Chitin Nanofibrils and Nanolignin as Functional Agents in Skin Regeneration
Abstract
:1. Introduction
2. Results
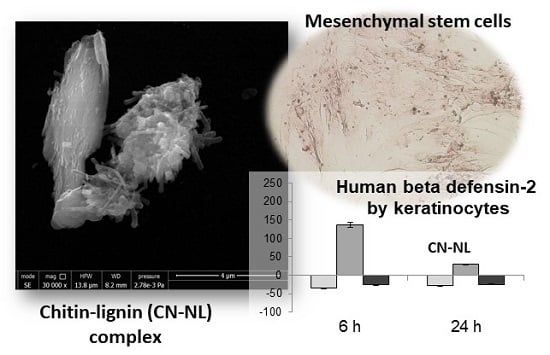
2.1. Morphological Characterization of CN, NL and CN-NL Complexes
2.2. Chemical Structure and Thermal Stability of CN, NL, GA and CN-NL/GA Complexes
2.3. HaCaT Cell Viability
2.4. HMSC Morphology, Viability and Osteodifferentiative Potential
2.5. Anti-Inflammatory and Immune Responses of HaCaT Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Morphological Characterization of CN, NL and CN-NL Complexes
4.3. Chemical Structure and Thermal Stability Charachterisation of CN, NL and CN-NL Complexes
4.4. In vitro Culture of hMSCs and HaCaT
4.5. MTT Assay
4.6. Evaluation of hMSCs Viability and Differentiation Potential
4.7. Anti-Inflammatory and Immune Responses Evaluation of HaCaT Cells
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CN | Chitin nanofibrlis |
| NL | Nanolignin |
| GA | Glycyrrhetinic acid |
| HaCaT | Human keratinocytes |
| HMSCs | Human mesenchymal stromal cells |
| IL | Interleukin |
| TNF-α | Tumor necrosis factor alpha |
| PEG | Poly(ethylene glycol) |
| HBD-2 | Human beta-defensin 2 |
| TGF-β | Transforming growth factor beta |
| DMEM | Dulbecco’s Minimal Essential Medium |
| DPBS | Dulbecco’s phosphate-buffered saline |
| RT-PCR | Reverse transcription polymerase chain reaction |
| DMSO | Dimethyl sulfoxide |
| RNA | Ribonucleic acid |
| cDNA | Complementary deoxyribonucleic acid |
| MgCl2 | Magnesium chloride |
| FESEM | Field Emission Scanning Electron Microscopy |
| ATR | Attenuated Total Reflection |
| TGA/SDTA | Thermogravimetric Analysis/Scanning Differential Thermal Analysis |
References
- Babu, R.P.; O’connor, K.; Seeram, R. Current progress on bio-based polymers and their future trends. Prog. Biomate. 2013, 2, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Morganti, P.; Danti, S.; Coltelli, M.B. Chitin and lignin to produce biocompatible tissues. Res. Clin. Dermatol. 2018, 1, 5–11. [Google Scholar]
- Morganti, P.; Febo, P.; Cardillo, M.; Donnarumma, G.; Baroni, A. Chitin nanofibril and nanolignin: Natural polymers of biomedical interest. J. Clin. Cosmet. Dermatol. 2017, 1. [Google Scholar] [CrossRef]
- Beisl, S.; Friedl, A.; Miltner, A. Lignin from micro-to nanosize: Applications. Int. J. Mol. Sci. 2017, 18, 2367. [Google Scholar] [CrossRef] [PubMed]
- Morganti, P.; Del Ciotto, P.; Stoller, M.; Chianese, A. Antibacterial and anti-inflammatory green nanocomposites. Chem. Eng. Trans. 2016, 47, 61–66. [Google Scholar] [CrossRef]
- Morganti, P.; Svolacchia, F.; Del Ciotto, P.; Carezzi, F. New insights on anti-aging activity of chitin nanofibril-hyaluronan block copolymers entrapping active ingredients: In vitro and in vivo study. J. Appl. Cosmetol. 2013, 31, 1–29. [Google Scholar]
- Morganti, P.; Palombo, M.; Tishchenko, G.; Yudin, V.E.; Guarneri, F.; Cardillo, M.; Del Ciotto, P.; Carezzi, F.; Morganti, G.; Fabrizi, G. Chitin-hyaluronan nanoparticles: A multifunctional carrier to deliver anti-aging active ingredients through the skin. Cosmet 2014, 1, 140–158. [Google Scholar] [CrossRef]
