Melatonin Influences Structural Plasticity in the Axons of Granule Cells in the Dentate Gyrus of Balb/C Mice
Abstract
:1. Introduction
2. Results
2.1. Distribution of Calbindin in the Dentate Gyrus in Male Balb/c Mice
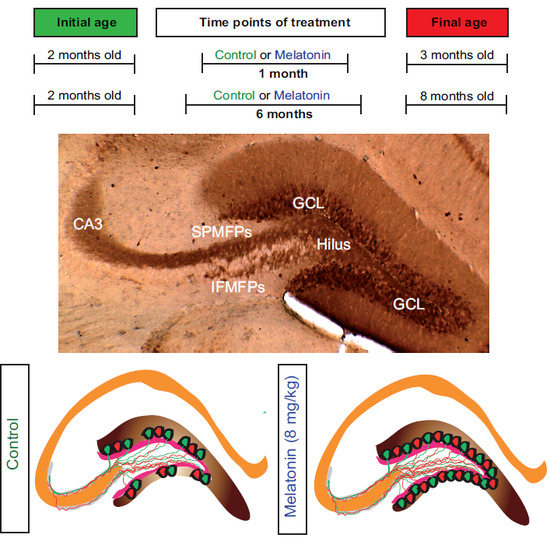
2.2. Melatonin Modulates Plasticity of Axons in Granule Cells in the Dentate Gyrus in Male Balb/c Mice
3. Discussion
4. Materials and Methods
4.1. Animals and Melatonin Treatment
4.2. Tissue Processing, Immunohistochemistry, Total Number of Calbindin-Labeled Cells in the Granular Cell Layer in the Dentate Gyrus, and Morphometric Analysis
4.3. Immunoblotting
4.4. Statistics
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DG | Dentate gyrus |
| CA | Cornus ammonis |
| VEH | Vehicle |
| PKC | Protein kinase C |
| CaM | Calmodulin |
| Akt | Protein kinase B |
References
- Dubocovich, M.L. Melatonin receptors: Role on sleep and circadian rhythm regulation. Sleep Med. 2007, 8 (Suppl. 3), 34–42. [Google Scholar] [CrossRef] [PubMed]
- Dubocovich, M.L.; Markowska, M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine 2005, 27, 101–110. [Google Scholar] [CrossRef]
- Benitez-King, G.; Anton-Tay, F. Calmodulin mediates melatonin cytoskeletal effects. Experientia 1993, 49, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Benitez-King, G.; Hernandez, M.E.; Tovar, R.; Ramirez, G. Melatonin activates PKC-alpha but not PKC-epsilon in N1E-115 cells. Neurochem. Int. 2001, 39, 95–102. [Google Scholar] [CrossRef]
- Benitez-King, G.; Huerto-Delgadillo, L.; Anton-Tay, F. Binding of 3H-melatonin to calmodulin. Life Sci. 1993, 53, 201–207. [Google Scholar] [CrossRef]
- Huerto-Delgadillo, L.; Anton-Tay, F.; Benitez-King, G. Effects of melatonin on microtubule assembly depend on hormone concentration: Role of melatonin as a calmodulin antagonist. J. Pineal Res. 1994, 17, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Soto-Vega, E.; Meza, I.; Ramirez-Rodriguez, G.; Benitez-King, G. Melatonin stimulates calmodulin phosphorylation by protein kinase C. J. Pineal Res. 2004, 37, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.P.; Garcia-Perganeda, A.; Guerrero, J.M.; Osuna, C. Membrane-bound calmodulin in Xenopus laevis oocytes as a novel binding site for melatonin. FASEB J. 1998, 12, 1401–1408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benitez-King, G.; Huerto-Delgadillo, L.; Anton-Tay, F. Melatonin effects on the cytoskeletal organization of MDCK and neuroblastoma N1E-115 cells. J. Pineal Res. 1990, 9, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Webster, S.V.; Newton, P.M. The biology of protein kinase C. Adv. Exp. Med. Biol. 2012, 740, 639–661. [Google Scholar]
- Bellon, A.; Ortiz-Lopez, L.; Ramirez-Rodriguez, G.; Anton-Tay, F.; Benitez-King, G. Melatonin induces neuritogenesis at early stages in N1E-115 cells through actin rearrangements via activation of protein kinase C and Rho-associated kinase. J. Pineal Res. 2007, 42, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Benitez-King, G. Melatonin as a cytoskeletal modulator: Implications for cell physiology and disease. J. Pineal Res. 2006, 40, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Alonso, A.; Ramirez-Rodriguez, G.; Benitez-King, G. Melatonin increases dendritogenesis in the hilus of hippocampal organotypic cultures. J. Pineal Res. 2012, 52, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Rodriguez, G.; Ortiz-Lopez, L.; Dominguez-Alonso, A.; Benitez-King, G.A.; Kempermann, G. Chronic treatment with melatonin stimulates dendrite maturation and complexity in adult hippocampal neurogenesis of mice. J. Pineal Res. 2011, 50, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Rodriguez, G.; Gomez-Sanchez, A.; Ortiz-Lopez, L. Melatonin maintains calcium-binding calretinin-positive neurons in the dentate gyrus during aging of Balb/C mice. Exp. Gerontol. 2014, 60, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Brandt, M.D.; Jessberger, S.; Steiner, B.; Kronenberg, G.; Reuter, K.; Bick-Sander, A.; von der Behrens, W.; Kempermann, G. Transient calretinin expression defines early postmitotic step of neuronal differentiation in adult hippocampal neurogenesis of mice. Mol. Cell. Neurosci. 2003, 24, 603–613. [Google Scholar] [CrossRef]
