Selenium Species: Current Status and Potentials in Cancer Prevention and Therapy
Abstract
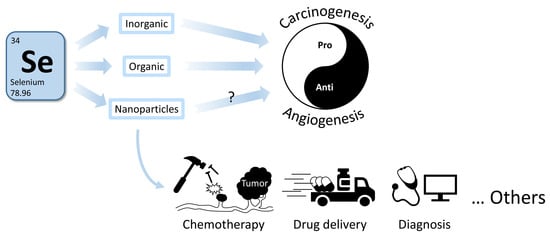
:1. Introduction
2. Anti- or Pro-Cancer?
3. Se-Containing Compounds and Their Usage in Oncology
3.1. Inorganic Se Compounds
3.2. Organic Se Compounds
3.3. SeNPs
4. Se and Epigenetics: Possible Roles in Cancer Prevention and Therapy
5. Other Potential Applications of Se Compounds
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| D-501036 | 2,5-bis(5-hydroxymethyl-2-selenienyl)-3-hydroxymethyl-N-methylpyrrole |
| MSA | Methylseleninic acid |
| MSC | Methylselenocysteine |
| ncRNA | non-coding RNA |
| NK | Natural killer |
| NPC | Nutritional prevention of cancer |
| PBM | Peripheral blood mononuclear |
| PTM | Post-translational modification |
| ROS | Reactive oxygen species |
| Se | Selenium |
| SELECT | Selenium and vitamin E cancer prevention trial |
| SeMet | Selenomethionine |
| SeNPs | Se-containing nanoparticles |
| SWOG | Southwest Oncology Group |
References
- Lau, A.T.Y.; Tan, H.W.; Xu, Y.M. Epigenetic effects of dietary trace elements. Curr. Pharmacol. Rep. 2017, 3, 232–241. [Google Scholar] [CrossRef]
- Wu, Q.; Rayman, M.P.; Lv, H.; Schomburg, L.; Cui, B.; Gao, C.; Chen, P.; Zhuang, G.; Zhang, Z.; Peng, X.; et al. Low population selenium status is associated with increased prevalence of thyroid disease. J. Clin. Endocrinol. Metab. 2015, 100, 4037–4047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Li, X.; Wong, Y.S.; Chen, T.; Zhang, H.; Liu, C.; Zheng, W. The reversal of cisplatin-induced nephrotoxicity by selenium nanoparticles functionalized with 11-mercapto-1-undecanol by inhibition of ROS-mediated apoptosis. Biomaterials 2011, 32, 9068–9076. [Google Scholar] [CrossRef] [PubMed]
- Forootanfar, H.; Adeli-Sardou, M.; Nikkhoo, M.; Mehrabani, M.; Amir-Heidari, B.; Shahverdi, A.R.; Shakibaie, M. Antioxidant and cytotoxic effect of biologically synthesized selenium nanoparticles in comparison to selenium dioxide. J. Trace Elem. Med. Biol. 2014, 28, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Huang, Q.; Zheng, Z.; Guan, H.; Liu, S. Construction of a Cordyceps sinensis exopolysaccharide-conjugated selenium nanoparticles and enhancement of their antioxidant activities. Int. J. Biol. Macromol. 2017, 99, 483–491. [Google Scholar] [CrossRef]
- Broome, C.S.; McArdle, F.; Kyle, J.A.; Andrews, F.; Lowe, N.M.; Hart, C.A.; Arthur, J.R.; Jackson, M.J. An increase in selenium intake improves immune function and poliovirus handling in adults with marginal selenium status. Am. J. Clin. Nutr. 2004, 80, 154–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narayan, V.; Ravindra, K.C.; Liao, C.; Kaushal, N.; Carlson, B.A.; Prabhu, K.S. Epigenetic regulation of inflammatory gene expression in macrophages by selenium. J. Nutr. Biochem. 2015, 26, 138–145. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Cao, W.; Xu, H.; Zhu, M.; Wang, J.; Ke, X. Treatment with a selenium-platinum compound induced T-cell acute lymphoblastic leukemia/lymphoma cells apoptosis through the mitochondrial signaling pathway. Oncol. Lett. 2017, 13, 1702–1710. [Google Scholar] [CrossRef] [Green Version]
- Kohler, L.N.; Florea, A.; Kelley, C.P.; Chow, S.; Hsu, P.; Batai, K.; Saboda, K.; Lance, P.; Jacobs, E.T. Higher plasma selenium concentrations are associated with increased odds of prevalent type 2 diabetes. J. Nutr. 2018, 148, 1333–1340. [Google Scholar] [CrossRef]
- Casaril, A.M.; Ignasiak, M.T.; Chuang, C.Y.; Vieira, B.; Padilha, N.B.; Carroll, L.; Lenardao, E.J.; Savegnago, L.; Davies, M.J. Selenium-containing indolyl compounds: Kinetics of reaction with inflammation-associated oxidants and protective effect against oxidation of extracellular matrix proteins. Free Radic. Biol. Med. 2017, 113, 395–405. [Google Scholar] [CrossRef]
- Mistry, H.D.; Broughton Pipkin, F.; Redman, C.W.; Poston, L. Selenium in reproductive health. Am. J. Obstet. Gynecol. 2012, 206, 21–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riaz, M.; Mahmood, Z.; Shahid, M.; Saeed, M.U.; Tahir, I.M.; Shah, S.A.; Munir, N.; El-Ghorab, A. Impact of reactive oxygen species on antioxidant capacity of male reproductive system. Int. J. Immunopathol. Pharmacol. 2016, 29, 421–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, S.; Jin, Y.; Hall, K.S.; Liang, C.; Unverzagt, F.W.; Ji, R.; Murrell, J.R.; Cao, J.; Shen, J.; Ma, F.; et al. Selenium level and cognitive function in rural elderly Chinese. Am. J. Epidemiol. 2007, 165, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Jin, N.; Zhu, H.; Liang, X.; Huang, W.; Xie, Q.; Xiao, P.; Ni, J.; Liu, Q. Sodium selenate activated Wnt/β-catenin signaling and repressed amyloid-β formation in a triple transgenic mouse model of Alzheimer′s disease. Exp. Neurol. 2017, 297, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Skröder, H.; Kippler, M.; Tofail, F.; Vahter, M. Early-life selenium status and cognitive function at 5 and 10 years of age in Bangladeshi children. Environ Health Perspect 2017, 125, 117003. [Google Scholar] [CrossRef] [PubMed]
- Vinceti, M.; Filippini, T.; Cilloni, S.; Bargellini, A.; Vergoni, A.V.; Tsatsakis, A.; Ferrante, M. Health risk assessment of environmental selenium: Emerging evidence and challenges. Mol. Med. Rep. 2017, 15, 3323–3335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinceti, M.; Wei, E.T.; Malagoli, C.; Bergomi, M.; Vivoli, G. Adverse health effects of selenium in humans. Rev. Environ. Health 2001, 16, 233–251. [Google Scholar] [CrossRef] [PubMed]
- Loscalzo, J. Keshan disease, selenium deficiency, and the selenoproteome. N. Engl. J. Med. 2014, 370, 1756–1760. [Google Scholar] [CrossRef]
- Yang, B.; Wang, K.; Zhang, D.; Sun, B.; Ji, B.; Wei, L.; Li, Z.; Wang, M.; Zhang, X.; Zhang, H.; et al. Light-activatable dual-source ROS-responsive prodrug nanoplatform for synergistic chemo-photodynamic therapy. Biomater. Sci. 2018, 6, 2965–2975. [Google Scholar] [CrossRef]
- Sutter, M.E.; Thomas, J.D.; Brown, J.; Morgan, B. Selenium toxicity: A case of selenosis caused by a nutritional supplement. Ann. Intern. Med. 2008, 148, 970–971. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; National Academy Press: Washington, DC, USA, 2000; pp. 284–324. [Google Scholar]
- Weisberger, A.S.; Suhrland, L.G. Studies on analogues of L-cysteine and L-cystine. II. The effect of selenium cystine on Murphy lymphosarcoma tumor cells in the rat. Blood 1956, 11, 11–18. [Google Scholar] [PubMed]
- Weisberger, A.S.; Suhrland, L.G. Studies on analogues of L-cysteine and L-cystine. III. The effect of selenium cystine on leukemia. Blood 1956, 11, 19–30. [Google Scholar] [PubMed]
- Mautner, H.G.; Jaffe, J.J. The activity of 6-selenopurine and related compounds against some experimental mouse tumors. Cancer Res. 1958, 18, 294–298. [Google Scholar] [PubMed]
- Weekley, C.M.; Harris, H.H. Which form is that? The importance of selenium speciation and metabolism in the prevention and treatment of disease. Chem. Soc. Rev. 2013, 42, 8870–8894. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Boylan, M.; Selvam, A.; Spallholz, J.E.; Bjornstedt, M. Redox-active selenium compounds–from toxicity and cell death to cancer treatment. Nutrients 2015, 7, 3536–3556. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.C.; Combs, G.F., Jr.; Turnbull, B.W.; Slate, E.H.; Chalker, D.K.; Chow, J.; Davis, L.S.; Glover, R.A.; Graham, G.F.; Gross, E.G.; et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA 1996, 276, 1957–1963. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.C.; Dalkin, B.; Krongrad, A.; Combs, G.F., Jr.; Turnbull, B.W.; Slate, E.H.; Witherington, R.; Herlong, J.H.; Janosko, E.; Carpenter, D.; et al. Decreased incidence of prostate cancer with selenium supplementation: Results of a double-blind cancer prevention trial. Br. J. Urol. 1998, 81, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Duffield-Lillico, A.J.; Reid, M.E.; Turnbull, B.W.; Combs, G.F., Jr.; Slate, E.H.; Fischbach, L.A.; Marshall, J.R.; Clark, L.C. Baseline characteristics and the effect of selenium supplementation on cancer incidence in a randomized clinical trial: A summary report of the Nutritional Prevention of Cancer Trial. Cancer Epidemiol. Biomarkers Prev. 2002, 11, 630–639. [Google Scholar] [PubMed]
