Photosensitive Melanopsin-Containing Retinal Ganglion Cells in Health and Disease: Implications for Circadian Rhythms
Abstract
:1. Introduction
2. Melanopsin-Containing Ganglion Cells in Rodents and Humans
3. Melanopsin-Containing Ganglion Cells in Aging
4. Melanopsin-Containing Ganglion Cells in Retinal Diseases
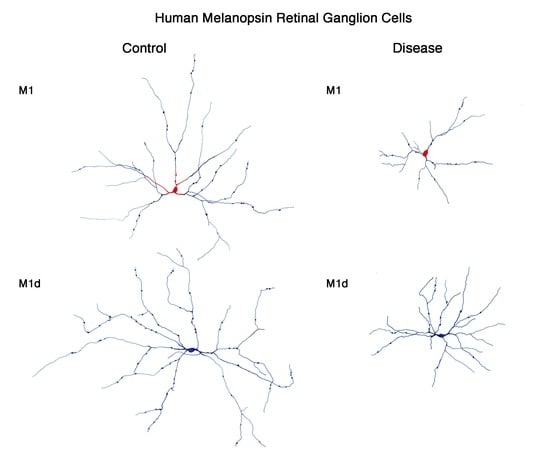
5. Melanopsin-Containing Ganglion Cells in Neurodegenerative Diseases
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| mRGCs | Melanopsin-containing ganglion cells |
| IPL | Inner plexiform layer |
| GCL | Ganglion cell layer |
| INL | Inner nuclear layer |
| M1d | Displaced M1 cells |
| AD | Alzheimer’s disease |
| PD | Parkinson’s disease |
References
- Provencio, I.; Rodriguez, I.R.; Jiang, G.; Hayes, W.P.; Moreira, E.F.; Rollag, M.D. A novel human opsin in the inner retina. J. Neurosci. 2000, 20, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Hattar, S.; Liao, H.W.; Takao, M.; Berson, D.M.; Yau, K.W. Melanopsin-containing retinal ganglion cells: Architecture, projections, and intrinsic photosensitivity. Science 2002, 295, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Dacey, D.M.; Liao, H.W.; Peterson, B.B.; Robinson, F.R.; Smith, V.C.; Pokorny, J.; Yau, K.W.; Gamlin, P.D. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature 2005, 433, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.M.; Gias, C.; Hatori, M.; Keding, S.R.; Semo, M.; Coffey, P.J.; Gigg, J.; Piggins, H.D.; Panda, S.; Lucas, R.J. Melanopsin contributions to irradiance coding in the thalamo-cortical visual system. PLoS Biol. 2010, 8, e1000558. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.M.; Wynne, J.; Piggins, H.D.; Lucas, R.J. Multiple hypothalamic cell populations encoding distinct visual information. J. Physiol. 2011, 589 Pt 5, 1173–1194. [Google Scholar] [CrossRef]
- Ecker, J.L.; Dumitrescu, O.N.; Wong, K.Y.; Alam, N.M.; Chen, S.K.; LeGates, T.; Renna, J.M.; Prusky, G.T.; Berson, D.M.; Hattar, S. Melanopsin-expressing retinal ganglion-cell photoreceptors: Cellular diversity and role in pattern vision. Neuron 2010, 67, 49–60. [Google Scholar] [CrossRef]
- Hannibal, J.; Hindersson, P.; Knudsen, S.M.; Georg, B.; Fahrenkrug, J. The photopigment melanopsin is exclusively present in pituitary adenylate cyclase-activating polypeptide-containing retinal ganglion cells of the retinohypothalamic tract. J. Neurosci. 2002, 22, RC191. [Google Scholar] [CrossRef]
- Baver, S.B.; Pickard, G.E.; Sollars, P.J. Two types of melanopsin retinal ganglion cell differentially innervate the hypothalamic suprachiasmatic nucleus and the olivary pretectal nucleus. Eur. J. Neurosci. 2008, 27, 1763–1770. [Google Scholar] [CrossRef]
- Chen, S.K.; Badea, T.C.; Hattar, S. Photoentrainment and pupillary light reflex are mediated by distinct populations of ipRGCs. Nature 2011, 476, 92–95. [Google Scholar] [CrossRef] [Green Version]
