-barbendol, and aloesaponarin II); phenolic naphthalene (feroxidin); phenolic dimer (feralolide); flavonoids (isovitexin and isorhamnetin; isoflavones (daidzein and genistein); hydroxycinnamic acids (chlorogenic acid, ferulic acid, and caffeic acid) | [49] | |
| A. calidophila | TLC, IR, MS, 1H NMR, and 13C NMR | Aloinoside, aloin, and microdontin | [55] |
| A. ferox | VLC fractionation, silica gel chromatography | Aloe emodin, chrysophanol, and aloin A | [56] |
| Solvent partitioning and chromatography | Aloe-emodin, p-hydroxybenzaldehyde, p-hydroxyacetophenone, pyrocatechol, 10-oxooctadecanoic acid, 10-hydroxyoctadecanoic acid, methyl 10-hydroxyoctadecanoate, 7-hydroxy-2,5-dimethylchromone, furoaloesone, and 2-acetonyl-8-(2-furoylmethyl)-7-hydroxy-5-methylchromone | [57] | |
| Phytochemical screening | Flavonoids, condensed tannins, and gallotannins | [58] | |
| Phytochemical screening | Phenols, flavonoids, flavonols, proanthocyanidins, tannins, alkaloids, and saponins | [59] | |
| Fractionation, chromatography | Aloe emodin, aloin A, aloin B, desoxyaloin, aloinoside B, aloinoside C, aloinoside D, aloenin aglycone, feroxidin, 7-hydroxy-5-(hydroxymethyl)-2-methylchromone, 5-methylresorcinol, aloe resin D, 7-O-methylaloesinol, aloeresin G, C-2′-decoumaroylaloeresin G, 5-((S)-2′-oxo-4′-hydrosypentyl)-2-hydroxymethylchromone, aloveroside A, and aloenin B | [60] | |
| A. greatheadii var. davyana | Solvent fractionation, GC-MS | Alkaloids (hypoxanthine), polyphenols/phenolic compounds (phenol, 4-ethylphenol, cholestenol, homovanilic, gentisic, 6,7-dihydroxycoumaric, o-hydroxycinnamic, protocatechuic, 3,4-dihydroxyphenylacetic, syringic, sinapic, caffeic, isoferulic, ferulic, benzoic, phenylacetic, 2-methoxybenzoic, o-toluic, phenylpropionic, 4-phenyllactic, 4-hydroxybenzoic, 2,3-dihydrobenzoic, 4-hydroxyphenylacetic, hydro-p-coumaric, and p-coumaric); phytosterols (cholestenol, campesterol, â-sitosterol, and stigmasterol) | [44] |
| A. lomatophyllloides | LC-UV-MS/MS | 3-O-Caffeoylquinic acid, 4-O-p-coumaroylquinic acid, isoorientin pentoside, isoorientin, vitexin/isovitexin hexoside, vitexin/isovitexin pentoside, vitexin/isovitexin, aloin or nataloin isomer, aloin B, aloin A, aloeresin A, malonylnataloin, and aloeemodin dianthrone di-O-hexoside | [47] |
| A. macra | LC-UV-MS/MS | 3-O-Caffeoylquinic acid, aloesin, 4-O-p-coumaroylquinic acid, vitexin/isovitexin hexoside, isoorientin pentoside, isoorientin, vitexin/isovitexin hexoside, vitexin/isovitexin pentoside, vitexin/isovitexin, aloin, 2″-O-trans-p-coumaroylaloenin, aloin B, aloin A, aloeresin A, and malonylnataloin | [47] |
| A. marlothii | FCC, TLC | 7-O-methylaloeresin A, 5-hydroxyaloin A 6′-O-acetate, and 5-hydroxyaloin A | [61] |
| A. rupestris | FCC, TLC | 7-O-methylaloesin and aloesin | [61] |
| A. sabaea | Phytochemical screening | Glycoprotein | [62] |
| A. striata | Phytochemical screening | Flavonoids, terpeneoids, and aromatic compounds | [50] |
| A. tormentorii | LC-UV-MS/MS | Aloesin, 4-O-p-coumaroylquinic acid, vitexin/isovitexin hexoside, isoorientin pentoside, isoorientin, vitexin/isovitexin hexoside, vitexin/isovitexin pentoside, vitexin/isovitexin, aloin or nataloin isomer, 2-O-trans-p-coumaroylaloenin, aloin B, aloin A, aloeresin A, malonylnataloin, aloeemodin dianthrone di-O-hexoside, and microdontin A or B | [47] |
| A. trichosantha | TLC | Aloin A/B and aloin-6′-O-acetate A/B | [63] |
| A. vera | Phytochemical screening | Steroids, terpenoids, carotenoids, anthraquinones, catechin, and tannins | [64] |
| Phytochemical analysis | Alkaloids, glycosides, reducing sugars, phenolic compounds, steroids, terpenoids, flavonoids, tannins, and saponin glycosides | [65] | |
| LC-UV-MS/MS | 3-O-Caffeoylquinic acid, aloesin, 4-O-p-coumaroylquinic acid, vitexin/isovitexin hexoside, isoorientin, vitexin/isovitexin hexoside, vitexin/isovitexin pentoside, vitexin/isovitexin, aloin, 2″-O-feruloylaloesin, 7-O-methylaloeresin A, aloin B, aloin A, malonylnataloin, and microdontin | [47] | |