- Morganti, P.; Carezzi, F.; Del Ciotto, P.; Tishchenco, G.; Chianese, A.; Yudin, V.E. A green multifunctional polymer from discarded material: Chitin Nanofibrils. Br. J. Appl. Sci. Technol. 2014, 4, 4175–4190. [Google Scholar] [CrossRef]
- Morganti, P.; Tishchenko, G.; Palombo, M.; Kelnar, I.; Brozova, L.; Spirkova, M.; Pavlova, H.; Kobera, L.; Carezzi, F. Chitin nanofibrils for biomimetic products: nanoparticles and nanocomposite chitosan films in health care. In Marine Biomaterials: Isolation, Characterization and Application, 1st ed.; Kim, S., Ed.; CRC Press: Boca Raton, FL, USA, 2013; pp. 681–715. ISBN 9781138076389. [Google Scholar]
- Morganti, P.; Coltelli, M.B.; Danti, S. Biobased Tissues for innovative Cosmetic products: Polybioskin as an EU Research project. Glob. J. Nano. 2018, 3, 555620. [Google Scholar] [CrossRef]
- Rizvi, R.; Cochrane, B.; Naguib, H.; Lee, P.C. Fabrication and characterization of melt-blended polylactide-chitin composites and their foams. J. Cell. Plast. 2011, 47, 283–300. [Google Scholar] [CrossRef]
- Herrera, N.; Singh, A.A.; Salaberria, A.M.; Labidi, J.; Mathew, A.P.; Oksman, K. Triethyl Citrate (TEC) as a Dispersing Aid in Polylactic Acid/Chitin Nanocomposites Prepared via Liquid-Assisted Extrusion. Polymers 2017, 9, 406. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.; Naguib, H.E. Fabrication and Characterization of PLA/PHBV-Chitin Nanocomposites and Their Foams. J. Polym. Environ. 2014, 22, 119–130. [Google Scholar] [CrossRef]
- Herrera, N.; Roch, H.; Salaberria, A.M.; Pino-Orellana, M.A.; Labidi, J.; Fernandes, S.C.M.; Radic, D.; Leiva, A.; Oksman, K. Functionalized blown films of plasticized polylactic acid/chitin nanocomposite: Preparation and characterization. Mater. Des. 2016, 92, 846–852. [Google Scholar] [CrossRef]
- Coltelli, M.B.; Cinelli, P.; Gigante, V.; Aliotta, L.; Morganti, P.; Panariello, L.; Lazzeri, A. Chitin Nanofibrils in Poly(Lactic Acid) (PLA) Nanocomposites: Dispersion and Thermo-Mechanical Properties. Int. J. Mol. Sci. 2019, 20, 504. [Google Scholar] [CrossRef]
- Morganti, P. Composition and material comprising chitin nanofibrils, lignin and a co-polymer and their uses. Patent WO 2016/042474 Al, 2015. [Google Scholar]
- Andersen, F.A. Final report on the safety assessment of Glycyrrhetinic Acid, Potassium Glycyrrhetinate, Disodium Succinoyl Glycyrrhetinate, Glyceryl Glycyrrhetinate, Glycyrrhetinyl Stearate, Stearyl Glycyrrhetinate, Glycyrrhizic Acid, Ammonium Glycyrrhizate, Dipotassium Glycyrrhizate, Disodium Glycyrrhizate, Trisodium Glycyrrhizate, Methyl Glycyrrhizate, and Potassium Glycyrrhizinate. Int. J. Toxicol. 2007, 26, 79–112. [Google Scholar] [CrossRef]
- Hussain, H.; Green, I.R.; Shamraiz, U.; Saleem, M.; Badshah, A.; Abbas, G.; Rehman, N.U.; Irshad, M. Therapeutic potential of glycyrrhetinic acids: a patent review (2010-2017). Expert. Opin. Ther. Pat. 2018, 28, 383–398. [Google Scholar] [CrossRef] [PubMed]
- Kumirska, J.; Czerwicka, M.; Kaczyński, Z.; Bychowska, A.; Brzozowski, K.; Thöming, J.; Stepnowski, P. Application of Spectroscopic Methods for Structural Analysis of Chitin and Chitosan. Mar. Drugs 2010, 8, 1567–1636. [Google Scholar] [CrossRef] [Green Version]
- Boeriu, C.G.; Bravo, D.; Gosselink, R.J.A.; Van Dam, J.E.G. Characterisation of structure-dependent functional properties of lignin with infrared spectroscopy. Ind. Crops Prod. 2004, 20, 205–218. [Google Scholar] [CrossRef]