- Vinceti, M.; Filippini, T.; Cilloni, S.; Crespi, C.M. The epidemiology of selenium and human cancer. Adv. Cancer Res. 2017, 136, 1–48. [Google Scholar]
- Collery, P. Strategies for the development of selenium-based anticancer drugs. J. Trace Elem. Med. Biol. 2018, 50, 498–507. [Google Scholar] [CrossRef]
- Lippman, S.M.; Klein, E.A.; Goodman, P.J.; Lucia, M.S.; Thompson, I.M.; Ford, L.G.; Parnes, H.L.; Minasian, L.M.; Gaziano, J.M.; Hartline, J.A.; et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2009, 301, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.R.; Tangen, C.M.; Sakr, W.A.; Wood, D.P., Jr.; Berry, D.L.; Klein, E.A.; Lippman, S.M.; Parnes, H.L.; Alberts, D.S.; Jarrard, D.F.; et al. Phase III trial of selenium to prevent prostate cancer in men with high-grade prostatic intraepithelial neoplasia: SWOG S9917. Cancer Prev. Res. 2011, 4, 1761–1769. [Google Scholar] [CrossRef] [PubMed]
- Karp, D.D.; Lee, S.J.; Keller, S.M.; Wright, G.S.; Aisner, S.; Belinsky, S.A.; Johnson, D.H.; Johnston, M.R.; Goodman, G.; Clamon, G.; et al. Randomized, double-blind, placebo-controlled, phase III chemoprevention trial of selenium supplementation in patients with resected stage I non-small-cell lung cancer: ECOG 5597. J. Clin. Oncol. 2013, 31, 4179–4187. [Google Scholar] [CrossRef] [PubMed]
- Mix, M.; Singh, A.K.; Tills, M.; Dibaj, S.; Groman, A.; Jaggernauth, W.; Rustum, R.; Jameson, M.B. Randomized phase Ⅱ trial of selenomethionine as a modulator of efficacy and toxicity of chemoradiation in squamous cell carcinoma of the head and neck. World J. Clin. Oncol. 2015, 6, 166. [Google Scholar] [CrossRef] [PubMed]
- Vinceti, M.; Vicentini, M.; Wise, L.A.; Sacchettini, C.; Malagoli, C.; Ballotari, P.; Filippini, T.; Malavolti, M.; Rossi, P.G. Cancer incidence following long-term consumption of drinking water with high inorganic selenium content. Sci. Total Environ. 2018, 635, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Akbaraly, N.T.; Arnaud, J.; Hininger-Favier, I.; Gourlet, V.; Roussel, A.M.; Berr, C. Selenium and mortality in the elderly: Results from the EVA study. Clin. Chem. 2005, 51, 2117–2123. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.P.; Gandin, V. Selenium compounds as therapeutic agents in cancer. Biochim. Biophys. Acta 2015, 1850, 1642–1660. [Google Scholar] [CrossRef]
- Rahmanto, A.S.; Davies, M.J. Selenium-containing amino acids as direct and indirect antioxidants. IUBMB Life 2012, 64, 863–871. [Google Scholar] [CrossRef] [Green Version]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K. Reactive oxygen species (ROS) and cancer: Role of antioxidative nutraceuticals. Cancer Lett. 2017, 387, 95–105. [Google Scholar] [CrossRef]
- Georgieva, E.; Ivanova, D.; Zhelev, Z.; Bakalova, R.; Gulubova, M.; Aoki, I. Mitochondrial dysfunction and redox imbalance as a diagnostic marker of “free radical diseases”. Anticancer Res. 2017, 37, 5373–5381. [Google Scholar]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Drake, E.N. Cancer chemoprevention: Selenium as a prooxidant, not an antioxidant. Med. Hypotheses 2006, 67, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, D.L.; Yoo, M.H.; Carlson, B.A.; Gladyshev, V.N. Selenoproteins that function in cancer prevention and promotion. Biochim. Biophys. Acta 2009, 1790, 1541–1545. [Google Scholar] [CrossRef] [Green Version]
- Brozmanová, J.; Mániková, D.; Vlčková, V.; Chovanec, M. Selenium: A double-edged sword for defense and offence in cancer. Arch. Toxicol. 2010, 84, 919–938. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R.; Flohé, L. Selenium and redox signaling. Arch. Biochem. Biophys. 2017, 617, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Varlamova, E.G.; Cheremushkina, I.V. Contribution of mammalian selenocysteine-containing proteins to carcinogenesis. J. Trace Elem. Med. Biol. 2017, 39, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Kang, G.; Wang, T.; Huang, H. Tumor angiogenesis and anti-angiogenic gene therapy for cancer. Oncol. Lett. 2018, 16, 687–702. [Google Scholar] [CrossRef] [PubMed]
- Unni, E.; Koul, D.; Yung, W.K.; Sinha, R. Se-methylselenocysteine inhibits phosphatidylinositol 3-kinase activity of mouse mammary epithelial tumor cells in vitro. Breast Cancer Res. 2005, 7, R699–R707. [Google Scholar] [CrossRef] [PubMed]
- Schroterova, L.; Kralova, V.; Voracova, A.; Haskova, P.; Rudolf, E.; Cervinka, M. Antiproliferative effects of selenium compounds in colon cancer cells: Comparison of different cytotoxicity assays. Toxicol. In Vitro 2009, 23, 1406–1411. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Endo, M.; Shinohara, F.; Echigo, S.; Rikiishi, H. Differential apoptotic response of human cancer cells to organoselenium compounds. Cancer Chemother. Pharmacol. 2010, 66, 475–484. [Google Scholar] [CrossRef]
- Cao, S.; Durrani, F.A.; Rustum, Y.M. Selective modulation of the therapeutic efficacy of anticancer drugs by selenium containing compounds against human tumor xenografts. Clin. Cancer Res. 2004, 10, 2561–2569. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Carrier, L.; Belame, A.; Thiyagarajah, A.; Salvo, V.A.; Burow, M.E.; Rowan, B.G. Combination of methylselenocysteine with tamoxifen inhibits MCF-7 breast cancer xenografts in nude mice through elevated apoptosis and reduced angiogenesis. Breast Cancer Res. Treat. 2009, 118, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Chintala, S.; Toth, K.; Cao, S.; Durrani, F.A.; Vaughan, M.M.; Jensen, R.L.; Rustum, Y.M. Se-methylselenocysteine sensitizes hypoxic tumor cells to irinotecan by targeting hypoxia-inducible factor 1α. Cancer Chemother. Pharmacol. 2010, 66, 899–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharya, A. Methylselenocysteine: A promising antiangiogenic agent for overcoming drug delivery barriers in solid malignancies for therapeutic synergy with anticancer drugs. Expert. Opin. Drug Deliv. 2011, 8, 749–763. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Durrani, F.A.; Toth, K.; Rustum, Y.M. Se-methylselenocysteine offers selective protection against toxicity and potentiates the antitumour activity of anticancer drugs in preclinical animal models. Br. J. Cancer 2014, 110, 1733–1743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McAuslan, B.R.; Reilly, W. Selenium-induced cell migration and proliferation: Relevance to angiogenesis and microangiopathy. Microvasc. Res. 1986, 32, 112–120. [Google Scholar] [CrossRef]
- Streicher, K.L.; Sylte, M.J.; Johnson, S.E.; Sordillo, L.M. Thioredoxin reductase regulates angiogenesis by increasing endothelial cell-derived vascular endothelial growth factor. Nutr. Cancer 2004, 50, 221–231. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Yan, X.; Zhang, L. Comparison of short-term toxicity between Nano-Se and selenite in mice. Life Sci. 2005, 76, 1099–1109. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Xu, T. Elemental selenium at nano size (Nano-Se) as a potential chemopreventive agent with reduced risk of selenium toxicity: Comparison with Se-methylselenocysteine in mice. Toxicol. Sci. 2008, 101, 22–31. [Google Scholar] [CrossRef]
- Chaudhary, S.; Umar, A.; Mehta, S.K. Surface functionalized selenium nanoparticles for biomedical applications. J. Biomed. Nanotechnol. 2014, 10, 3004–3042. [Google Scholar] [CrossRef]
- Bai, K.; Hong, B.; He, J.; Hong, Z.; Tan, R. Preparation and antioxidant properties of selenium nanoparticles-loaded chitosan microspheres. Int. J. Nanomed. 2017, 12, 4527–4539. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.D.; Vardhanabhuti, B.; Lin, M.J.; Mustapha, A. Antibacterial properties of selenium nanoparticles and their toxicity to Caco-2 cells. Food Control 2017, 77, 17–24. [Google Scholar] [CrossRef]
- Sharma, G.; Sharma, A.R.; Bhavesh, R.; Park, J.; Ganbold, B.; Nam, J.S.; Lee, S.S. Biomolecule-mediated synthesis of selenium nanoparticles using dried Vitis vinifera (raisin) extract. Molecules 2014, 19, 2761–2770. [Google Scholar] [CrossRef] [PubMed]