| Chromatography | Aloeresin G, isoaloeresin D, aloeemodin, babarloin A, 8-O-methyl-7-hydroxyaloin B, elgonica-dimer A, elgonica-dimer B, feralolide, hopan-3-ol, β-sitosterol, and daucosterol | [66] | |
| Solvent fractionation, TLC, GC-MS | Pyrocatechol, cinnamic acid, p-coumaric acid, and ascorbic acid | [67] | |
| Roots | |||
| A. pulcherrima | Fractionation, column chromatography | Chrysophanol, aloesaponarin II, and aloesaponarin I | [68] |
| A. megalacantha | Column chromatography | Chrysophanol, helminthosporin, and methyl 26-O-feruloyl-oxyhexacosanoate; asphodelin, aloesaponarin III, and 10-(chrysophanol-70-yl)-10-hydroxychrysophanol-9-anthrone; aloesaponarin I, aloesaponarin II, 4,7-dihydroxy-5-methylcoumarin, 1,8-dimethoxynepodinol, and aloe emodin; 10-O-methylchrysalodin and chrysalodin, aloesaponol | [69] |
| Whole Plant | |||
| A. turkanensis | Phytochemical screening | Tannins, anthraquinones, terpenoids/steroids, saponins, and alkaloids | [70] |
| A. barberae | TLC | Aloin and chrysophanol | [71] |
| Leaves and roots | |||
| A. arborescens var. natalensis | Silica gel chromatography, TLC, GC-MS | Aloe-emodin, barbaloin, 2″,-O-feruloylaloesin, aloenin, aloesin, succinic acid, d-glucose, fatty acid methyl esters, n-triacontanol, n-dotriacontanol, and â-sitosterol | [72] |
| Aloe Species | Phytochemicals | Reference |
|---|---|---|
| Leaves | ||
| A. aageodonta | 7-O-Methylaloesin, aloeresin D, aloin A, aloin B, aloinoside A, aloinoside B, microdontin A, and microdontin B | [33] |
| A. barbadensis | Cycloartenol, 24-methylene-cycloartanol, lophenol, 24-methyl-lophenol, and 24-ethyl-lophenol | [73,74,75] |
| Anthraglycosides, sugars, cardiotonic glycosides, mucilages, pectin, sterols type Δ5, anthraquinones, saponins, sterols, and triterpenoids | [76] | |
| A. castanea | 6′-O-Coumaroylaloesin | [77] |
| A. claviflora | Oxanthrone, 10-hydroxyaloin B 6′-O-acetate | [78] |
| A. excelsa | Aloe-emodin and aloin A | [79] |
| Aloctin A and aloctin B | [80] | |
| 1,8-Dihydroxy-3-methylanthracenedione (chrysophanol), | [79] | |
| A. ferox | Aloeresin D (a C-glucosylated 5-methylchromone), feroxidin (1-methyltetralin derivative), and feralolide (a dihydroisocoumarin) | [81,82,83] |
| 5-Hydroxy-3-methylnaphtho[2,3-c]furan-4(9H)-one, 5-hydroxy-3-methylnaphtho[2,3-c]furan-4,9-dione, and 5-hydroxy-3-methylnaphtho[2,3-c]furan-4(1H)-one | [84] | |
| A. microstigma | 5-Hydroxyaloin A and microstigmin A, | [85] |
| A. nyeriensis | Aloesin, 7-O-methylaloesin, aloenin, aloeresin D, aloin B, and aloin A | [34] |
| A. purpurea | Phenols and trace amounts of saponins, tannins, alkaloids, anthraquinones, terpenes, coumarins, and flavonoids | [41] |
| A. rabaiensis | Aloe-emodin-11-O-rhamnoside, aloe-emodin anthrone-10-C-glucoside, aloe-emodin anthrone-10-C-rhamnoside, aloeresin D, and rabaichromone | [86] |
| A. rubroviolacea | Phytosterols (cholesterol, 24-methylcholesta-5,22-dien-3β-ol, campesterol, campestanol, stigmasterol, 15holesteno, and sitostanol); anthraquinones (aloin A and aloe-emodin); anthrone-anthraquinones (elgonica A and elgonica B); C-glycosyl chromones (8-C-glycosyl-(2’-O-cinnamoyl)-7-O-methylaloediol B, aloeresin E, and 8-C-glycosyl-7-O-methyl-I-aloesol) | [87] |
| A. sabaea | Coniine, γ-coniceine, N-4′-chlorobutylbutyramide, and N,N-dimethylconiine | [88] |
| A. vera | Tannins, saponins, and flavonoids | [89] |
| β-sitosterol | [25] | |
| Roots | ||
| A. berhana | Aloesaponol I, laccaic acid D methyl ester, aloesaponol III, aloesaponarin I, chrysophanol-8-methyl ether, chrysophanol, and aloechrysone | [90] |
| Stems | ||
| A. saponaria | Aloesaponarin I, aloesaponarin II, desoxyerythrolaccin, helminthosporin, isoxanthorin, and laccaic acid D methyl ester, aloesaponol III, aloesaponol IV, chrysophanol, helminthosporin, and isoxanthorin | [91] |
