Polycystins in Colorectal Cancer
Abstract
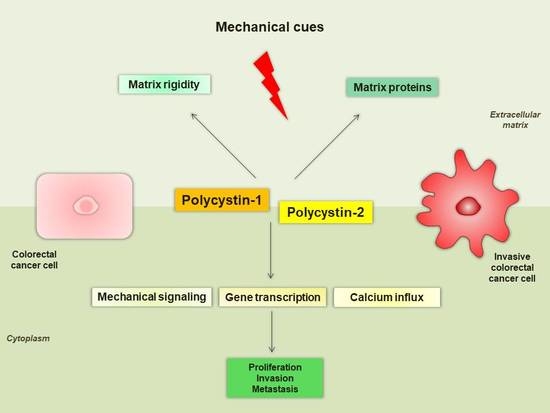
:1. Introduction
2. Structure and Function of Polycystin-1 and Polycystin-2
3. Polycystins Implication in Cancer Progression
4. Data Linking Polycystins to Colorectal Cancer Progression
5. Polycystins as Putative Therapeutic Targets in CRC
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 4EBP1 | 4E binding protein 1 |
| ADPKD | Autosomal dominant polycystic kidney disease |
| AP-1 | Activator protein-1 |
| CRC | Colorectal cancer |
| CTT | C-terminal tail |
| ECM | Extracellular matrix |
| EGFR | Epidermal growth factor receptor |
| EMT | Epithelial-to-mesenchymal transition |
| ER | Endoplasmic reticulum |
| ERK | Extracellular signal-regulated kinase |
| FAK | Focal adhesion kinase |
| IP3 | Inositol 1,4,5-trisphosphate |
| miRNAs | MicroRNAs |
| MSI | Microsatellite instability |
| mTOR | Mammalian target of rapamycin |
| NF-κB | Nuclear factor-kappaB |
| p | Phospho |
| PC1 | Polycystin-1 |
| PC2 | Polycystin-2 |
| PKD1 | Polycystic kidney disease 1 |
| PKD2 | Polycystic kidney disease 2 |
| p70S6K | p70S6 kinase |
| RTKs | Receptor tyrosine kinases |
| TAZ | Transcriptional coactivator with PDZ-binding motif |
| TOP | Tetragonal opening for polycystins |
| TRP | Transient receptor potential |
| VEGFR | Vascular endothelial growth factor receptor |
References
- Gargalionis, A.N.; Basdra, E.K.; Papavassiliou, A.G. Cancer mechanobiology: Effects and therapeutic perspectives. Int. J. Cancer 2018, 142, 1298–1299. [Google Scholar] [CrossRef] [PubMed]
- Gargalionis, A.N.; Basdra, E.K.; Papavassiliou, A.G. Tumor mechanosensing and its therapeutic potential. J. Cell Biochem. 2018, 119, 4304–4308. [Google Scholar] [CrossRef] [PubMed]
- Gargalionis, A.N.; Basdra, E.K.; Papavassiliou, A.G. Mechanosignalling in tumour progression. J. Cell. Mol. Med. 2018, 22, 704–705. [Google Scholar] [CrossRef] [PubMed]
- Ciasca, G.; Papi, M.; Minelli, E.; Palmieri, V.; De Spirito, M. Changes in cellular mechanical properties during onset or progression of colorectal cancer. World J. Gastroenterol. 2016, 22, 7203–7214. [Google Scholar] [CrossRef] [PubMed]
- Katsianou, M.A.; Skondra, F.G.; Gargalionis, A.N.; Piperi, C.; Basdra, E.K. The role of transient receptor potential polycystin channels in bone diseases. Ann. Transl. Med. 2018, 6, 246. [Google Scholar] [CrossRef] [PubMed]
- Malakou, L.S.; Gargalionis, A.N.; Piperi, C.; Papadavid, E.; Papavassiliou, A.G.; Basdra, E.K. Molecular mechanisms of mechanotransduction in psoriasis. Ann. Transl. Med. 2018, 6, 245. [Google Scholar] [CrossRef] [PubMed]
- Ong, A.C.; Harris, P.C. A polycystin-centric view of cyst formation and disease: The polycystins revisited. Kidney Int. 2015, 88, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Gargalionis, A.N.; Papavassiliou, K.A.; Basdra, E.K.; Papavassiliou, A.G. Polycystins: Mechanosensors with Diagnostic and Prognostic Potential in Cancer. Trends Mol. Med. 2016, 22, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Gargalionis, A.N.; Korkolopoulou, P.; Farmaki, E.; Piperi, C.; Dalagiorgou, G.; Adamopoulos, C.; Levidou, G.; Saetta, A.; Fragkou, P.; Tsioli, P.; et al. Polycystin-1 and polycystin-2 are involved in the acquisition of aggressive phenotypes in colorectal cancer. Int. J. Cancer 2015, 136, 1515–1527. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Ye, C.; Zhou, Q.; Zheng, R.; Lv, X.; Chen, Y.; Hu, Z.; Guo, H.; Zhang, Z.; Wang, Y.; et al. PKD1 inhibits cancer cells migration and invasion via Wnt signaling pathway in vitro. Cell. Biochem. Funct. 2007, 25, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Zhang, Z.; Lv, X.; Fan, J.; Chen, Y.; Wang, Y.; Tan, R.; Liu, Y.; Zhou, Q. Polycystin-1 induced apoptosis and cell cycle arrest in G0/G1 phase in cancer cells. Cell. Biol. Int. 2008, 32, 427–435. [Google Scholar] [CrossRef]