- Fairweather-Tait, S.J.; Collings, R.; Hurst, R. Selenium bioavailability: Current knowledge and future research requirements. Am. J. Clin. Nutr. 2010, 91, 1484S–1491S. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Selenium in cancer prevention: A review of the evidence and mechanism of action. Proc. Nutr. Soc. 2005, 64, 527–542. [Google Scholar] [CrossRef] [PubMed]
- Papp, L.V.; Lu, J.; Holmgren, A.; Khanna, K.K. From selenium to selenoproteins: Synthesis, identity, and their role in human health. Antioxid. Redox Signal. 2007, 9, 775–806. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.I.; Combs, G.F., Jr. Selenium and anticarcinogenesis: Underlying mechanisms. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 718–726. [Google Scholar] [CrossRef]
- Ronai, Z.; Tillotson, J.K.; Traganos, F.; Darzynkiewicz, Z.; Conaway, C.C.; Upadhyaya, P.; el-Bayoumy, K. Effects of organic and inorganic selenium compounds on rat mammary tumor cells. Int. J. Cancer 1995, 63, 428–434. [Google Scholar] [CrossRef]
- Sanmartín, C.; Plano, D.; Sharma, A.K.; Palop, J.A. Selenium compounds, apoptosis and other types of cell death: An overview for cancer therapy. Int. J. Mol. Sci. 2012, 13, 9649–9672. [Google Scholar] [CrossRef]
- Choi, A.; Jo, M.J.; Jung, M.J.; Kim, H.S.; Yoon, S. Selenate specifically sensitizes drug-resistant cancer cells by increasing apoptosis via G2 phase cell cycle arrest without P-GP inhibition. Eur. J. Pharmacol. 2015, 764, 63–69. [Google Scholar] [CrossRef]
- Takahashi, M.; Sato, T.; Shinohara, F.; Echigo, S.; Rikiishi, H. Possible role of glutathione in mitochondrial apoptosis of human oral squamous cell carcinoma caused by inorganic selenium compounds. Int. J. Oncol. 2005, 27, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Endo, M.; Hasegawa, H.; Kaneko, T.; Kanno, C.; Monma, T.; Kano, M.; Shinohara, F.; Takahashi, T. Antitumor activity of selenium compounds and its underlying mechanism in human oral squamous cell carcinoma cells: A preliminary study. J. Oral Maxillofac. Surg. Med. Pathol. 2017, 29, 17–23. [Google Scholar] [CrossRef]
- Kieliszek, M.; Lipinski, B.; Blazejak, S. Application of sodium selenite in the prevention and treatment of cancers. Cells 2017, 6, 39. [Google Scholar] [CrossRef]
- Tan, H.W.; Xu, Y.M.; Wu, D.D.; Lau, A.T.Y. Recent insights into human bronchial proteomics – how are we progressing and what is next? Expert. Rev. Proteomics 2018, 15, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Brodin, O.; Eksborg, S.; Wallenberg, M.; Asker-Hagelberg, C.; Larsen, E.H.; Mohlkert, D.; Lenneby-Helleday, C.; Jacobsson, H.; Linder, S.; Misra, S.; et al. Pharmacokinetics and toxicity of sodium selenite in the treatment of patients with carcinoma in a phase I clinical trial: The SECAR Study. Nutrients 2015, 7, 4978–4994. [Google Scholar] [CrossRef] [PubMed]
- Berthier, S.; Arnaud, J.; Champelovier, P.; Col, E.; Garrel, C.; Cottet, C.; Boutonnat, J.; Laporte, F.; Faure, P.; Hazane-Puch, F. Anticancer properties of sodium selenite in human glioblastoma cell cluster spheroids. J. Trace Elem. Med. Biol. 2017, 44, 161–176. [Google Scholar] [CrossRef]
- Lipinski, B. Sodium selenite as an anticancer agent. Anticancer Agents Med. Chem. 2017, 17, 658–661. [Google Scholar] [CrossRef]
- Chen, W.; An, J.; Guo, J.; Wu, Y.; Yang, L.; Dai, J.; Gong, K.; Miao, S.; Xi, S.; Du, J. Sodium selenite attenuates lung adenocarcinoma progression by repressing SOX2-mediated stemness. Cancer Chemother. Pharmacol. 2018, 81, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Olm, E.; Fernandes, A.P.; Hebert, C.; Rundlöf, A.K.; Larsen, E.H.; Danielsson, O.; Björnstedt, M. Extracellular thiol-assisted selenium uptake dependent on the xc- cystine transporter explains the cancer-specific cytotoxicity of selenite. PNAS 2009, 106, 11400–11405. [Google Scholar] [CrossRef] [PubMed]
- Enqvist, M.; Nilsonne, G.; Hammarfjord, O.; Wallin, R.P.A.; Bjorkstrom, N.K.; Bjornstedt, M.; Hjerpe, A.; Ljunggren, H.G.; Dobra, K.; Malmberg, K.J.; et al. Selenite induces posttranscriptional blockade of HLA-E expression and sensitizes tumor cells to CD94/NKG2A-Positive NK Cells. J. Immunol. 2011, 187, 3546–3554. [Google Scholar] [CrossRef]
- Rigobello, M.P.; Gandin, V.; Folda, A.; Rundlof, A.K.; Fernandes, A.P.; Bindoli, A.; Marzano, C.; Bjornstedt, M. Treatment of human cancer cells with selenite or tellurite in combination with auranofin enhances cell death due to redox shift. Free Radic. Biol. Med. 2009, 47, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Xu, W.; Sun, R.; Yin, H.; Dong, C.; Zeng, H. Synergism between thioredoxin reductase inhibitor ethaselen and sodium selenite in inhibiting proliferation and inducing death of human non-small cell lung cancer cells. Chem. Biol. Interact. 2017, 275, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, K.; Ni, L.; Wang, X.; Wang, D.; Zhang, J. Sodium selenosulfate at an innocuous dose markedly prevents cisplatin-induced gastrointestinal toxicity. Toxicol. Appl. Pharmacol. 2012, 258, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M.; Alves, V.; Sarmento-Ribeiro, A.B.; Mota-Pinto, A. Combined effect of sodium selenite and docetaxel on PC3 metastatic prostate cancer cell line. Biochem. Biophys. Res. Commun. 2011, 408, 713–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroeder, C.P.; Goeldner, E.M.; Schulze-Forster, K.; Eickhoff, C.A.; Holtermann, P.; Heidecke, H. Effect of selenite combined with chemotherapeutic agents on the proliferation of human carcinoma cell lines. Biol. Trace Elem. Res. 2004, 99, 17–25. [Google Scholar] [CrossRef]
- Björkhem-Bergman, L.; Jönsson, K.; Eriksson, L.C.; Olsson, J.M.; Lehmann, S.; Paul, C.; Björnstedt, M. Drug-resistant human lung cancer cells are more sensitive to selenium cytotoxicity. Effects on thioredoxin reductase and glutathione reductase. Biochem. Pharmacol. 2002, 63, 1875–1884. [Google Scholar] [CrossRef]
- Jönsson-Videsäter, K.; Björkhem-Bergman, L.; Hossain, A.; Söderberg, A.; Eriksson, L.C.; Paul, C.; Rosén, A.; Björnstedt, M. Selenite-induced apoptosis in doxorubicin-resistant cells and effects on the thioredoxin system. Biochem. Pharmacol. 2004, 67, 513–522. [Google Scholar] [CrossRef]
- Hinrichsen, S.; Planer-Friedrich, B. Cytotoxic activity of selenosulfate versus selenite in tumor cells depends on cell line and presence of amino acids. Environ. Sci. Pollut. Res. Int. 2016, 23, 8349–8357. [Google Scholar] [CrossRef]
- Hu, B.; Cheng, R.; Gao, X.; Pan, X.; Kong, F.; Liu, X.; Xu, K.; Tang, B. Targetable mesoporous Silica nanoprobes for mapping the subcellular distribution of H2Se in cancer cells. ACS Appl. Mater. Interfaces 2018, 10, 17345–17351. [Google Scholar] [CrossRef]
- Storkey, C.; Davies, M.J.; White, J.M.; Schiesser, C.H. Synthesis and antioxidant capacity of 5-selenopyranose derivatives. Chem. Commun. 2011, 47, 9693–9695. [Google Scholar] [CrossRef]
- Jariwalla, R.J.; Gangapurkar, B.; Nakamura, D. Differential sensitivity of various human tumour-derived cell types to apoptosis by organic derivatives of selenium. Br. J. Nutr. 2009, 101, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.C.; Tsai, M.L.; Kuo, F.L.; Lai, C.S.; Badmaev, V.; Ho, C.T.; Pan, M.H. Se-Methyl-L-selenocysteine induces apoptosis via endoplasmic reticulum stress and the death receptor pathway in human colon adenocarcinoma COLO 205 Cells. J. Agricult. Food Chem. 2015, 63, 5008–5016. [Google Scholar] [CrossRef] [PubMed]
- Domracheva, I.; Kanepe-Lapsa, I.; Jackevica, L.; Vasiljeva, J.; Arsenyan, P. Selenopheno quinolinones and coumarins promote cancer cell apoptosis by ROS depletion and caspase-7 activation. Life Sci. 2017, 186, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Zheng, W.; Fu, X.; Li, X.; Wong, Y.S.; Chen, T. Strategy to enhance the therapeutic effect of doxorubicin in human hepatocellular carcinoma by selenocystine, a synergistic agent that regulates the ROS-mediated signaling. Oncotarget 2014, 5, 2853–2863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deepagan, V.G.; Kwon, S.; You, D.G.; Nguyen, V.Q.; Um, W.; Ko, H.; Lee, H.; Jo, D.G.; Kang, Y.M.; Park, J.H. In situ diselenide-crosslinked polymeric micelles for ROS-mediated anticancer drug delivery. Biomaterials 2016, 103, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Çetin, E.S.; Naziroğlu, M.; Çiğ, B.; Övey, I.S.; Koşar, P.A. Selenium potentiates the anticancer effect of cisplatin against oxidative stress and calcium ion signaling-induced intracellular toxicity in MCF-7 breast cancer cells: Involvement of the TRPV1 channel. J. Recept. Signal. Transduct. Res. 2017, 37, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Gandin, V.; Khalkar, P.; Braude, J.; Fernandes, A.P. Organic selenium compounds as potential chemotherapeutic agents for improved cancer treatment. Free Radic. Biol. Med. 2018, 127, 80–97. [Google Scholar] [CrossRef]
