Quantitative Proteomic Analysis Reveals Novel Insights into Intracellular Silicate Stress-Responsive Mechanisms in the Diatom Skeletonema dohrnii
Abstract
:1. Introduction
2. Results
2.1. Physiological Responses of the Cell
2.2. iTRAQ Results
2.3. Gene Ontology and COG Analysis
2.4. KEGG Analysis of the DEPs in Skeleteonema dohrnii
2.5. Photosynthesis Metabolism
2.6. Carbon Metabolism
2.7. Response of Cellular Respiration
2.8. Identification PCD Related Proteins
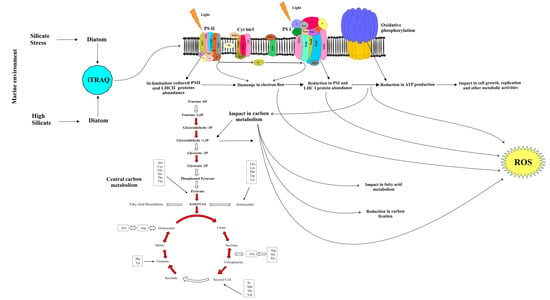
3. Discussion
3.1. Physiological Changes
3.2. Downregulation of Photosynthesis
3.3. Reduction in the Photosynthetic Carbon Fixation
3.4. The Response of Cellular Respiration to Possible ROS Accumulation
3.5. Metabolic Regulation of PCD Related Proteins
4. Materials and Methods
4.1. Species Description
4.2. Culture Condition
4.3. Analysis of Physiological Measurements
4.4. Protein Extraction and Preparation
4.5. iTRAQ Labeling and Fraction
4.6. LC-MS/MS Analysis
4.7. Proteomic Data Analysis
4.8. Functional Annotation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| iTRAQ | Isobaric tag for relative and absolute quantification |
| ROS | Reactive Oxygen Species |
| ATP | Adenosine triphosphate |
| PSII | Photosystem II |
| PSI | Photosystem I |
| PCD | Programmed Cell Death |
| LC-MS/MS | liquid chromatography-tandem mass spectrometry |
| NPP | Net Primary Productivity |
| GO | Gene Ontology |
| COG | Clusters of Orthologous Groups |
| KEGG | The Kyoto Encyclopedia of Genes and Genomes |
| DEPs | Diffrenccilaly Expressed Proteins |
| FCPs | Fucoxanthin-chlorophyll proteins |
| GTP | Guanosine triphosphate |
| FADH2 | flavin adenine dinucleotide |
| TCA | The citric acid cycle |
| NADH | Nicotinamide adenine dinucleotide |
| RuBisCO | Ribulose-1,5-bisphosphate carboxylase/oxygenase |
References
- Armbrust, E.V. The Life of Diatoms in the World’s Oceans. Nature 2009, 459, 185–192. [Google Scholar] [CrossRef]
- Bowler, C.; Vardi, A.; Allen, A.E. Oceanographic and Biogeochemical Insights from Diatom Genomes. Annu. Rev. Mar. Sci. 2010, 2, 333–365. [Google Scholar] [CrossRef] [PubMed]
- Rosenwasser, S.; Van Creveld, S.G.; Schatz, D.; Malitsky, S.; Tzfadia, O.; Aharoni, A.; Levin, Y.; Gabashvili, A.; Feldmesser, E.; Vardi, A. Mapping the Diatom Redox-Sensitive Proteome Provides Insight into Response to Nitrogen Stress in the Marine Environment. Proc. Natl. Acad. Sci. USA 2014, 111, 2740–2745. [Google Scholar] [CrossRef]
- Du, C.; Liang, J.-R.; Chen, D.-D.; Xu, B.; Zhuo, W.-H.; Gao, Y.-H.; Chen, C.-P.; Bowler, C.; Zhang, W. iTRAQ-Based Proteomic Analysis of the Metabolism Mechanism Associated with Silicon Response in the Marine Diatom Thalassiosira Pseudonana. J. Proteome Res. 2014, 13, 720–734. [Google Scholar] [CrossRef] [PubMed]
- Brzezinski, M.A.; Olson, R.J.; Chisholm, S.W. Silicon Availability and Cell-Cycle Progression in Marine Diatoms. Mar. Ecol. Prog. Ser. 1990, 67, 83–96. [Google Scholar] [CrossRef]