- Fedeles, S.V.; Gallagher, A.R.; Somlo, S. Polycystin-1: A master regulator of intersecting cystic pathways. Trends Mol. Med. 2014, 20, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Merrick, D.; Bertuccio, C.A.; Chapin, H.C.; Lal, M.; Chauvet, V.; Caplan, M.J. Polycystin-1 cleavage and the regulation of transcriptional pathways. Pediatr. Nephrol. 2014, 29, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Grieben, M.; Pike, A.C.; Shintre, C.A.; Venturi, E.; El-Ajouz, S.; Tessitore, A.; Shrestha, L.; Mukhopadhyay, S.; Mahajan, P.; Chalk, R.; et al. Structure of the polycystic kidney disease TRP channel Polycystin-2 (PC2). Nat. Struct. Mol. Biol. 2017, 24, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Retailleau, K.; Duprat, F. Polycystins and partners: Proposed role in mechanosensitivity. J. Physiol. 2014, 592, 2453–2471. [Google Scholar] [CrossRef]
- Dalagiorgou, G.; Piperi, C.; Adamopoulos, C.; Georgopoulou, U.; Gargalionis, A.N.; Spyropoulou, A.; Zoi, I.; Nokhbehsaim, M.; Damanaki, A.; Deschner, J.; et al. Mechanosensor polycystin-1 potentiates differentiation of human osteoblastic cells by upregulating Runx2 expression via induction of JAK2/STAT3 signaling axis. Cell. Mol. Life Sci. 2017, 74, 921–936. [Google Scholar] [CrossRef] [PubMed]
- Nauli, S.M.; Kawanabe, Y.; Kaminski, J.J.; Pearce, W.J.; Ingber, D.E.; Zhou, J. Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation 2008, 117, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Nauli, S.M.; Alenghat, F.J.; Luo, Y.; Williams, E.; Vassilev, P.; Li, X.; Elia, A.E.; Lu, W.; Brown, E.M.; Quinn, S.J.; et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat. Genet. 2003, 33, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Seeger-Nukpezah, T.; Geynisman, D.M.; Nikonova, A.S.; Benzing, T.; Golemis, E.A. The hallmarks of cancer: Relevance to the pathogenesis of polycystic kidney disease. Nat. Rev. Nephrol. 2015, 11, 515–534. [Google Scholar] [CrossRef] [PubMed]
- Barakat, M.T.; Scott, M.P. Tail wags dog: Primary cilia and tumorigenesis. Cancer Cell. 2009, 16, 276–277. [Google Scholar] [CrossRef]
- Harris, P.C.; Watson, M.L. Autosomal dominant polycystic kidney disease: Neoplasia in disguise? Nephrol. Dial. Transpl. 1997, 12, 1089–1090. [Google Scholar] [CrossRef]
- Liberati, S.; Morelli, M.B.; Nabissi, M.; Santoni, M.; Santoni, G. Oncogenic and anti-oncogenic effects of transient receptor potential channels. Curr. Top. Med. Chem. 2013, 13, 344–366. [Google Scholar] [CrossRef] [PubMed]
- Santoni, G.; Farfariello, V. TRP channels and cancer: New targets for diagnosis and chemotherapy. Endocr. Metab. Immune Disord. Drug Targets 2011, 11, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Drummond, I.A. Polycystins, focal adhesions and extracellular matrix interactions. Biochim. Biophys. Acta 2011, 1812, 1322–1326. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Xu, D.; Mei, C. The association between autosomal dominant polycystic kidney disease and cancer. Int. Urol Nephrol. 2018. [Google Scholar] [CrossRef]
- Adamopoulos, C.; Gargalionis, A.N.; Piperi, C.; Papavassiliou, A.G. Recent Advances in Mechanobiology of Osteosarcoma. J. Cell. Biochem. 2017, 118, 232–236. [Google Scholar] [CrossRef]
- Dere, R.; Wilson, P.D.; Sandford, R.N.; Walker, C.L. Carboxy terminal tail of polycystin-1 regulates localization of TSC2 to repress mTOR. PLoS ONE 2010, 5, e9239. [Google Scholar] [CrossRef]
- Distefano, G.; Boca, M.; Rowe, I.; Wodarczyk, C.; Ma, L.; Piontek, K.B.; Germino, G.G.; Pandolfi, P.P.; Boletta, A. Polycystin-1 regulates extracellular signal-regulated kinase-dependent phosphorylation of tuberin to control cell size through mTOR and its downstream effectors S6K and 4EBP1. Mol. Cell. Biol. 2009, 29, 2359–2371. [Google Scholar] [CrossRef]