- Álvarez-Pérez, M.; Ali, W.; Marć, M.A.; Handzlik, J.; Domínguez-Álvarez, E. Selenides and diselenides: A review of their anticancer and chemopreventive activity. Molecules 2018, 23, 628. [Google Scholar] [CrossRef]
- Ali, W.; Álvarez-Pérez, M.; Marć, M.A.; Salardón-Jiménez, N.; Handzlik, J.; Domínguez-Álvarez, E. The anticancer and chemopreventive activity of selenocyanate-containing compounds. Curr. Pharmacol. Rep. 2018, 4, 468–481. [Google Scholar] [CrossRef]
- de Bruin, E.C.; Medema, J.P. Apoptosis and non-apoptotic deaths in cancer development and treatment response. Cancer Treat. Rev. 2008, 34, 737–749. [Google Scholar] [CrossRef]
- Poerschke, R.L.; Franklin, M.R.; Moos, P.J. Modulation of redox status in human lung cell lines by organoselenocompounds: Selenazolidines, selenomethionine, and methylseleninic acid. Toxicol. In Vitro 2008, 22, 1761–1767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, R.; Zhao, L.; Liu, X.; Rowan, B.G.; Wabitsch, M.; Edwards, D.P.; Nishi, Y.; Yanase, T.; Yu, Q.; Dong, Y. Methylseleninic acid is a novel suppressor of aromatase expression. J. Endocrinol. 2012, 212, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Fu, X.; Xiong, Z.; Zhang, H.; Hill, S.M.; Rowan, B.G.; Dong, Y. Methylseleninic acid enhances paclitaxel efficacy for the treatment of triple-negative breast cancer. PLoS ONE 2012, 7, e31539. [Google Scholar] [CrossRef] [PubMed]
- Lennicke, C.; Rahn, J.; Bukur, J.; Hochgräfe, F.; Wessjohann, L.A.; Lichtenfels, R.; Seliger, B. Modulation of MHC class I surface expression in B16F10 melanoma cells by methylseleninic acid. Oncoimmunology 2017, 6, e1259049. [Google Scholar] [CrossRef] [PubMed]
- Li, G.X.; Lee, H.J.; Wang, Z.; Hu, H.; Liao, J.D.; Watts, J.C.; Combs, G.F.; Lü, J. Superior in vivo inhibitory efficacy of methylseleninic acid against human prostate cancer over selenomethionine or selenite. Carcinogenesis 2008, 29, 1005–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Bonorden, M.J.L.; Li, G.; Lee, H.J.; Hu, H.; Zhang, Y.; Liao, J.D.; Cleary, M.P.; Lü, J. Methyl-selenium compounds inhibit prostate carcinogenesis in the transgenic adenocarcinoma of mouse prostate model with survival benefit. Cancer Prev. Res. 2009, 2, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.O.; Chun, J.Y.; Nadiminty, N.; Trump, D.L.; Ip, C.; Dong, Y.; Gao, A.C. Monomethylated selenium inhibits growth of LNCaP human prostate cancer xenograft accompanied by a decrease in the expression of androgen receptor and prostate-specific antigen (PSA). Prostate 2006, 66, 1070–1075. [Google Scholar] [CrossRef] [PubMed]
- Lobb, R.J.; Jacobson, G.M.; Cursons, R.T.; Jameson, M.B. The interaction of selenium with chemotherapy and radiation on normal and malignant human mononuclear blood cells. Int. J. Mol. Sci. 2018, 19, 3167. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, J.; Yang, Q.; Si, Y.; Liu, Y.; Wang, Q.; Han, F.; Huang, Z. Synergistic effects of SAM and selenium compounds on proliferation, migration and adhesion of HeLa cells. Anticancer Res. 2017, 37, 4433–4441. [Google Scholar] [PubMed]
- Li, Z.; Carrier, L.; Rowan, B.G. Methylseleninic acid synergizes with tamoxifen to induce caspase-mediated apoptosis in breast cancer cells. Mol. Cancer Ther. 2008, 7, 3056–3063. [Google Scholar] [CrossRef] [Green Version]
- Khalkar, P.; Diaz-Argelich, N.; Antonio Palop, J.; Sanmartín, C.; Fernandes, A.P. Novel methylselenoesters induce programed cell death via entosis in pancreatic cancer cells. Int. J. Mol. Sci. 2018, 19, 2849. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Yu, L.; Yang, F.; Yan, J.; Zeng, H. A novel organoselenium compound induces cell cycle arrest and apoptosis in prostate cancer cell lines. Biochem. Biophys. Res. Commun. 2003, 309, 578–583. [Google Scholar] [CrossRef]
- Sharma, V.; Tewari, R.; Sk, U.H.; Joseph, C.; Sen, E. Ebselen sensitizes glioblastoma cells to Tumor Necrosis Factor (TNFα)-induced apoptosis through two distinct pathways involving NF-κβ downregulation and Fas-mediated formation of death inducing signaling complex. Int. J. Cancer 2008, 123, 2204–2212. [Google Scholar] [CrossRef] [PubMed]
- Shiah, H.S.; Lee, W.S.; Juang, S.H.; Hong, P.C.; Lung, C.C.; Chang, C.J.; Chou, K.M.; Chang, J.Y. Mitochondria-mediated and p53-associated apoptosis induced in human cancer cells by a novel selenophene derivative, D-501036. Biochem. Pharmacol. 2007, 73, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Juang, S.H.; Lung, C.C.; Hsu, P.C.; Hsu, K.S.; Li, Y.C.; Hong, P.C.; Shiah, H.S.; Kuo, C.C.; Huang, C.W.; Wang, Y.C.; et al. D-501036, a novel selenophene-based triheterocycle derivative, exhibits potent in vitro and in vivo antitumoral activity which involves DNA damage and ataxia telangiectasia-mutated nuclear protein kinase activation. Mol. Cancer Ther. 2007, 6, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.N.; Chou, K.M.; Pan, W.Y.; Chen, Y.W.; Tsou, T.C.; Yeh, S.C.; Cheung, C.H.; Chen, L.T.; Chang, J.Y. Enhancement of non-homologous end joining DNA repair capacity confers cancer cells resistance to the novel selenophene compound, D-501036. Cancer Lett. 2011, 309, 110–118. [Google Scholar] [CrossRef]
- Gumulec, J.; Balvan, J.; Sztalmachova, M.; Raudenska, M.; Dvorakova, V.; Knopfova, L.; Polanska, H.; Hudcova, K.; Ruttkay-Nedecky, B.; Babula, P.; et al. Cisplatin-resistant prostate cancer model: Differences in antioxidant system, apoptosis and cell cycle. Int. J. Oncol. 2014, 44, 923–933. [Google Scholar] [CrossRef]
- Bartolini, D.; Sancineto, L.; Fabro de Bem, A.; Tew, K.D.; Santi, C.; Radi, R.; Toquato, P.; Galli, F. Selenocompounds in cancer therapy: An overview. Adv. Cancer Res. 2017, 136, 259–302. [Google Scholar]
- Ho, B.N.; Pfeffer, C.M.; Singh, A.T.K. Update on nanotechnology-based drug delivery systems in cancer treatment. Anticancer Res. 2017, 37, 5975–5981. [Google Scholar]
- Gao, F.; Yuan, Q.; Gao, L.; Cai, P.; Zhu, H.; Liu, R.; Wang, Y.; Wei, Y.; Huang, G.; Liang, J.; et al. Cytotoxicity and therapeutic effect of irinotecan combined with selenium nanoparticles. Biomaterials 2014, 35, 8854–8866. [Google Scholar] [CrossRef]
- Shamsi, M.M.; Chekachak, S.; Soudi, S.; Gharakhanlou, R.; Quinn, L.S.; Ranjbar, K.; Rezaei, S.; Shirazi, F.J.; Allahmoradi, B.; Yazdi, M.H.; et al. Effects of exercise training and supplementation with selenium nanoparticle on T-helper 1 and 2 and cytokine levels in tumor tissue of mice bearing the 4 T1 mammary carcinoma. Nutrition 2018, 57, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Skalickova, S.; Milosavljevic, V.; Cihalova, K.; Horky, P.; Richtera, L.; Adam, V. Selenium nanoparticles as a nutritional supplement. Nutrition 2017, 33, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Teng, Z.; Yuan, Y.; Zeng, Q.Z.; Lou, Z.; Lee, S.H.; Wang, Q. Development, physicochemical characterization and cytotoxicity of selenium nanoparticles stabilized by beta-lactoglobulin. Int. J. Biol. Macromol. 2018, 107, 1406–1413. [Google Scholar] [CrossRef] [PubMed]
- Menon, S.; KS, S.D.; Santhiya, R.; Rajeshkumar, S.; Kumar, V. Selenium nanoparticles: A potent chemotherapeutic agent and an elucidation of its mechanism. Colloids Surf B Biointerfaces 2018, 170, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Wadhwani, S.A.; Gorain, M.; Banerjee, P.; Shedbalkar, U.U.; Singh, R.; Kundu, G.C.; Chopade, B.A. Green synthesis of selenium nanoparticles using Acinetobacter sp. SW30: Optimization, characterization and its anticancer activity in breast cancer cells. Int. J. Nanomed. 2017, 12, 6841–6855. [Google Scholar] [CrossRef] [PubMed]