- Shrestha, R.P.; Tesson, B.; Norden-Krichmar, T.; Federowicz, S.; Hildebrand, M.; Allen, A.E. Whole Transcriptome Analysis of the Silicon Response of the Diatom Thalassiosira Pseudonana. BMC Genom. 2012, 13, 499. [Google Scholar] [CrossRef] [PubMed]
- Tirichine, L.; Bowler, C. Decoding Algal Genomes: Tracing Back the History of Photosynthetic Life on Earth. Plant J. 2011, 66, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Parkhill, J.P.; Maillet, G.; Cullen, J.J. Fluorescence-Based Maximal Quantum Yield for PSII as a Diagnostic of Nutrient Stress. J. Phycol. 2001, 37, 517–529. [Google Scholar] [CrossRef]
- Hockin, N.L.; Mock, T.; Mulholland, F.; Kopriva, S.; Malin, G. The Response of Diatom Central Carbon Metabolism to Nitrogen Starvation is Different from that of Green Algae and Higher Plants. Plant Physiol. 2012, 158, 299–312. [Google Scholar] [CrossRef]
- Jian, J.; Zeng, D.; Wei, W.; Lin, H.; Li, P.; Liu, W. The Combination of RNA and Protein Profiling Reveals the Response to Nitrogen Depletion in Thalassiosira Pseudonana. Sci. Rep. 2017, 7, 8989. [Google Scholar] [CrossRef]
- Nunn, B.L.; Faux, J.F.; Hippmann, A.A.; Maldonado, M.T.; Harvey, H.R.; Goodlett, D.R.; Boyd, P.W.; Strzepek, R.F. Diatom Proteomics Reveals Unique Acclimation Strategies to Mitigate Fe Limitation. PLoS ONE 2013, 8, e75653. [Google Scholar] [CrossRef]
- Brembu, T.; Muhlroth, A.; Alipanah, L.; Bones, A.M. The Effects of Phosphorus Limitation on Carbon Metabolism in Diatoms. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160406. [Google Scholar] [CrossRef]
- Mock, T.; Samanta, M.P.; Iverson, V.; Berthiaume, C.; Robison, M.; Holtermann, K.; Durkin, C.; BonDurant, S.S.; Richmond, K.; Rodesch, M. Whole-Genome Expression Profiling of the Marine Diatom Thalassiosira Pseudonana Identifies Genes Involved in Silicon Bioprocesses. Proc. Natl. Acad. Sci. USA 2008, 105, 1579–1584. [Google Scholar] [CrossRef]
- Lin, Q.; Liang, J.-R.; Huang, Q.-Q.; Luo, C.-S.; Anderson, D.M.; Bowler, C.; Chen, C.-P.; Li, X.-S.; Gao, Y.-H. Differential Cellular Responses Associated with Oxidative Stress and Cell Fate Decision under Nitrate and Phosphate Limitations in Thalassiosira Pseudonana: Comparative Proteomics. PLoS ONE 2017, 12, e0184849. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.-S.; Liang, J.-R.; Lin, Q.; Li, C.; Bowler, C.; Anderson, D.M.; Wang, P.; Wang, X.-W.; Gao, Y.-H. Cellular Responses Associated with ROS Production and Cell Fate Decision in Early Stress Response to Iron Limitation in the Diatom Thalassiosira Pseudonana. J. Proteome Res. 2014, 13, 5510–5523. [Google Scholar] [CrossRef]
- Javaheri, N.; Dries, R.; Burson, A.; Stal, L.; Sloot, P.; Kaandorp, J. Temperature Affects the Silicate Morphology in a Diatom. Sci. Rep. 2015, 5, 11652. [Google Scholar] [CrossRef]
- Casotti, R.; Mazza, S.; Brunet, C.; Vantrepotte, V.; Ianora, A.; Miralto, A. Growth Inhibition and Toxicity of the Diatom Aldehyde 2-Trans, 4-Trans-Decadienal Onthalassiosira Weissflogii (Bacillariophyceae). J. Phycol. 2005, 41, 7–20. [Google Scholar] [CrossRef]