| Flowers | ||
| A. perryi | Glycosides, phytosterols, proteins, and amino acids, flavonoids, phenols, and carbohydrates | [92] |
| Leaves and roots | ||
| A. hijazensis | Aloe-emodin, emodin, chrysophanol, aloesaponarin II, 3-methyl ether, ziganein, ziganein-5-methyl ether, aloesaponarin I, chrysophanein, feralolide, 4,7-dichloro-quinoline, lupeol, aloin, aloenin, ethylidene-aloenin, aloenin B, quercetin, kaempferol, cosmosiin, isovitexin, cinnamic acid, caffeic acid, and ferulic acid | [93] |
| A. arborescens var. natalensis | 2′-O-p-Coumaroylaloesin and 2′-O-feruloylaloesin | [94] |
| Biological Activities | Observed Effects | Active Molecules | References |
|---|---|---|---|
| Wound healing and cell proliferation | Skin damage treatment, cell type proliferation stimulation, cell phagocytic activity stimulation, wound contraction rate, collagen and elastin synthesis increase, fibroblast proliferation, hyaluronic acid, and hydroxyproline production | Mannose-6-phosphate, polysaccharides, glycoproteins, saponins, acemannan | [5,33,135,174,175,176,178,179,180,184,185,186] |
| Intestinal absorption and purgative action | Drug permeability increase (opening tight junctions), intestinal water absorption reduction, mucus secretion stimulation, reduce visceral fat accumulation, reduce large-sized intestinal polyps, intestinal motility improvement | Anthraquinones (aloin, aloe-emodin, emodin), pytosterols | [5,133,188,189,190,191,192,194] |
| Anti-inflammatory and immunomodulatory | Phagocytic and prolifertive activity raise (through cyclooxygenase (COX) pathways inhibition and prostaglandins production reduction), abolish albumin gene transcription, inflammatory processes inhibition (leukocyte adhesion and pro-inflammatory cytokines production reduction), cerebral ischemia and reperfusion injury attenuation (inhibition of systemic inflammatory response, leukocyte aggregation, and lipid peroxidation) | Aloe-emodin, polysaccharides, aloesin, anthraquinones, chromones | [2,148,199,200,201,202,203,204,205,206,207,208] |
| Hepatoprotective | Morphofunctional and molecular changes reduction, protection against hepatocyte death and lipid peroxidation, liver fatty acid synthesis downregulation and oxidation upregulation, cytokine level reduction, mRNA lipogenic gene expression suppression | Aloe-emodin, anthraquinones, phytosterols (lophenol, cycloartanol) | [74,133,209] |
| Antioxidant | Free radical scavenging activity, free radicals’ generation and reactive oxygen species (ROS) production suppression, lipid peroxidation reduction, superoxide dismutase (SOD) activity raise | Aloesin, aloeresin A, and aloesone | [44,49,198,212,213,214,215,216,217,218] |
| Antibacterial, antifungal and antiviral | Phagocytic leukocyte activity stimulation, cytotoxic effects, alkalization promotion and constipation alleviation, virus replication inhibition | Emodin, aloin A, aloe-emodin, saponins, chrysophanol, acemannan, pyrocatechol, polysaccharides | [6,56,58,67,71,76,79,136,146,148,170,181,192,198,219,220,221,222,223,224,225,226,227,228,229,230,231,232,233] |
| Anticancer | Chemopreventive activity, VEGF secretion inhibition, tumor angiogenesis and angiogenic response inhibition, proliferation inhibition and endothelial cell migration, N-acetyl transferase activity and gene expression inhibition, STAT3 activation blocking, benzopyrene binding, iNOS, NFkB, and P53 activity inhibition, TNF-α, IL-1, and interferon production stimulation | Aloin, aloe-emodin, rhein, acemannan, barbaloin, physcion, chrysophanol, aloesin, diethyl hexylphthalate and an N-terminal octapeptide | [148,184,225,234,235,236,237,238,239,240,241,242,243,244,245,246,247,248,249,250,251,252,253,254,255] |