- Todkar, K.; Scotti, A.L.; Schwaller, B. Absence of the calcium-binding protein calretinin, not of calbindin D-28k, causes a permanent impairment of murine adult hippocampal neurogenesis. Front. Mol. Neurosci. 2012, 5, 56. [Google Scholar] [CrossRef]
- Bu, J.; Sathyendra, V.; Nagykery, N.; Geula, C. Age-related changes in calbindin-D28k, calretinin, and parvalbumin-immunoreactive neurons in the human cerebral cortex. Exp. Neurol. 2003, 182, 220–231. [Google Scholar] [CrossRef]
- Lee, C.H.; Hwang, I.K.; Choi, J.H.; Yoo, K.Y.; Park, O.K.; Huh, S.O.; Lee, Y.L.; Shin, H.C.; Won, M.H. Age-dependent changes in calretinin immunoreactivity and its protein level in the gerbil hippocampus. Neurochem. Res. 2010, 35, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Mirochnic, S.; Wolf, S.; Staufenbiel, M.; Kempermann, G. Age effects on the regulation of adult hippocampal neurogenesis by physical activity and environmental enrichment in the APP23 mouse model of Alzheimer disease. Hippocampus 2009, 19, 1008–1018. [Google Scholar] [CrossRef]
- Jinno, S. Decline in adult neurogenesis during aging follows a topographic pattern in the mouse hippocampus. J. Comp. Neurol. 2011, 519, 451–466. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Lopez, L.; Perez-Beltran, C.; Ramirez-Rodriguez, G. Chronic administration of a melatonin membrane receptor antagonist, luzindole, affects hippocampal neurogenesis without changes in hopelessness-like behavior in adult mice. Neuropharmacology 2016, 103, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Rodriguez, G.; Klempin, F.; Babu, H.; Benitez-King, G.; Kempermann, G. Melatonin modulates cell survival of new neurons in the hippocampus of adult mice. Neuropsychopharmacology 2009, 34, 2180–2191. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Rodriguez, G.; Vega-Rivera, N.M.; Benitez-King, G.; Castro-Garcia, M.; Ortiz-Lopez, L. Melatonin supplementation delays the decline of adult hippocampal neurogenesis during normal aging of mice. Neurosci. Lett. 2012, 530, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Rodriguez, G.; Vega-Rivera, N.M.; Oikawa-Sala, J.; Gomez-Sanchez, A.; Ortiz-Lopez, L.; Estrada-Camarena, E. Melatonin synergizes with citalopram to induce antidepressant-like behavior and to promote hippocampal neurogenesis in adult mice. J. Pineal Res. 2014, 56, 450–461. [Google Scholar] [CrossRef]
- Kempermann, G.; Krebs, J.; Fabel, K. The contribution of failing adult hippocampal neurogenesis to psychiatric disorders. Curr. Opin. Psychiatry 2008, 21, 290–295. [Google Scholar] [CrossRef]
- Couillard-Despres, S.; Wuertinger, C.; Kandasamy, M.; Caioni, M.; Stadler, K.; Aigner, R.; Bogdahn, U.; Aigner, L. Ageing abolishes the effects of fluoxetine on neurogenesis. Mol. Psychiatry 2009, 14, 856–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brusco, L.I.; Marquez, M.; Cardinali, D.P. Melatonin treatment stabilizes chronobiologic and cognitive symptoms in Alzheimer’s disease. Neuro Endocrinol. Lett. 2000, 21, 39–42. [Google Scholar] [PubMed]
- Toni, N.; Laplagne, D.A.; Zhao, C.; Lombardi, G.; Ribak, C.E.; Gage, F.H.; Schinder, A.F. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat. Neurosci. 2008, 11, 901–907. [Google Scholar] [CrossRef] [Green Version]