- Sonkusre, P.; Cameotra, S.S. Biogenic selenium nanoparticles induce ROS-mediated necroptosis in PC-3 cancer cells through TNF activation. J. Nanobiotechnol. 2017, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Yan, C.; Miao, J.; Zhang, X.; Chen, J.; Sun, L.; Meng, L.; Liang, T.; Li, Q. Synthesis, characterization and antitumor properties of selenium nanoparticles coupling with ferulic acid. Mater. Sci. Eng. C 2018, 90, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Shahverdi, A.R.; Shahverdi, F.; Faghfuri, E.; Reza Khoshayand, M.; Mavandadnejad, F.; Yazdi, M.H.; Amini, M. Characterization of folic acid surface-coated selenium nanoparticles and corresponding in vitro and in vivo effects against breast cancer. Arch. Med. Res. 2018, 49, 10–17. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, Q.; Zhao, Z.; He, L.; Chan, L.; Liu, Y.; Chen, Y.; Bai, M.; Pan, T.; Qu, Y.; et al. Functionalized selenium nanosystem as radiation sensitizer of 125I seeds for precise cancer therapy. ACS Appl. Mater. Interfaces 2017, 9, 25857–25869. [Google Scholar] [CrossRef]
- Qiu, W.Y.; Wang, Y.Y.; Wang, M.; Yan, J.K. Construction, stability, and enhanced antioxidant activity of pectin-decorated selenium nanoparticles. Colloids Surf. B Biointerfaces 2018, 170, 692–700. [Google Scholar] [CrossRef]
- Huang, G.; Liu, Z.; He, L.; Luk, K.H.; Cheung, S.T.; Wong, K.H.; Chen, T. Autophagy is an important action mode for functionalized selenium nanoparticles to exhibit anti-colorectal cancer activity. Biomater. Sci. 2018, 6, 2508–2517. [Google Scholar] [CrossRef] [PubMed]
- Gautam, P.K.; Kumar, S.; Tomar, M.S.; Singh, R.K.; Acharya, A.; Kumar, S.; Ram, B. Selenium nanoparticles induce suppressed function of tumor associated macrophages and inhibit Dalton′s lymphoma proliferation. Biochem. Biophys. Rep. 2017, 12, 172–184. [Google Scholar] [PubMed]
- Zhao, G.; Wu, X.; Chen, P.; Zhang, L.; Yang, C.S.; Zhang, J. Selenium nanoparticles are more efficient than sodium selenite in producing reactive oxygen species and hyper-accumulation of selenium nanoparticles in cancer cells generates potent therapeutic effects. Free Radic. Biol. Med. 2018, 126, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Zhai, S.; Hu, X.; Hu, Y.; Wu, B.; Xing, D. Visible light-induced crosslinking and physiological stabilization of diselenide-rich nanoparticles for redox-responsive drug release and combination chemotherapy. Biomaterials 2017, 121, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Chen, X.; Gong, H.; Qiu, P.; Xiao, X.; Dang, S.; Hong, A.; Ma, Y. Delivery of a TNF-α–derived peptide by nanoparticles enhances its antitumor activity by inducing cell-cycle arrest and caspase-dependent apoptosis. FASEB J. 2018. [Google Scholar] [CrossRef] [PubMed]
- Luesakul, U.; Puthong, S.; Neamati, N.; Muangsin, N. pH-responsive selenium nanoparticles stabilized by folate-chitosan delivering doxorubicin for overcoming drug-resistant cancer cells. Carbohydr. Polym. 2018, 181, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Jalalian, S.H.; Ramezani, M.; Abnous, K.; Taghdisi, S.M. Targeted co-delivery of epirubicin and NAS-24 aptamer to cancer cells using selenium nanoparticles for enhancing tumor response in vitro and in vivo. Cancer Lett. 2018, 416, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Nonsuwan, P.; Puthong, S.; Palaga, T.; Muangsin, N. Novel organic/inorganic hybrid flower-like structure of selenium nanoparticles stabilized by pullulan derivatives. Carbohydr. Polym. 2018, 184, 9–19. [Google Scholar] [CrossRef]
- Xia, Y.; Guo, M.; Xu, T.; Li, Y.; Wang, C.; Lin, Z.; Zhao, M.; Zhu, B. siRNA-loaded selenium nanoparticle modified with hyaluronic acid for enhanced hepatocellular carcinoma therapy. Int. J. Nanomed. 2018, 13, 1539–1552. [Google Scholar] [CrossRef] [Green Version]
- Abd-Rabou, A.A.; Shalby, A.B.; Ahmed, H.H. Selenium nanoparticles induce the chemo-sensitivity of fluorouracil nanoparticles in breast and colon cancer cells. Biol. Trace Elem. Res. 2018. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, L.; Qiu, N.; Liu, Y.; Xu, B.; Zhu, H. On the hypoxic tumor targeting ability of two chitosan micelles loaded with oil-soluble CdSe quantum dots. Pharm. Dev. Technol. 2018, 23, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Pi, J.; Jiang, J.; Cai, H.; Yang, F.; Jin, H.; Yang, P.; Cai, J.; Chen, Z.W. GE11 peptide conjugated selenium nanoparticles for EGFR targeted oridonin delivery to achieve enhanced anticancer efficacy by inhibiting EGFR-mediated PI3K/AKT and Ras/Raf/MEK/ERK pathways. Drug Deliv. 2017, 24, 1549–1564. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, M.; Zheng, W.; Xu, T.; Deng, H.; Liu, J. Se/Ru-decorated porous metal-organic framework nanoparticles for the delivery of pooled siRNAs to reversing multidrug resistance in taxol-resistant breast cancer cells. ACS Appl. Mater. Interfaces 2017, 9, 6712–6724. [Google Scholar] [CrossRef] [PubMed]
- Cadkova, M.; Kovarova, A.; Dvorakova, V.; Metelka, R.; Bilkova, Z.; Korecka, L. Electrochemical quantum dots-based magneto-immunoassay for detection of HE4 protein on metal film-modified screen-printed carbon electrodes. Talanta 2018, 182, 111–115. [Google Scholar] [CrossRef]
- Liu, M.L.; Zou, H.Y.; Li, C.M.; Li, R.S.; Huang, C.Z. Aptamer-modified selenium nanoparticles for dark-field microscopy imaging of nucleolin. Chem. Commun. 2017, 53, 13047–13050. [Google Scholar] [CrossRef]
- Moulick, A.; Milosavljevic, V.; Vlachova, J.; Podgajny, R.; Hynek, D.; Kopel, P.; Adam, V. Using CdTe/ZnSe core/shell quantum dots to detect DNA and damage to DNA. Int. J. Nanomed. 2017, 12, 1277–1291. [Google Scholar] [CrossRef] [PubMed]
- Madhankumar, A.B.; Mrowczynski, O.D.; Patel, S.R.; Weston, C.L.; Zacharia, B.E.; Glantz, M.J.; Siedlecki, C.A.; Xu, L.C.; Connor, J.R. Interleukin-13 conjugated quantum dots for identification of glioma initiating cells and their extracellular vesicles. Acta Biomater. 2017, 58, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sun, Q.; Zhang, X.; Baeyens, J.; Su, H. Self-assembled selenium nanoparticles and their application in the rapid diagnostic detection of small cell lung cancer biomarkers. Soft Matter 2018, 14, 481–489. [Google Scholar] [CrossRef]
- Zhu, C.N.; Chen, G.; Tian, Z.Q.; Wang, W.; Zhong, W.Q.; Li, Z.; Zhang, Z.L.; Pang, D.W. Near-infrared fluorescent Ag2Se–cetuximab nanoprobes for targeted imaging and therapy of cancer. Small 2017, 13. [Google Scholar] [CrossRef]
- Purohit, M.P.; Verma, N.K.; Kar, A.K.; Singh, A.; Ghosh, D.; Patnaik, S. Inhibition of thioredoxin reductase by targeted selenopolymeric nanocarriers synergizes the therapeutic efficacy of doxorubicin in MCF7 human breast cancer cells. ACS Appl. Mater. Interfaces 2017, 9, 36493–36512. [Google Scholar] [CrossRef]
- Zhao, S.; Yu, Q.; Pan, J.; Zhou, Y.; Cao, C.; Ouyang, J.M.; Liu, J. Redox-responsive mesoporous selenium delivery of doxorubicin targets MCF-7 cells and synergistically enhances its anti-tumor activity. Acta Biomater. 2017, 54, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Zhu, X.; Chen, X.; Zhao, Y.; Liu, J. Gd3+-Doped MoSe2 nanosheets used as a theranostic agent for bimodal imaging and highly efficient photothermal cancer therapy. Biomater. Sci. 2018, 6, 372–387. [Google Scholar] [CrossRef] [PubMed]
- Bidkar, A.P.; Sanpui, P.; Ghosh, S.S. Efficient induction of apoptosis in cancer cells by paclitaxel-loaded selenium nanoparticles. Nanomedicine 2017, 12, 2641–2651. [Google Scholar] [CrossRef] [PubMed]
- Hauksdóttir, H.L.; Webster, T.J. Selenium and iron oxide nanocomposites for magnetically-targeted anti-cancer applications. J. Biomed. Nanotechnol. 2018, 14, 510–525. [Google Scholar] [CrossRef] [PubMed]