- Chung, C.C.; Hwang, S.P.; Chang, J. Cooccurrence of ScDSP Gene Expression, Cell Death, and DNA Fragmentation in a Marine Diatom, Skeletonema Costatum. Appl. Environ. Microbiol. 2005, 71, 8744–8751. [Google Scholar] [CrossRef]
- Berges, J.A.; Falkowski, P.G. Physiological Stress and Cell Death in Marine Phytoplankton: Induction of Proteases in Response to Nitrogen or Light Limitation. Limnol. Oceanogr. 1998, 43, 129–135. [Google Scholar] [CrossRef]
- Brussaard, C.P.; Noordeloos, A.A.; Riegman, R. Autolysis Kinetics of the Marine Diatom Ditylum Brightwellii (Bacillariophyceae) under Nitrogen and Phosphorus Limitation and Starvation 1. J. Phycol. 1997, 33, 980–987. [Google Scholar] [CrossRef]
- Mackey, K.; Morris, J.J.; Morel, F.; Kranz, S. Response of Photosynthesis to Ocean Acidification. Oceanography 2015, 25, 74–91. [Google Scholar] [CrossRef]
- Tolonen, A.C.; Aach, J.; Lindell, D.; Johnson, Z.I.; Rector, T.; Steen, R.; Church, G.M.; Chisholm, S.W. Global Gene Expression of Prochlorococcus Ecotypes in Response to Changes in Nitrogen Availability. Mol. Syst. Biol. 2006, 2, 53. [Google Scholar] [CrossRef]
- Shen, W.; Wei, Y.; Dauk, M.; Tan, Y.; Taylor, D.C.; Selvaraj, G.; Zou, J. Involvement of a Glycerol-3-Phosphate Dehydrogenase in Modulating the NADH/NAD+ Ratio Provides Evidence of a Mitochondrial Glycerol-3-Phosphate Shuttle in Arabidopsis. Plant Cell 2006, 18, 422–441. [Google Scholar] [CrossRef]
- Fernie, A.R.; Stitt, M. On the Discordance of Metabolomics with Proteomics and Transcriptomics: Coping with Increasing Complexity in Logic, Chemistry, and Network Interactions Scientific Correspondence. Plant Physiol. 2012, 158, 1139–1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dyhrman, S.T.; Jenkins, B.D.; Rynearson, T.A.; Saito, M.A.; Mercier, M.L.; Alexander, H.; Whitney, L.P.; Drzewianowski, A.; Bulygin, V.V.; Bertrand, E.M.; et al. The Transcriptome and Proteome of the Diatom Thalassiosira Pseudonana Reveal a Diverse Phosphorus Stress Response. PLoS ONE 2012, 7, e33768. [Google Scholar] [CrossRef] [PubMed]
- Lommer, M.; Specht, M.; Roy, A.-S.; Kraemer, L.; Andreson, R.; Gutowska, M.A.; Wolf, J.; Bergner, S.V.; Schilhabel, M.B.; Klostermeier, U.C.; et al. Genome and Low-Iron Response of an Oceanic Diatom Adapted to Chronic Iron Limitation. Genome Biol. 2012, 13, R66. [Google Scholar] [CrossRef]
- Takabayashi, M.; Lew, K.; Johnson, A.; Marchi, A.; Dugdale, R.; Wilkerson, F.P. The Effect of Nutrient Availability and Temperature on Chain Length of the Diatom, Skeletonema Costatum. J. Plankton Res. 2006, 28, 831–840. [Google Scholar] [CrossRef]
- Anning, T.; Harris, G.; Geider, R. Thermal Acclimation in the Marine Diatom Chaetoceros Calcitrans (Bacillariophyceae). Eur. J. Phycol. 2001, 36, 233–241. [Google Scholar] [CrossRef]