- Zu, Y.; Meng, L.; Zhao, X.; Ge, Y.; Yu, X.; Zhang, Y.; Deng, Y. Preparation of 10-hydroxycamptothecin- loaded glycyrrhizic acid-conjugated bovine serum albumin nanoparticles for hepatocellular carcinoma-targeted drug delivery. Int. J. Nanomed. 2013, 8, 1207–1222. [Google Scholar] [CrossRef]
- Cheng, M.; Gao, X.; Wang, Y.; Chen, H.; He, B.; Xu, H.; Li, Y.; Han, J.; Zhang, Z. Synthesis of Glycyrrhetinic Acid-Modified Chitosan, 5-Fluorouracil Nanoparticles and Its Inhibition of Liver Cancer Characteristics in Vitro and in Vivo. Mar. Drugs 2013, 11, 3517–3536. [Google Scholar] [CrossRef]
- Ablis, M.; Leininger-Muller, B.; Wong, C.D.; Siest, G.; Loppinet, V.; Visvikis, S. Synthesis and in Vitro Antioxidant Activity of Glycyrrhetinic Acid Derivatives Tested with the Cytochrome P450/NADPH System. Chem. Pharm. Bull. 2004, 52, 1436–1439. [Google Scholar] [CrossRef]
- Nilforoushzadeh, M.A.; Ahmadi Ashtiani, H.R.; Jaffary, F.; Jahangiri, F.; Nikkhah, N.; Mahmoudbeyk, M.; Fard, M.; Ansaria, Z.; Zare, S. Dermal Fibroblast Cells: Biology and Function in Skin Regeneration. J. Skin Stem Cell 2017, 4, e69080. [Google Scholar] [CrossRef]
- Danti, S.; D’Acunto, M.; Trombi, L.; Berrettini, S.; Pietrabissa, A. A micro/nanoscale surface mechanical study on morpho-functional changes occurred to multilineage-differentiated human mesenchymal stem cells. Macromol. Biosci. 2007, 7, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Donnarumma, G.; Paoletti, I.; Fusco, A.; Perfetto, B.; Buommino, E.; de Gregorio, V.; Baroni, A. β-Defensins: Work in Progress. Adv. Exp. Med. Biol. 2016, 901, 59–76. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Interleukin-1. Cytokine Growth Factor Rev. 1997, 8, 253–265. [Google Scholar] [CrossRef]
- Esposito, E.; Cuzzocrea, S. TNF-alpha as a therapeutic target in inflammatory diseases, ischemia-reperfusion injury and trauma. Curr. Med. Chem. 2009, 16, 3152–3167. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.E.; Polverini, P.J.; Kunkel, S.L.; Harlow, L.A.; DiPietro, L.A.; Elner, V.M.; Elner, S.G.; Strieter, R.M. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science 1992, 258, 1798–1801. [Google Scholar] [CrossRef]
- Morganti, P.; Fusco, A.; Paoletti, I.; Perfetto, B.; Del Ciotto, P.; Palombo, M.; Chianese, A.; Baroni, A.; Donnarumma, G. Anti-inflammatory, immunomodulatory, and tissue repair activity on human keratinocytes by green innovative nanocomposites. Materials 2017, 10, 843. [Google Scholar] [CrossRef] [PubMed]
- Oyama, K.; Kawada-Matsuo, M.; Oogai, Y.; Hayashi, T.; Nakamura, N.; Komatsuzawa, H. Antibacterial effects of glycyrrhetinic acid and its derivatives on staphylococcus aureus. PLoS ONE 2016, 11, e0165831. [Google Scholar] [CrossRef]
- Morganti, P.; Muzzarelli, C. Spray-dried chitin nanofibrils, method for production and uses thereof. Patent WO2007060628A1, 23 November 2005. [Google Scholar]
- Morganti, P. Method of preparation of chitin and active principles complexes and the so obtained complexes. Patent WO2012143875A1, 19 April 2011. [Google Scholar]