- Römer, B.; Krebs, J.; Overall, R.W.; Fabel, K.; Babu, H.; Overstreet-Wadiche, L.; Brandt, M.; Williams, R.W.; Jessberger, S.; Kempermann, G. Adult hippocampal neurogenesis and plasticity in the infrapyramidal bundle of the mossy fiber projection: I. Co-regulation by activity. Front. Neurosci. 2011, 5, 107. [Google Scholar] [CrossRef]
- Crusio, W.E.; Schwegler, H. Hippocampal mossy fiber distribution covaries with open-field habituation in the mouse. Behav. Brain Res. 1987, 26, 153–158. [Google Scholar] [CrossRef]
- Schwegler, H.; Crusio, W.E.; Brust, I. Hippocampal mossy fibers and radial-maze learning in the mouse: A correlation with spatial working memory but not with non-spatial reference memory. Neuroscience 1990, 34, 293–298. [Google Scholar] [CrossRef]
- Schopke, R.; Wolfer, D.P.; Lipp, H.P.; Leisinger-Trigona, M.C. Swimming navigation and structural variations of the infrapyramidal mossy fibers in the hippocampus of the mouse. Hippocampus 1991, 1, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Berggård, T.; Miron, S.; Önnerfjord, P.; Thulin, E.; Åkerfeldt, K.S.; Enghild, J.J.; Akke, M.; Linse, S. Calbindin D28k exhibits properties characteristic of a Ca2+ sensor. J. Biol. Chem. 2002, 277, 16662–16672. [Google Scholar] [CrossRef] [PubMed]
- Sloviter, R.S. Calcium-binding protein (calbindin-D28k) and parvalbumin immunocytochemistry: Localization in the rat hippocampus with specific reference to the selective vulnerability of hippocampal neurons to seizure activity. J. Comp. Neurol. 1989, 280, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Kempermann, G.; Kuhn, H.G.; Gage, F.H. Genetic influence on neurogenesis in the dentate gyrus of adult mice. Proc. Natl. Acad. Sci. USA 1997, 94, 10409–10414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, P.J.; Kohman, R.A.; Miller, D.S.; Bhattacharya, T.K.; Brzezinska, W.J.; Rhodes, J.S. Genetic influences on exercise-induced adult hippocampal neurogenesis across 12 divergent mouse strains. Genes Brain Behav. 2011, 10, 345–353. [Google Scholar] [CrossRef]
- Gómez-Corvera, A.; Cerrillo, I.; Molinero, P.; Naranjo, M.C.; Lardone, P.J.; Sanchez-Hidalgo, M.; Carrascosa-Salmoral, M.P.; Medrano-Campillo, P.; Guerrero, J.M.; Rubio, A. Evidence of immune system melatonin production by two pineal melatonin deficient mice, C57BL/6 and Swiss strains. J. Pineal Res. 2009, 47, 15–22. [Google Scholar] [CrossRef]
- Vivien-Roels, B.; Malan, A.; Rettori, M.C.; Delagrange, P.; Jeanniot, J.P.; Pevet, P. Daily variations in pineal melatonin concentrations in inbred and outbred mice. J. Biol. Rhythms. 1998, 13, 403–409. [Google Scholar] [CrossRef]
- Liu, J.; Somera-Molina, K.C.; Hudson, R.L.; Dubocovich, M.L. Melatonin potentiates running wheel-induced neurogenesis in the dentate gyrus of adult C3H/HeN mice hippocampus. J. Pineal Res. 2013, 54, 222–231. [Google Scholar] [CrossRef]
- Reiter, R.J. Melatonin, active oxygen species and neurological damage. Drug News Perspect. 1998, 11, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Reiter, R.J.; Qi, W.; Kim, S.J.; El-Sokkary, G.H. Melatonin protects hippocampal neurons in vivo against kainic acid-induced damage in mice. J. Neurosci. Res. 1998, 54, 382–389. [Google Scholar] [CrossRef]