- Falkowski, P.G. Light-Shade Adaptation in Marine Phytoplankton. In Primary Productivity in the Sea; Springer: Berlin, Germany, 1980; pp. 99–119. [Google Scholar]
- Gururani, M.A.; Venkatesh, J.; Tran, L.S. Regulation of Photosynthesis during Abiotic Stress-Induced Photoinhibition. Mol. Plant 2015, 8, 1304–1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dang, K.V.; Plet, J.; Tolleter, D.; Jokel, M.; Cuine, S.; Carrier, P.; Auroy, P.; Richaud, P.; Johnson, X.; Alric, J.; et al. Combined Increases in Mitochondrial Cooperation and Oxygen Photoreduction Compensate for Deficiency in Cyclic Electron Flow in Chlamydomonas Reinhardtii. Plant Cell 2014, 26, 3036–3050. [Google Scholar] [CrossRef]
- Munekage, Y.; Hojo, M.; Meurer, J.; Endo, T.; Tasaka, M.; Shikanai, T. PGR5 is Involved in Cyclic Electron Flow Around Photosystem I and is Essential for Photoprotection in Arabidopsis. Cell 2002, 110, 361–371. [Google Scholar] [CrossRef]
- Tikkanen, M.; Mekala, N.R.; Aro, E.-M. Photosystem II Photoinhibition-Repair Cycle Protects Photosystem I from Irreversible Damage. Biochim. Biophys. Acta 2014, 1837, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Jordan, P.; Fromme, P.; Witt, H.T.; Klukas, O.; Saenger, W.; Krauß, N. Three-Dimensional Structure of Cyanobacterial Photosystem I at 2.5 Å Resolution. Nature 2001, 411, 909. [Google Scholar] [CrossRef]
- Sunda, W.; Kieber, D.; Kiene, R.; Huntsman, S. An Antioxidant Function for DMSP and DMS in Marine Algae. Nature 2002, 418, 317. [Google Scholar] [CrossRef] [PubMed]
- Stefels, J. Physiological Aspects of the Production and Conversion of DMSP in Marine Algae and Higher Plants. J. Sea Res. 2000, 43, 183–197. [Google Scholar] [CrossRef]
- Allen, A.E.; Vardi, A.; Bowler, C. An Ecological and Evolutionary Context for Integrated Nitrogen Metabolism and Related Signaling Pathways in Marine Diatoms. Curr. Opin. Plant Biol. 2006, 9, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Sweetlove, L.J.; Beard, K.F.; Nunes-Nesi, A.; Fernie, A.R.; Ratcliffe, R.G. Not Just a Circle: Flux Modes in the Plant TCA Cycle. Trends Plant Sci. 2010, 15, 462–470. [Google Scholar] [CrossRef]
- St-Pierre, J.; Buckingham, J.A.; Roebuck, S.J.; Brand, M.D. Topology of Superoxide Production from Different Sites in the Mitochondrial Electron Transport Chain. J. Biol. Chem. 2002, 277, 44784–44790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrero, A.; Barja, G. Sites and Mechanisms Responsible for the Low Rate of Free Radical Production of Heart Mitochondria in the Long-Lived Pigeon. Mech. Ageing Dev. 1997, 98, 95–111. [Google Scholar] [CrossRef]
- Babcock, G.T.; Wikström, M. Oxygen Activation and the Conservation of Energy in Cell Respiration. Nature 1992, 356, 301–309. [Google Scholar] [CrossRef]