- Kolmas, J.; Pajor, K.; Pajchel, L.; Przekora, A.; Ginalska, G.; Oledzka, E.; Sobczak, M. Fabrication and physicochemical characterization of porous composite microgranules with selenium oxyanions and risedronate sodium for potential applications in bone tumors. Int. J. Nanomed. 2017, 12, 5633–5642. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Li, C.; Zheng, L.; Yang, F.; Chen, T. Dual-targeted selenium nanoparticles for synergistic photothermal therapy and chemotherapy of tumors. Chem. Asian J. 2018, 13, 996–1004. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, X.H.; Hu, X.D.; Liu, P.D.; Zhang, H.Q. The effects of combined selenium nanoparticles and radiation therapy on breast cancer cells in vitro. Artif. Cells Nanomed. Biotechnol. 2018, 46, 937–948. [Google Scholar] [CrossRef]
- Maiyo, F.; Singh, M. Selenium nanoparticles: Potential in cancer gene and drug delivery. Nanomedicine 2017, 12, 1075–1089. [Google Scholar] [CrossRef]
- Zhai, X.; Zhang, C.; Zhao, G.; Stoll, S.; Ren, F.; Leng, X. Antioxidant capacities of the selenium nanoparticles stabilized by chitosan. J. Nanobiotechnol. 2017, 15, 1–12. [Google Scholar] [CrossRef]
- Kumari, M.; Ray, L.; Purohit, M.P.; Patnaik, S.; Pant, A.B.; Shukla, Y.; Kumar, P.; Gupta, K.C. Curcumin loading potentiates the chemotherapeutic efficacy of selenium nanoparticles in HCT116 cells and Ehrlich′s ascites carcinoma bearing mice. Eur. J. Pharm. Biopharm. 2017, 117, 346–362. [Google Scholar] [CrossRef]
- Wadhwani, S.A.; Shedbalkar, U.U.; Singh, R.; Chopade, B.A. Biogenic selenium nanoparticles: Current status and future prospects. Appl. Microbiol. Biotechnol. 2016, 100, 2555–2566. [Google Scholar] [CrossRef] [PubMed]
- Vetchinkina, E.; Loshchinina, E.; Kursky, V.; Nikitina, V. Reduction of organic and inorganic selenium compounds by the edible medicinal basidiomycete Lentinula edodes and the accumulation of elemental selenium nanoparticles in its mycelium. J. Microbiol. 2013, 51, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Tugarova, A.V.; Kamnev, A.A. Proteins in microbial synthesis of selenium nanoparticles. Talanta 2017, 174, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.S.; Patel, H.; Patel, T.; Patel, K.; Selvaraj, K. Biosynthesis of Se nanoparticles and its effect on UV-induced DNA damage. Colloids Surf. B Biointerfaces 2013, 103, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Zonaro, E.; Piacenza, E.; Presentato, A.; Monti, F.; Dell′Anna, R.; Lampis, S.; Vallini, G. Ochrobactrum sp. MPV1 from a dump of roasted pyrites can be exploited as bacterial catalyst for the biogenesis of selenium and tellurium nanoparticles. Microb. Cell Fact. 2017, 16, 215. [Google Scholar] [CrossRef] [PubMed]
- Ramya, S.; Shanmugasundaram, T.; Balagurunathan, R. Biomedical potential of actinobacterially synthesized selenium nanoparticles with special reference to anti-biofilm, anti-oxidant, wound healing, cytotoxic and anti-viral activities. J. Trace Elem. Med. Biol. 2015, 32, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Saratale, R.G.; Karuppusamy, I.; Saratale, G.D.; Pugazhendhi, A.; Kumar, G.; Park, Y.; Ghodake, G.S.; Bharagava, R.N.; Banu, J.R.; Shin, H.S. A comprehensive review on green nanomaterials using biological systems: Recent perception and their future applications. Colloids Surf. B Biointerfaces 2018, 170, 20–35. [Google Scholar] [CrossRef]
- Eswayah, A.S.; Smith, T.J.; Scheinost, A.C.; Hondow, N.; Gardiner, P.H.E. Microbial transformations of selenite by methane-oxidizing bacteria. Appl. Microbiol. Biotechnol. 2017, 101, 6713–6724. [Google Scholar] [CrossRef] [Green Version]
- Song, D.; Li, X.; Cheng, Y.; Xiao, X.; Lu, Z.; Wang, Y.; Wang, F. Aerobic biogenesis of selenium nanoparticles by Enterobacter cloacae Z0206 as a consequence of fumarate reductase mediated selenite reduction. Sci. Rep. 2017, 7, 3239. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Llamosas, H.; Castro, L.; Blazquez, M.L.; Diaz, E.; Carmona, M. Speeding up bioproduction of selenium nanoparticles by using Vibrio natriegens as microbial factory. Sci. Rep. 2017, 7, 16046. [Google Scholar] [CrossRef]
- Quintana, M.; Haro-Poniatowski, E.; Morales, J.; Batina, N. Synthesis of selenium nanoparticles by pulsed laser ablation. Appl. Surf. Sci. 2002, 195, 175–186. [Google Scholar] [CrossRef]
- Chang, S.Q.; Dai, Y.D.; Kang, B.; Han, W.; Chen, D. Gamma-radiation synthesis of silk fibroin coated CdSe quantum dots and their biocompatibility and photostability in living cells. J. Nanosci. Nanotechnol. 2009, 9, 5693–5700. [Google Scholar] [CrossRef] [PubMed]
- Guisbiers, G.; Lara, H.H.; Mendoza-Cruz, R.; Naranjo, G.; Vincent, B.A.; Peralta, X.G.; Nash, K.L. Inhibition of Candida albicans biofilm by pure selenium nanoparticles synthesized by pulsed laser ablation in liquids. Nanomedicine 2017, 13, 1095–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasinski, A.L.; Slack, F.J. Epigenetics and genetics. MicroRNAs en route to the clinic: Progress in validating and targeting microRNAs for cancer therapy. Nat. Rev. Cancer 2011, 11, 849–864. [Google Scholar] [CrossRef] [PubMed]
- Berndsen, R.H.; Abdul, U.K.; Weiss, A.; Zoetemelk, M.; Te Winkel, M.T.; Dyson, P.J.; Griffioen, A.W.; Nowak-Sliwinska, P. Epigenetic approach for angiostatic therapy: Promising combinations for cancer treatment. Angiogenesis 2017, 20, 245–267. [Google Scholar] [CrossRef] [PubMed]
- Speckmann, B.; Grune, T. Epigenetic effects of selenium and their implications for health. Epigenetics 2015, 10, 179–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jablonska, E.; Reszka, E. Selenium and epigenetics in cancer: Focus on DNA methylation. Adv. Cancer Res. 2017, 136, 193–234. [Google Scholar]
- Xiang, N.; Zhao, R.; Song, G.; Zhong, W. Selenite reactivates silenced genes by modifying DNA methylation and histones in prostate cancer cells. Carcinogenesis 2008, 29, 2175–2181. [Google Scholar] [CrossRef] [Green Version]
- de Miranda, J.X.; Andrade Fde, O.; Conti, A.; Dagli, M.L.; Moreno, F.S.; Ong, T.P. Effects of selenium compounds on proliferation and epigenetic marks of breast cancer cells. J. Trace Elem. Med. Biol. 2014, 28, 486–491. [Google Scholar] [CrossRef]
- Hu, C.; Liu, M.; Zhang, W.; Xu, Q.; Ma, K.; Chen, L.; Wang, Z.; He, S.; Zhu, H.; Xu, N. Upregulation of KLF4 by methylseleninic acid in human esophageal squamous cell carcinoma cells: Modification of histone H3 acetylation through HAT/HDAC interplay. Mol. Carcinog. 2015, 54, 1051–1059. [Google Scholar] [CrossRef]
- Wu, J.C.; Wang, F.Z.; Tsai, M.L.; Lo, C.Y.; Badmaev, V.; Ho, C.T.; Wang, Y.J.; Pan, M.H. Se-Allylselenocysteine induces autophagy by modulating the AMPK/mTOR signaling pathway and epigenetic regulation of PCDH17 in human colorectal adenocarcinoma cells. Mol. Nutr. Food Res. 2015, 59, 2511–2522. [Google Scholar] [CrossRef] [PubMed]
- Silvers, A.L.; Lin, L.; Bass, A.J.; Chen, G.; Wang, Z.; Thomas, D.G.; Lin, J.; Giordano, T.J.; Orringer, M.B.; Beer, D.G.; et al. Decreased selenium-binding protein 1 in esophageal adenocarcinoma results from posttranscriptional and epigenetic regulation and affects chemosensitivity. Clin. Cancer Res. 2010, 16, 2009–2021. [Google Scholar] [CrossRef] [PubMed]
- Khalkar, P.; Ali, H.A.; Codo, P.; Argelich, N.D.; Martikainen, A.; Arzenani, M.K.; Lehmann, S.; Walfridsson, J.; Ungerstedt, J.; Fernandes, A.P. Selenite and methylseleninic acid epigenetically affects distinct gene sets in myeloid leukemia: A genome wide epigenetic analysis. Free Radic. Biol. Med. 2018, 117, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Hosnedlova, B.; Kepinska, M.; Skalickova, S.; Fernandez, C.; Ruttkay-Nedecky, B.; Peng, Q.; Baron, M.; Melcova, M.; Opatrilova, R.; Zidkova, J.; et al. Nano-selenium and its nanomedicine applications: A critical review. Int. J. Nanomed. 2018, 13, 2107–2128. [Google Scholar] [CrossRef] [PubMed]
- Sakr, T.M.; Korany, M.; Katti, K.V. Selenium nanomaterials in biomedicine—An overview of new opportunities in nanomedicine of selenium. J. Drug Deliv. Sci. Tec. 2018, 46, 223–233. [Google Scholar] [CrossRef]

| SeNP | Material | Shape and Size (nm) | Effects | Dosage | Pathway | Model | Reference |
|---|---|---|---|---|---|---|---|
| Acinetobacter sp. SW30 SeNPs | Acinetobacter sp. SW30 | Amorphous nanospheres, 78 nm; Polygonal-shaped, 79 nm | Selectively against breast cancer cells and non-toxic to normal cells | - | - | Breast cancer cells (4T1, MCF-7) and noncancer cells (NIH/3T3, HEK293) | [126] |