- Brooker, A.D.M.; Vaccaro, M.; Scialla, S.; Walker, S.J.; Morganti, P.; Carezzi, F.; Benjelloun-Mlayah, B.; Crestini, C.; Lange, H.; Bartzoka, E. A consumer goods product comprising chitin nanofibrils, lignin and a polymer or co-polymer. Patent EP2995321B1, 15 April 2014. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 1, 55–63. [Google Scholar] [CrossRef]
- Fusco, A.; Coretti, L.; Savio, V.; Buommino, E.; Lembo, F.; Donnarumma, G. Biofilm formation and immunomodulatory activity of Proteus mirabilis clinically isolated strains. Int. J. Mol. Sci. 2017, 18, 414. [Google Scholar] [CrossRef] [PubMed]










| Onset 1 (°C) | Mass Loss 1 (%) | Onset 2 (°C) | Mass Loss 2 (%) | Onset 3 (°C) | Mass Loss 3 (%) | Residue (%) | |
|---|---|---|---|---|---|---|---|
| GA | 386.95 | −98.92 | - | - | - | - | 1.97 |
| NL | 40 | −5.26 | 262 | −55.63 | - | - | 38.93 |
| CN | 159 | −5.35 | 300 | −57. 54 | 387 | −17.85 | 17.42 |
| CN-NL | 114 | −2.60 | 266 | −69.25 | 406 | −10.39 | 12.37 |
| CN-NL/GA | 127 | −1.92 | 251 | −68.53 | 472 | −11.63 | 13.84 |
| CN-NL | 0.5 µg/mL | 0.2 µg/mL | 0.1 µg/mL |
| GA | 1 µg/mL | 0.5 µg/mL | 0.2 µg/mL |
| CN-NL/GA | 1 µg/mL | 0.5 µg/mL | 0.2 µg/mL |
| Gene | Primers Sequence | Conditions | Product Size (bp) |
|---|---|---|---|
| IL-1α | 5’-CATGTCAAATTTCACTGCTTCATCC -3’ 5’-GTCTCTGAATCAGAAATCCTTCTATC -3’ | 5 s at 95 °C, 8 s at 55 °C, 17 s at 72 °C for 45 cycles | 421 |
| TNF-α | 5’-CAGAGGGAAGAGTTCCCCAG -3’ 5’-CCTTGGTCTGGTAGGAGACG -3’ | 5 s at 95°C, 6 s at 57°C, 13 s at 72°C for 40 cycles | 324 |
| IL-6 | 5’-ATGAACTCCTTCTCCACAAGCGC-3’ 5’-GAAGAGCCCTCAGGCTGGACTG-3’ | 5 s at 95°C, 13 s at 56°C, 25 s at 72°C for 40 cycles | 628 |
| IL-8 | 5-ATGACTTCCAAGCTGGCCGTG -3’ 5-TGAATTCTCAGCCCTCTTCAAAAACTTCTC-3’ | 5 s at 94°C, 6 s at 55°C, 12 s at 72°C for 40 cycles | 297 |
| TGF-β | 5’-CCGACTACTACGCCAAGGAGGTCAC-3’ 5’-AGGCCGGTTCATGCCATGAATGGTG-3’ | 5 s at 94°C, 9 s at 60°C, 18 s at 72°C for 40 cycles | 439 |
| IL-1β | 5’-GCATCCAGCTACGAATCTCC-3’ 5’-CCACATTCAGCACAGGACTC-3’ | 5 s at 95°C, 14 s at 58°C, 28 s at 72°C for 40 cycles | 708 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danti, S.; Trombi, L.; Fusco, A.; Azimi, B.; Lazzeri, A.; Morganti, P.; Coltelli, M.-B.; Donnarumma, G. Chitin Nanofibrils and Nanolignin as Functional Agents in Skin Regeneration. Int. J. Mol. Sci. 2019, 20, 2669. https://doi.org/10.3390/ijms20112669
Danti S, Trombi L, Fusco A, Azimi B, Lazzeri A, Morganti P, Coltelli M-B, Donnarumma G. Chitin Nanofibrils and Nanolignin as Functional Agents in Skin Regeneration. International Journal of Molecular Sciences. 2019; 20(11):2669. https://doi.org/10.3390/ijms20112669
Chicago/Turabian StyleDanti, Serena, Luisa Trombi, Alessandra Fusco, Bahareh Azimi, Andrea Lazzeri, Pierfrancesco Morganti, Maria-Beatrice Coltelli, and Giovanna Donnarumma. 2019. "Chitin Nanofibrils and Nanolignin as Functional Agents in Skin Regeneration" International Journal of Molecular Sciences 20, no. 11: 2669. https://doi.org/10.3390/ijms20112669
APA StyleDanti, S., Trombi, L., Fusco, A., Azimi, B., Lazzeri, A., Morganti, P., Coltelli, M. -B., & Donnarumma, G. (2019). Chitin Nanofibrils and Nanolignin as Functional Agents in Skin Regeneration. International Journal of Molecular Sciences, 20(11), 2669. https://doi.org/10.3390/ijms20112669