| Antidiabetic | Glucose transporter mRNA expression modulation, reduce fasting blood glucose levels, glucose transport improvement through proximal and distal marker modulation | Polysaccharides, phytosterols (lophenol, 24-methyl-lophenol, 24-ethyl-lophenol, cycloartanol and 24-methylene cycloartenol), aloe-emodin-8-O-glycoside | [37,73,74,148,183,255,256,257,258,259,260] |
| Antihyperlipidemic | Reduce visceral fat mass, total cholesterol, triglycerides, LDL and VLDL levels, glucose intolerance and lipid metabolizing enzymes improvement and abnormal estrous cyclicity reversion | Phytosterols | [261,262,263,264,265] |
| Estrogen status | Suppress breast cancer cells proliferation, estrogen receptor-α inhibition | Emodin, aloe-emodin | [52,263] |
| Antiulcer | Promote digestion, cytoprotection, dose-dependent gastric acid secretion inhibition | Plant extract | [5,266,267,268,269] |
| Skin use | Increase type I and type III collagen synthesis gene expression and hyaluronic acid levels, tyrosinase inhibitory activity, | Sterols, aloin, aloesin | [270,271,272,273,274,275] |
| Antiallergic | Reduce histamine release, stimulate leukotriene synthesis and secretion, protein kinase C and phospholipase C activities inhibition, Ca2+ influx blocking during mast cell activation | Glycoprotein | [276] |
| Biological Activities | Observed Effects | Active Molecules | References |
|---|---|---|---|
| Wound healing and cell proliferation | Tyrosinase activity inhibition, re-epithelialization, wound healing promotion | Arbutin, aloesin | [310,311,312,313,314,315,316,317] |
| Anti-inflammatory and immunomodulatory effects | Decrease pain score and aphthous wound healing period, promote eye external part treatment, downregulate lipopolysaccharide-induced inflammatory cytokine production and NLRP3 inflammasome expression, potentiate lymphocyte response, phagocytosis and circulating monocyte and macrophage levels | Acemannan, polysaccharides | [75,254,266,318,319,320,321] |
| Antidiabetic effects | Lower blood glucose levels, reduce body weight, body fat mass and insulin resistance, revert impaired fasting glucose levels and impaired glucose tolerance | Plant extracts | [322,323] |
| Antihyperlipidemic effects | Reduce atherosclerosis, total serum cholesterol and LDL levels | Plant extracts | [323] |
| Acquired immune deficiency syndrome (AIDS) treatment | Sooth wound and burn of internal organs, inhibit HIV-1 virus | Mannose-6-phosphate, plant extract | [324] |
| Dental and oral diseases treatment | Heal aphthous ulcers and reduce pain, plaque and gingivitis | Acemannan | [325,326] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salehi, B.; Albayrak, S.; Antolak, H.; Kręgiel, D.; Pawlikowska, E.; Sharifi-Rad, M.; Uprety, Y.; Tsouh Fokou, P.V.; Yousef, Z.; Amiruddin Zakaria, Z.; et al. Aloe Genus Plants: From Farm to Food Applications and Phytopharmacotherapy. Int. J. Mol. Sci. 2018, 19, 2843. https://doi.org/10.3390/ijms19092843
Salehi B, Albayrak S, Antolak H, Kręgiel D, Pawlikowska E, Sharifi-Rad M, Uprety Y, Tsouh Fokou PV, Yousef Z, Amiruddin Zakaria Z, et al. Aloe Genus Plants: From Farm to Food Applications and Phytopharmacotherapy. International Journal of Molecular Sciences. 2018; 19(9):2843. https://doi.org/10.3390/ijms19092843
Chicago/Turabian StyleSalehi, Bahare, Sevil Albayrak, Hubert Antolak, Dorota Kręgiel, Ewelina Pawlikowska, Mehdi Sharifi-Rad, Yadav Uprety, Patrick Valere Tsouh Fokou, Zubaida Yousef, Zainul Amiruddin Zakaria, and et al. 2018. "Aloe Genus Plants: From Farm to Food Applications and Phytopharmacotherapy" International Journal of Molecular Sciences 19, no. 9: 2843. https://doi.org/10.3390/ijms19092843
APA StyleSalehi, B., Albayrak, S., Antolak, H., Kręgiel, D., Pawlikowska, E., Sharifi-Rad, M., Uprety, Y., Tsouh Fokou, P. V., Yousef, Z., Amiruddin Zakaria, Z., Varoni, E. M., Sharopov, F., Martins, N., Iriti, M., & Sharifi-Rad, J. (2018). Aloe Genus Plants: From Farm to Food Applications and Phytopharmacotherapy. International Journal of Molecular Sciences, 19(9), 2843. https://doi.org/10.3390/ijms19092843