- Rennie, K.; De Butte, M.; Pappas, B.A. Melatonin promotes neurogenesis in dentate gyrus in the pinealectomized rat. J. Pineal Res. 2009, 47, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Kempermann, G.; Jessberger, S.; Steiner, B.; Kronenberg, G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004, 27, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Hoogenraad, C.C.; Bradke, F. Control of neuronal polarity and plasticity—A renaissance for microtubules? Trends Cell Biol. 2009, 19, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Wallace Gt Reiter, R.J.; Varma, A.K.; Ray, S.K.; Banik, N.L. Overexpression of melatonin membrane receptors increases calcium-binding proteins and protects VSC4.1 motoneurons from glutamate toxicity through multiple mechanisms. J. Pineal Res. 2013, 54, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, D.P.; Srinivasan, V.; Brzezinski, A.; Brown, G.M. Melatonin and its analogs in insomnia and depression. J. Pineal Res. 2012, 52, 365–375. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, L.A.; Gorenstein, C.; Moreno, R.; Pariante, C.; Markus, R.P. Effect of antidepressants on melatonin metabolite in depressed patients. J. Psychopharmacol. 2009, 23, 315–321. [Google Scholar] [CrossRef]
- Crupi, R.; Mazzon, E.; Marino, A.; La Spada, G.; Bramanti, P.; Cuzzocrea, S.; Spina, E. Melatonin treatment mimics the antidepressant action in chronic corticosterone-treated mice. J. Pineal Res. 2010, 49, 123–129. [Google Scholar] [CrossRef]
- Crupi, R.; Mazzon, E.; Marino, A.; La Spada, G.; Bramanti, P.; Spina, E.; Cuzzocrea, S. Melatonin’s stimulatory effect on adult hippocampal neurogenesis in mice persists after ovariectomy. J. Pineal Res. 2011, 51, 353–360. [Google Scholar] [CrossRef]
- Liu, D.; Wei, N.; Man, H.Y.; Lu, Y.; Zhu, L.Q.; Wang, J.Z. The MT2 receptor stimulates axonogenesis and enhances synaptic transmission by activating Akt signaling. Cell Death Differ. 2015, 22, 583–596. [Google Scholar] [CrossRef]
- Corrales, A.; Vidal, R.; García, S.; Vidal, V.; Martínez, P.; García, E.; Flórez, J.; Sanchez-Barceló, E.J.; Martínez-Cué, C.; Rueda, N. Chronic melatonin treatment rescues electrophysiological and neuromorphological deficits in a mouse model of Down syndrome. J. Pineal Res. 2014, 56, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Schwaller, B. Calretinin: From a “simple” Ca buffer to a multifunctional protein implicated in many biological processes. Front. Neuroanat. 2014, 8, 3. [Google Scholar] [CrossRef]
- Kuznicki, J.; Isaacs, K.R.; Jacobowitz, D.M. The expression of calretinin in transfected PC12 cells provides no protection against Ca2+-overload or trophic factor deprivation. Biochim. Biophys. Acta 1996, 1313, 194–200. [Google Scholar] [CrossRef]
- De Faria Poloni, J.; Feltes, B.C.; Bonatto, D. Melatonin as a central molecule connecting neural development and calcium signaling. Funct. Integr. Genom. 2011, 11, 383–388. [Google Scholar] [CrossRef]
- Schmidt, H. Three functional facets of calbindin D-28k. Front. Mol. Neurosci. 2012, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Waldhauser, F.; Ehrhart, B.; Forster, E. Clinical aspects of the melatonin action: Impact of development, aging, and puberty, involvement of melatonin in psychiatric disease and importance of neuroimmunoendocrine interactions. Experientia 1993, 49, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Waldhauser, F.; Kovacs, J.; Reiter, E. Age-related changes in melatonin levels in humans and its potential consequences for sleep disorders. Exp. Gerontol. 1998, 33, 759–772. [Google Scholar] [CrossRef]
- Waldhauser, F.; Steger, H. Changes in melatonin secretion with age and pubescence. J. Neural Transm. Suppl. 1986, 21, 183–197. [Google Scholar]
- Waldhauser, F.; Weiszenbacher, G.; Tatzer, E.; Gisinger, B.; Waldhauser, M.; Schemper, M.; Frisch, H. Alterations in nocturnal serum melatonin levels in humans with growth and aging. J. Clin. Endocrinol. Metab. 1988, 66, 648–652. [Google Scholar] [CrossRef]
- Catapano, F.; Monteleone, P.; Fuschino, A.; Maj, M.; Kemali, D. Melatonin and cortisol secretion in patients with primary obsessive-compulsive disorder. Psychiatry Res. 1992, 44, 217–225. [Google Scholar] [CrossRef]