| Bacillus licheniformis JS2 derived biogenic SeNPs | Bacillus licheniformis JS2, aerobic condition in 1.8 mM Na2SeO3 stress | Spherical, 110 nm | Stimulated ROS production and caused damage to the mitochondria without affecting the cell membrane integrity. Induced overexpression of necroptotic genes and promoted RIP3-independent necroptosis | Concentration of 2 μg Se/mL | - | Human prostate adenocarcinoma cell line (PC-3) | [127] |
| Blg stabilized SeNPs | Ascorbic acid, Blg, Na2SeO3 | Spherical, mean particle size of 36.8 ± 4.1 nm | Lower cytotoxicity than Na2SeO3. Similar cell growth inhibition on both colon cancer cell and corresponding normal cell | - | - | Human colon adenocarcinoma cells (HCT116) and colon normal cell (CCD112) | [124] |
| Ferulic acid-modified SeNPs | Na2SeO3, ascorbic acid, ferulic acid solution | Amorphous, average diameter of 109 nm | Induced intracellular ROS generation and MMP disruption | >100 μg/mL | Caspase-3/9, mitochondrial pathway | HepG2 | [128] |
| Folic acid surface-coated Se nanoparticles (FA@-SeNPs) | Folic acid, SeO2 solution, ascorbic acid solution | Rod-shaped (400 × 100 nm) | Showed antiproliferative effect against 4T1 cells. Significantly increased the lifespan and reduced the tumor size of cancerous animals. Had better absorption toward cancer cells. Exhibited a better in vivo anticancer effect compared to SeNPs | 200 μg/mL in vitro and 300 mg/week in vivo | - | 4T1 breast cancer cell line and inbred Balb/c mice | [129] |
| Folic acid-conjugated SeNPs (FA@SeNPs) | Folic acid, CTS, Na2SeO3, ascorbic acid | Spherical, ~192 nm | Able to synergistically enhance the anticancer efficacy and colony formation inhibition ability of radioactive 125I seeds. Increased ROS overproduction. Induced DNA damage and activated the mitogen-activated protein kinase and TP53 signaling pathways | 5 mg/kg of FA@SeNPs with an intratumor injection strategy every other day and/ or implanted with radioactive 125I seeds | DNA damage-mediated p53 and MAPK signaling pathways | Michigan Cancer Foundation-7 cell (MCF-7) and female nude mice | [130] |
| PEC-decorated Se nanoparticles | Selenite and ascorbic acid, PEC | Spherical, average size of ~41 nm | PEC as a surface decorator could be effectively used to improve the stability and antioxidant capacity of SeNPs | - | - | Cancer cells (SPCA-1 and HeLa) and normal cells (RWPE-1) | [131] |
| Pleurotus tuber-regium (PTR)-conjugated SeNPs (PTR-SeNPs) | Sclerotia of tiger milk mushrooms, Na2SeO3, ascorbic acid | Se concentration: 1.35 ± 0.12 μM; particle size: 80.0 ± 12.3 nm | Triggered intracellular G2/M phase arrest and apoptosis. Activated autophagy to promote the death of cancer cells | - | Beclin 1-related signaling pathways | Human colon cancer cells (HCT 116) | [132] |
| SeNPs | Se powder, Na2SO4, acetic acid | Spherical, 12–30 nm | Induced TAMs isolated from DL-bearing mice. Induced ROS generation, macrophage polykaryon formation, and adhesion molecules (CD54 or ICAM-1), and fusion receptors (CD47 and CD172α) expression on TAMs. Decreased tumor cell proliferation | 20–50 ng for 106 cells | - | Daltons lymphoma cells and DL-bearing BALB/c (H2d) strain of mice | [133] |
| SeNPs | SeO2 | - | Combination of AET and SeNP supplementation effects anti-tumor immune responses in splenocytes | 6 weeks of AET and SeNP administration (100 mg three times/week). Oral administration in doses of 100 and 200 mL per mouse | - | Mice bearing the 4T1 mammary carcinoma | [122] |
| SeNPs | Na2SeO3, GSH, BSA | 20–70 nm, average size of 40 nm | Able to rapidly, massively, and selectively accumulate in cancer cells. Showed stronger pro-oxidant property than selenite | - | - | Male Kunming mice and Murine H22 hepatocarcinoma cells | [134] |
| SeNP | Material | Shape and Size (nm) | Effects | Dosage | Pathway | Model | Reference |
|---|---|---|---|---|---|---|---|
| CPT and DOX-loaded PEG-b-PBSe core crosslinked micelles (CPT/DOX-CCM) | Diselenide diols precursors, PEG45-based RAFT agent, CPT, DOX | Spherical, ~129 nm | Features include high drug loading, visible light-induced in situ crosslinking, improved physiological stability, optimized pharmacokinetics, and tumor-specific combined drug release | 1 mg CPT or DOX equivalent per kg every four days in 24 days | - | Human breast cancer cell line (MCF-7) and mouse mammary tumor cell line (EMT-6) | [135] |
| CTS-modified Se nanoparticle | Na2SeO3, ascorbic acid solution (vitamin C), CTS | 400–4000 cm-1 | Slow-release carrier conjugated to the TNF-α-derived peptide P16, G0/G1 cell-cycle arrest, and apoptosis | - | p38MAPK/JNK pathway | Prostate cancer cells (DU145) and normal human prostate epithelial cells (RWPE-1) | [136] |
| DOX-SeNPs@TMC-FA (pH-sensitive) | Selenite, ascorbic acid, folic acid-N trimethyl CTS (TMC-FA) | An average diameter of 50 nm | Enhanced the activity of DOX by approximately 10-fold for a reduced IC50 value compared to free DOX | - | Apoptosis pathway involved caspase-3 and PARP proteins | Ovarian cancer DOX sensitive (OVCAR8) and resistant (NCI/ADR-RES) cells | [137] |
| EPI-loaded-NAS-24-functionalized PEIPEG-5TR1 aptamer coated SeNPs (ENPPASe complex) | Na2SeO3, EPI-loaded-NAS-24-functionalized PPA complex | 68.2 ± 6 nm | Able to provide high loading of EPI and NAS-24. Reduced the toxicity in non-target cells. Reduced the cell viability in the target cancer cells. Reduced the tumor growth in cancer-bearing mice compared to EPI treatment alone | - | - | Human breast carcinoma cell (MCF7), murine colon carcinoma cell (C26) and human hepatocellular carcinoma cell (HepG2) | [138] |
| FA-CP/SeNPs | Na2SeO3, folic acid decorated cationic pullulan (FA-CP) | Flower-like structure, approximately 50 nm | Higher loading capacity of DOX. Less toxicity against normal cells | - | - | KB cancer cells line and normal cell line (L292) | [139] |
| Hyaluronic acid Se-PEI nanoparticle | Na2SeO3, ascorbic acid, hyaluronic acid, PEI | 70–180 nm | Showed higher transfection efficiency, greater gene silencing ability, and stronger cytotoxicity | - | - | HepG2 cell, Lo2 cell and xenograft mouse model | [140] |
| Nano-Se + Nano-fluorouracil | Na2SeO3, GSH, BSA | Spherical, ranged from 66.43 nm to 98.9 nm | Induced chemo-sensitivity of 5-fluorouracil-encapsulated poly (D, L-lactide-co-glycolide) nanoparticles (nano-FU) in cancer cells | 0, 2, 4, 6, 8, 10, 30, and 50 μM | Glucose uptake slight blockage, interaction with Zn | Human breast cancer (MCF7) and human colorectal cancer Cell (Caco-2) | [141] |
| Oil-soluble CdSe QD | CdO, mineral oil, oleic acid, Se | DG-PEG-OC-9R, near spherical, 112.0 ± 1.63 nm; FA-PEG-OC-9R, near spherical, 115.2 ± 1.94 nm | Could be used to evaluate the hypoxic tumor cell-targeting properties of the wrapped CTS-based micelles | - | - | Normoxic/hypoxic HepG2 and HeLa cells | [142] |
| Oridonin-loaded and GE11 peptide conjugated SeNPs (GE11-Ori-SeNPs) | Na2SeO3, oridonin, ascorbic acid, GE11 polypeptide | Near-spherical, average diameter of 70 nm | The GE11 surface modification provides targeting towards cancer cells: oridonin releasing induced cancer cell apoptosis. Inhibited tumor growth via inhibition of tumor angiogenesis by reducing the angiogenesis-marker CD31 and activation of the immune system by enhancing IL-2 and TNF-a production | 2.5, 5, and 7.5 mg/kg/day for 15 days through tail intravenous injection | EGFR-mediated PI3K/AKT and Ras/Raf/MEK/ERK pathway, mitochondria-dependent pathway | Human esophageal cancer cell lines (KYSE-150 and EC9706) and KYSE-150 xenograft mice model | [143] |
| Se@MIL-101-(P + V) siRNA | MIL-101(Fe), cysteine, Na2SeO3, siRNA | Spherical, particle size 160 nm, pore diameter 2.19 nm | Enhanced protection of siRNAs against nuclease degradation. Increased siRNA cellular uptake and promoted siRNA escape from endosomes/lysosome to silence MDR genes in MCF-7/T (Taxol-resistance) cells. Enhanced cancer therapeutic efficacy and decreased systemic toxicity in vivo | 10 mg/kg by intravenous injection for 15 d (12 μg of siRNA per mouse) | p53, MAPK, and PI3K/Akt | MCF-7/T cells, paclitaxel resistance MCF-7/T cells and nude mice | [144] |
| SeNP | Material | Shape and Size (nm) | Effects | Dosage | Model | Reference |