- Francipane, M.G.; Lagasse, E. mTOR pathway in colorectal cancer: An update. Oncotarget 2014, 5, 49–66. [Google Scholar] [CrossRef]
- Swierczynski, S.; Klieser, E.; Illig, R.; Alinger-Scharinger, B.; Kiesslich, T.; Neureiter, D. Histone deacetylation meets miRNA: Epigenetics and post-transcriptional regulation in cancer and chronic diseases. Expert Opin. Biol. Ther. 2015, 15, 651–664. [Google Scholar] [CrossRef]
- Vaiopoulos, A.G.; Papachroni, K.K.; Papavassiliou, A.G. Colon carcinogenesis: Learning from NF-kappaB and AP-1. Int. J. Biochem. Cell Biol. 2010, 42, 1061–1065. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Baudry, J.; Cao, L.; Huang, J.; Chen, H.; Yates, C.R.; Li, W.; Dong, B.; Waters, C.M.; Smith, J.C.; et al. Polycystin-1 interacts with TAZ to stimulate osteoblastogenesis and inhibit adipogenesis. J. Clin. Invest. 2018, 128, 157–174. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shi, S.; Guo, Z.; Zhang, X.; Han, S.; Yang, A.; Wen, W.; Zhu, Q. Overexpression of YAP and TAZ is an independent predictor of prognosis in colorectal cancer and related to the proliferation and metastasis of colon cancer cells. PLoS ONE 2013, 8, e65539. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, S.X.; Liao, W.; Farhoodi, H.P.; Wong, C.W.; Chen, C.C.; Segaliny, A.I.; Chacko, J.V.; Nguyen, L.P.; Lu, M.; et al. Mechanoresponsive stem cells to target cancer metastases through biophysical cues. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.X.; Zhao, B.; Panupinthu, N.; Jewell, J.L.; Lian, I.; Wang, L.H.; Zhao, J.; Yuan, H.; Tumaneng, K.; Li, H.; et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 2012, 150, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Martini, G.; Troiani, T.; Cardone, C.; Vitiello, P.; Sforza, V.; Ciardiello, D.; Napolitano, S.; Della Corte, C.M.; Morgillo, F.; Raucci, A.; et al. Present and future of metastatic colorectal cancer treatment: A review of new candidate targets. World J. Gastroenterol. 2017, 23, 4675–4688. [Google Scholar] [CrossRef] [PubMed]
- Gargalionis, A.N.; Karamouzis, M.V.; Papavassiliou, A.G. The molecular rationale of Src inhibition in colorectal carcinomas. Int. J. Cancer 2014, 134, 2019–2029. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.Y. Inhibiting focal adhesion kinase: A potential target for enhancing therapeutic efficacy in colorectal cancer therapy. World J. Gastrointest. Oncol. 2018, 10, 290–292. [Google Scholar] [CrossRef]


| Expression of Polycystins in CRC Human Samples | Clinical and Pathological Correlations |
|---|---|
| PC1 increased expression (≥ median) in CRC human samples | Mucinous carcinomas |
| Grade III carcinomas | |
| T2-T4 stage carcinomas | |
| Reduced 5-year survival | |
| Increased 5-year recurrence rate | |
| Reduced recurrence-free survival | |
| PC2 increased expression (≥ median) in CRC human samples | Mucinous carcinomas |
| Grade III carcinomas | |
| PC2 expression | p-mTOR expression |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gargalionis, A.N.; Basdra, E.K.; Papavassiliou, A.G. Polycystins in Colorectal Cancer. Int. J. Mol. Sci. 2019, 20, 104. https://doi.org/10.3390/ijms20010104
Gargalionis AN, Basdra EK, Papavassiliou AG. Polycystins in Colorectal Cancer. International Journal of Molecular Sciences. 2019; 20(1):104. https://doi.org/10.3390/ijms20010104
Chicago/Turabian StyleGargalionis, Antonios N., Efthimia K. Basdra, and Athanasios G. Papavassiliou. 2019. "Polycystins in Colorectal Cancer" International Journal of Molecular Sciences 20, no. 1: 104. https://doi.org/10.3390/ijms20010104
APA StyleGargalionis, A. N., Basdra, E. K., & Papavassiliou, A. G. (2019). Polycystins in Colorectal Cancer. International Journal of Molecular Sciences, 20(1), 104. https://doi.org/10.3390/ijms20010104