- Ken, C.-F.; Hsiung, T.-M.; Huang, Z.-X.; Juang, R.-H.; Lin, C.-T. Characterization of Fe/Mn—Superoxide Dismutase from Diatom Thallassiosira Weissflogii: Cloning, Expression, and Property. J. Agric. Food Chem. 2005, 53, 1470–1474. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.E.; Laroche, J.; Maheswari, U.; Lommer, M.; Schauer, N.; Lopez, P.J.; Finazzi, G.; Fernie, A.R.; Bowler, C. Whole-Cell Response of the Pennate Diatom Phaeodactylum Tricornutum to Iron Starvation. Proc. Natl. Acad. Sci. USA 2008, 105, 10438–10443. [Google Scholar] [CrossRef]
- Levine, R.L.; Mosoni, L.; Berlett, B.S.; Stadtman, E.R. Methionine Residues as Endogenous Antioxidants in Proteins. Proc. Natl. Acad. Sci. USA 1996, 93, 15036–15040. [Google Scholar] [CrossRef] [PubMed]
- Dietz, K.J. Plant Peroxiredoxins. Annu. Rev. Plant Biol. 2003, 54, 93–107. [Google Scholar] [CrossRef]
- Murata, H.; Ihara, Y.; Nakamura, H.; Yodoi, J.; Sumikawa, K.; Kondo, T. Glutaredoxin Exerts an Antiapoptotic Effect by Regulating the Redox State of Akt. J. Biol. Chem. 2003, 278, 50226–50233. [Google Scholar] [CrossRef] [Green Version]
- Kaufman, B.A.; Kolesar, J.E.; Perlman, P.S.; Butow, R.A. A Function for the Mitochondrial Chaperonin Hsp60 in the Structure and Transmission of Mitochondrial DNA Nucleoids in Saccharomyces Cerevisiae. J. Cell Biol. 2003, 163, 457–461. [Google Scholar] [CrossRef]
- Parker, M.S.; Armbrust, E.V. Synergistic Effects of Light, Temperature, and Nitrogen Source on Transcription of Genes for Carbon and Nitrogen Metabolism in the Centric Diatom Thalassiosira Pseudonana (Bacillariophyceae)1. J. Phycol. 2005, 41, 1142–1153. [Google Scholar] [CrossRef]
- Hedstrom, L. Serine Protease Mechanism and Specificity. Chem. Rev. 2002, 102, 4501–4524. [Google Scholar] [CrossRef]
- Coffeen, W.C.; Wolpert, T.J. Purification and Characterization of Serine Proteases that Exhibit Caspase-Like Activity and are Associated with Programmed Cell Death in Avena Sativa. Plant Cell 2004, 16, 857–873. [Google Scholar] [CrossRef]
- Zhang, G.B.; Meng, S.; Gong, J.M. The Expected and Unexpected Roles of Nitrate Transporters in Plant Abiotic Stress Resistance and Their Regulation. Int. J. Mol. Sci. 2018, 19, 3535. [Google Scholar] [CrossRef] [PubMed]
- Hippler, F.W.R.; Mattos-Jr, D.; Boaretto, R.M.; Williams, L.E. Copper Excess Reduces Nitrate Uptake by Arabidopsis Roots with Specific Effects on Gene Expression. J. Plant Physiol. 2018, 228, 158–165. [Google Scholar] [CrossRef]
- Chen, X.H.; Li, Y.Y.; Zhang, H.; Liu, J.L.; Xie, Z.X.; Lin, L.; Wang, D.Z. Quantitative Proteomics Reveals Common and Specific Responses of a Marine Diatom Thalassiosira Pseudonana to Different Macronutrient Deficiencies. Front. Microbiol. 2018, 9, 2761. [Google Scholar] [CrossRef]
- Zrenner, R.; Stitt, M.; Sonnewald, U.; Boldt, R. Pyrimidine and Purine Biosynthesis and Degradation in Plants. Annu. Rev. Plant Biol. 2006, 57, 805–836. [Google Scholar] [CrossRef] [PubMed]
- Alipanah, L.; Rohloff, J.; Winge, P.; Bones, A.M.; Brembu, T. Whole-Cell Response to Nitrogen Deprivation in the Diatom Phaeodactylum Tricornutum. J. Exp. Bot. 2015, 66, 6281–6296. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. Biochemistry; WH Freeman: New York, NY, USA, 2002. [Google Scholar]