|---|---|---|---|---|---|---|
| Anti-HE4 IgG-HE4-anti-HE4CdSe/ZnS immunocomplex | Anti-HE4 IgG antibodies, CdSe/ZnS QD | - | Electrochemical immunosensor for HE4 protein detection using QD as electrochemically active labels of specific antibodies. Contributed significantly to the analytical performance of tumor marker detection and met the exacting requirements for HE4 protein clinical monitoring | - | HE4 in human serum | [145] |
| Aptamer-modified SeNPs (Apt-SeNPs) | - | Spherical structures, 88 ± 30 nm | Good chemical stability, water solubility, and biocompatibility. Strong green scattering light with a characterized scattering peak at 570 nm. Precisely and specifically target and image nucleolin overexpressed cancer cells after being modified with aptamers | - | Human epidermoid cancer (Hep-2) cells | [146] |
| CdTe/ZnSe core/shell QDs | CdTe, Na2TeO3, Zn(CH3CO2)2, Na2SeO3 | QD (10 ± 2 nm), QD+T (13 ± 2 nm), QD+C (18 ± 2 nm), QD+A (71 ± 2 nm), QD+G (95 ± 2 nm) | Able to detect DNAs (directly from cell extracts), damages to the DNA, and mutations | - | Prostate cancer (PC3) and normal human cells (PNT1A) | [147] |
| IL13 conjugated QD (IL13QD) | CdSe-based QD, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC), interleukin-13 (IL13) | Core-shell structure, a size range of 15–20 nm | IL13Rα2 can be detected in cerebrospinal fluid by IL13QD. A higher force of binding interaction between the IL13QD and IL13Rα2 expressing glioma cells and exosomes secreted by glioma stem cells was observed | - | U251 human glioma cells and CD133 positive glioma initiating cells (T3691) | [148] |
| SeNP loaded imprinted core-shell microcomposites (SIMs) | CTS, zeolite, TiO2, Na2SeO4, Na2SeO3 | Spherical, average size of 80 nm | Could be used for dot-blot immunoassays for rapid serodiagnosis of human lung cancer. The detection time of the colloidal Se dot test for the progastrin-releasing peptide (as a tumor marker for small cell lung cancers) was only 5 min | Linear with the concentration of antigen within the concentration range of 0–105 pg/mL. The lowest concentration to distinguish significant positive results was observed to be 75 pg/mL | Human progastrin releasing-peptide | [149] |
| SeNPs | Material | Shape and Size (nm) | Function 1 | Effects | Dosage | Pathway | Model | Reference |
|---|---|---|---|---|---|---|---|---|
| Ag2Se-cetuximab nanoprobes | Bis(trimethylsilyl)selenide, silver acetate, cetuximab | Spherical, diameter of 2.8 ± 0.5 nm | C and E | Displayed faster and more enrichment at the site of cancer. Inhibited the tumor growth and improved the survival rate of the cancer-bearing nude mice model. Combined targeted imaging and therapy | - | - | Human tongue squamous cell carcinoma cells (CAL-27) and human immortalized noncancerous keratinocytes cells (HaCaT) and Balb/c mice | [150] |
| DOX-loaded selenopolymeric nanocarriers (Se@CMHA-DOX NPs) | Na2SeO3, ascorbic acid, poly (ethylene glycol) (PEG), cetyl-modified hyaluronic acid, DOX | Spherical, 244 ± 6.8 nm | A and C | Inhibited TrxR activity and augmented the anticancer efficacy of DOX. Induced G2/M cell cycle arrest and TP53-mediated caspase-independent apoptosis. Reduced tumor activity in a three-dimensional tumor sphere model | 5 μg/mL for 48 h | Apoptotic pathway | MCF7 breast adenocarcinoma cells and MCF7 tumor sphere model | [151] |
| HSAMSe@DOX | Na2SeO3, L-ascorbic acid, Human serum albumin, DOX | Homogeneous spherical, ∼80 nm | A and C | Synergistically enhanced the antitumor activity of DOX and decreased the side effects associated with DOX. Increased tumor-targeting effects and enhanced cellular uptake through nanoparticle interact with SPARC protein | 10 mg/mL, 100 μL into the veins of the tails | - | MCF-7, MCF-10A, MDA-MB-231, SKBR3 and female BALB/C nude mice | [152] |
| MoSe2(Gd3+)-PEG nanosheets | NaMoO4·2H2O, Se, NaBH4, gadolinium(III) chloride hexahydrate | Lamellar, 100–150 nm | B, F and G | Able to provide a strong contrast for T1 weighted magnetic resonance imaging. Could be used as contrast agent for photoacoustic imaging (PAI). Increased the temperature to help kill cancer cells under laser irradiation. Enhanced permeation and retention effect in the tumor using magnetic resonance photoacoustic bimodal imaging in vivo. Suppressed tumors in mice by injection with laser irradiation | - | - | Hep G2 human hepatoma carcinoma cells, BALB/c nude mice | [153] |
| Paclitaxel-loaded SeNPs | SeO2, paclitaxel, ascorbic acid, pluronic F-127 | Hydrodynamic diameter, 87 nm, spherical | A and B | Significant antiproliferative activity against cancer cells. G2/M phase arrest in a dose-dependent manner leading to apoptosis. Disruption of mitochondrial membrane potential orchestrated with the induction of reactive oxygen species leading to the activation of caspases | - | MMP, caspases | Lung cells (L-132), cervical cancer cells (HeLa), breast cancer cells, (MCF7), non-small lung carcinoma cells (A549) and colorectal adenocarcinoma cells (HT29) | [154] |
| PPa@CTX-Se-OA/DSPE-PEG2k | Cabazitaxel, PPa, oleic acid, Se powder, DSPE-PEG2k | Spherical, average diameter of 104.1 ± 3.1 nm | C and D | Light irradiation disassembles the structure of ROS-responsive prodrug nanosystems by cleaving the ROS-responsive linkers to accelerate the release of the parent drug | 200 ng/mL for 4 h or 24 h | - | 4T1 murine breast cancer cells | [19] |
| Se/iron oxide nanoparticles (Se:IONP) | Na2SeO3, acetic acid, CTS, hydrophobic IONP | Spherical in a transmission electron microscope, irregular in a scanning electron microscope, 5–9 nm | A and H | An iron oxide core produced by thermal decomposition, followed by a silane ligand exchange, a CTS coating, and Se decoration. Reduced cancer cell viability | - | - | MB-231 breast cancer cells | [155] |
| Se-containing hydroxyapatite/alginate (SeHA/ALG) composite granules | (NH4)2HPO4, Na2SeO3⋅5H2O, Ca(NO3)2⋅4H2O, hydroxyapatite, alginate sodium, RIS | Spherical, 1.1–1.5 mm | A and C | Biphasic process of releasing sufficient Se and RIS against osteosarcoma cells | - | - | Human osteoblast-like cell line (Saos-2) and normal human fetal osteoblasts (hFOB 1.19) | [156] |
| SeNPs-DOX-ICG-RP | L-ascorbic acid, Na2SeO3, dual-target (RC-12 and PG-6 peptides), loaded with both DOX and ICG | Sphere-like morphology with an average size of 110 nm | B and C | NIR-laser irradiation that raised the temperature of the nanosystem and allowed nanoparticles to decompose and release drugs accurately in the tumor site. Reduced the damage of chemotherapy drugs to normal tissue | - | - | HepG2 and normal L02 cells | [157] |
| Ultra-small Nano-Se | Na2SeO3, GSH, BSA | Monodisperse spherical shape with a diameter about 27.5 ± 4.3 nm | A and F | Reinforced the toxic effects of irradiation, leading to a higher mortality rate than either treatment used alone. Induced cell cycle arrest at the G2/M phase and the activation of autophagy. Increased both endogenous and irradiation-induced ROS formation. Improved cancer cell sensitivity to the toxic effects of irradiation | 0.15 and 0.3 μg/mL, X-rays (6-MeV, 200 cGy/min) | - | MCF-7 breast carcinoma cells | [158] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, H.W.; Mo, H.-Y.; Lau, A.T.Y.; Xu, Y.-M. Selenium Species: Current Status and Potentials in Cancer Prevention and Therapy. Int. J. Mol. Sci. 2019, 20, 75. https://doi.org/10.3390/ijms20010075
Tan HW, Mo H-Y, Lau ATY, Xu Y-M. Selenium Species: Current Status and Potentials in Cancer Prevention and Therapy. International Journal of Molecular Sciences. 2019; 20(1):75. https://doi.org/10.3390/ijms20010075
Chicago/Turabian StyleTan, Heng Wee, Hai-Ying Mo, Andy T. Y. Lau, and Yan-Ming Xu. 2019. "Selenium Species: Current Status and Potentials in Cancer Prevention and Therapy" International Journal of Molecular Sciences 20, no. 1: 75. https://doi.org/10.3390/ijms20010075
APA StyleTan, H. W., Mo, H.-Y., Lau, A. T. Y., & Xu, Y.-M. (2019). Selenium Species: Current Status and Potentials in Cancer Prevention and Therapy. International Journal of Molecular Sciences, 20(1), 75. https://doi.org/10.3390/ijms20010075