- Ferrari, E.; Fioravanti, M.; Magri, F.; Solerte, S.B. Variability of interactions between neuroendocrine and immunological functions in physiological aging and dementia of the Alzheimer’s type. Ann. N. Y. Acad. Sci. 2000, 917, 582–596. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, P.; Maj, M.; Fusco, M.; Kemali, D.; Reiter, R.J. Depressed nocturnal plasma melatonin levels in drug-free paranoid schizophrenics. Schizophr. Res. 1992, 7, 77–84. [Google Scholar] [CrossRef]
- Ohashi, Y.; Okamoto, N.; Uchida, K.; Iyo, M.; Mori, N.; Morita, Y. Daily rhythm of serum melatonin levels and effect of light exposure in patients with dementia of the Alzheimer’s type. Biol. Psychiatry 1999, 45, 1646–1652. [Google Scholar] [CrossRef]
- Shigeta, H.; Yasui, A.; Nimura, Y.; Machida, N.; Kageyama, M.O.; Miura, M.; Menjo, M.; Ikeda, K. Postoperative delirium and melatonin levels in elderly patients. Am. J. Surg. 2001, 182, 449–454. [Google Scholar] [CrossRef]
- Nakamura, T.J.; Nakamura, W.; Yamazaki, S.; Kudo, T.; Cutler, T.; Colwell, C.S.; Block, G.D. Age-related decline in circadian output. J. Neurosci. 2011, 31, 10201–10205. [Google Scholar] [CrossRef]
- Skene, D.J.; Vivien-Roels, B.; Sparks, D.L.; Hunsaker, J.C.; Pevet, P.; Ravid, D.; Swaab, D.F. Daily variation in the concentration of melatonin and 5-methoxytryptophol in the human pineal gland: Effect of age and Alzheimer’s disease. Brain Res. 1990, 528, 170–174. [Google Scholar] [CrossRef]
- Skene, D.J.; Swaab, D.F. Melatonin rhythmicity: Effect of age and Alzheimer’s disease. Exp. Gerontol. 2003, 38, 199–206. [Google Scholar] [CrossRef]
- Wu, Y.H.; Zhou, J.N.; Van Heerikhuize, J.; Jockers, R.; Swaab, D.F. Decreased MT1 melatonin receptor expression in the suprachiasmatic nucleus in aging and Alzheimer’s disease. Neurobiol. Aging 2007, 28, 1239–1247. [Google Scholar] [CrossRef]
- Wu, Y.H.; Swaab, D.F. Disturbance and strategies for reactivation of the circadian rhythm system in aging and Alzheimer’s disease. Sleep Med. 2007, 8, 623–636. [Google Scholar] [CrossRef]
- Caballero, B.; Vega-Naredo, I.; Sierra, V.; Huidobro-Fernández, C.; Soria-Valles, C.; De Gonzalo-Calvo, D.; Tolivia, D.; Pallás, M.; Camins, A.; Rodríguez-Colunga, M.J.; et al. Melatonin alters cell death processes in response to age-related oxidative stress in the brain of senescence-accelerated mice. J. Pineal Res. 2009, 46, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Kempermann, G.; Gast, D.; Kronenberg, G.; Yamaguchi, M.; Gage, F.H. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development 2003, 130, 391–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Rodríguez, G.B.; Olvera-Hernández, S.; Vega-Rivera, N.M.; Ortiz-López, L. Melatonin Influences Structural Plasticity in the Axons of Granule Cells in the Dentate Gyrus of Balb/C Mice. Int. J. Mol. Sci. 2019, 20, 73. https://doi.org/10.3390/ijms20010073
Ramírez-Rodríguez GB, Olvera-Hernández S, Vega-Rivera NM, Ortiz-López L. Melatonin Influences Structural Plasticity in the Axons of Granule Cells in the Dentate Gyrus of Balb/C Mice. International Journal of Molecular Sciences. 2019; 20(1):73. https://doi.org/10.3390/ijms20010073
Chicago/Turabian StyleRamírez-Rodríguez, Gerardo Bernabé, Sandra Olvera-Hernández, Nelly Maritza Vega-Rivera, and Leonardo Ortiz-López. 2019. "Melatonin Influences Structural Plasticity in the Axons of Granule Cells in the Dentate Gyrus of Balb/C Mice" International Journal of Molecular Sciences 20, no. 1: 73. https://doi.org/10.3390/ijms20010073
APA StyleRamírez-Rodríguez, G. B., Olvera-Hernández, S., Vega-Rivera, N. M., & Ortiz-López, L. (2019). Melatonin Influences Structural Plasticity in the Axons of Granule Cells in the Dentate Gyrus of Balb/C Mice. International Journal of Molecular Sciences, 20(1), 73. https://doi.org/10.3390/ijms20010073