- Sarno, D.; Kooistra, W.H.; Medlin, L.K.; Percopo, I.; Zingone, A. Diversity in the Genus Skeletonema (Bacillariophyceae). ii. An Assessment of the Taxonomy of S. Costatum-Like Species with the Description of Four New Species 1. J. Phycol. 2005, 41, 151–176. [Google Scholar] [CrossRef]
- Zingone, A.; Percopo, I.; Sims, P.A.; Sarno, D. Diversity in the Genus Skeletonema (Bacillariophyceae). I. A Reexamination of the Type Material of S. Costatum with the Description of S. Grevillei Sp. NOV. 1. J. Phycol. 2005, 41, 140–150. [Google Scholar] [CrossRef]
- Sunda, W.G.; Price, N.M.; Morel, F.M. Trace Metal Ion Buffers and Their Use in Culture Studies. Algal Cult. Tech. 2005, 4, 35–63. [Google Scholar]
- Sun, J.; Ning, X. Marine Phytoplankton Specific Growth Rate. Adv. Earth Sci. 2005, 20, 939–945. [Google Scholar]
- Wei, Y.; Zhao, X.; Sun, J. Fast Repetition Rate Fluorometry (FRRF) Derived Phytoplankton Primary Productivity in the Bay of Bengal. Front. Microbiol. 2019. [Google Scholar] [CrossRef]
- Charbonneau, M.-È.; Girard, V.; Nikolakakis, A.; Campos, M.; Berthiaume, F.; Dumas, F.; Lépine, F.; Mourez, M. O-Linked Glycosylation Ensures the Normal Conformation of the Autotransporter Adhesin Involved in Diffuse Adherence. J. Bacteriol. 2007, 189, 8880–8889. [Google Scholar] [CrossRef] [Green Version]
- Van Domselaar, G.H.; Stothard, P.; Shrivastava, S.; Cruz, J.A.; Guo, A.; Dong, X.; Lu, P.; Szafron, D.; Greiner, R.; Wishart, D.S. BASys: A Web Server for Automated Bacterial Genome Annotation. Nucleic Acids Res. 2005, 33, W455–W459. [Google Scholar] [CrossRef] [PubMed]
- Unwin, R.D. Quantification of Proteins by iTRAQ. Methods Mol. Biol. 2010, 658, 205–215. [Google Scholar] [PubMed]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thangaraj, S.; Shang, X.; Sun, J.; Liu, H. Quantitative Proteomic Analysis Reveals Novel Insights into Intracellular Silicate Stress-Responsive Mechanisms in the Diatom Skeletonema dohrnii. Int. J. Mol. Sci. 2019, 20, 2540. https://doi.org/10.3390/ijms20102540
Thangaraj S, Shang X, Sun J, Liu H. Quantitative Proteomic Analysis Reveals Novel Insights into Intracellular Silicate Stress-Responsive Mechanisms in the Diatom Skeletonema dohrnii. International Journal of Molecular Sciences. 2019; 20(10):2540. https://doi.org/10.3390/ijms20102540
Chicago/Turabian StyleThangaraj, Satheeswaran, Xiaomei Shang, Jun Sun, and Haijiao Liu. 2019. "Quantitative Proteomic Analysis Reveals Novel Insights into Intracellular Silicate Stress-Responsive Mechanisms in the Diatom Skeletonema dohrnii" International Journal of Molecular Sciences 20, no. 10: 2540. https://doi.org/10.3390/ijms20102540
APA StyleThangaraj, S., Shang, X., Sun, J., & Liu, H. (2019). Quantitative Proteomic Analysis Reveals Novel Insights into Intracellular Silicate Stress-Responsive Mechanisms in the Diatom Skeletonema dohrnii. International Journal of Molecular Sciences, 20(10), 2540. https://doi.org/10.3390/ijms20102540