- Vugler, A.; Semo, M.; Ortin-Martinez, A.; Rojanasakul, A.; Nommiste, B.; Valiente-Soriano, F.J.; Garcia-Ayuso, D.; Coffey, P.; Vidal-Sanz, M.; Gias, C. A role for the outer retina in development of the intrinsic pupillary light reflex in mice. Neuroscience 2015, 286, 60–78. [Google Scholar] [CrossRef] [Green Version]
- Bonmati-Carrion, M.A.; Hild, K.; Isherwood, C.M.; Sweeney, S.J.; Revell, V.L.; Madrid, J.A.; Rol, M.A.; Skene, D.J. Effect of single and combined monochromatic light on the human pupillary light response. Front. Neurol. 2018, 9, 1019. [Google Scholar] [CrossRef] [PubMed]
- Bonmati-Carrion, M.A.; Hild, K.; Isherwood, C.; Sweeney, S.J.; Revell, V.L.; Skene, D.J.; Rol, M.A.; Madrid, J.A. Relationship between human pupillary light reflex and circadian system status. PLoS ONE 2016, 11, e0162476. [Google Scholar] [CrossRef] [PubMed]
- LeGates, T.A.; Altimus, C.M.; Wang, H.; Lee, H.K.; Yang, S.; Zhao, H.; Kirkwood, A.; Weber, E.T.; Hattar, S. Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature 2012, 491, 594–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monteggia, L.M.; Kavalali, E.T. Circadian rhythms: Depression brought to light. Nature 2012, 491, 537–538. [Google Scholar] [CrossRef] [PubMed]
- Lazzerini Ospri, L.; Prusky, G.; Hattar, S. Mood, the circadian system, and melanopsin retinal ganglion cells. Annu. Rev. Neurosci. 2017, 40, 539–556. [Google Scholar] [CrossRef] [PubMed]
- Valiente-Soriano, F.J.; Garcia-Ayuso, D.; Ortin-Martinez, A.; Jimenez-Lopez, M.; Galindo-Romero, C.; Villegas-Perez, M.P.; Agudo-Barriuso, M.; Vugler, A.A.; Vidal-Sanz, M. Distribution of melanopsin positive neurons in pigmented and albino mice: Evidence for melanopsin interneurons in the mouse retina. Front. Neuroanat. 2014, 8, 131. [Google Scholar] [CrossRef] [PubMed]
- Duncan, W.C., Jr. Circadian rhythms and the pharmacology of affective illness. Pharmacol. Ther. 1996, 71, 253–312. [Google Scholar] [CrossRef]
- Harper, D.G.; Stopa, E.G.; McKee, A.C.; Satlin, A.; Harlan, P.C.; Goldstein, R.; Volicer, L. Differential circadian rhythm disturbances in men with Alzheimer disease and frontotemporal degeneration. Arch. Gen. Psychiatry 2001, 58, 353–360. [Google Scholar] [CrossRef]
- Germain, A.; Kupfer, D.J. Circadian rhythm disturbances in depression. Hum. Psychopharmacol. 2008, 23, 571–585. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, J.S.; Hong, H.K.; Ko, C.H.; McDearmon, E.L. The genetics of mammalian circadian order and disorder: Implications for physiology and disease. Nat. Rev. Genet. 2008, 9, 764–775. [Google Scholar] [CrossRef]
- Wulff, K.; Gatti, S.; Wettstein, J.G.; Foster, R.G. Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat. Rev. Neurosci. 2010, 11, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Turek, F.W.; Penev, P.; Zhang, Y.; van Reeth, O.; Zee, P. Effects of age on the circadian system. Neurosci. Biobehav. Rev. 1995, 19, 53–58. [Google Scholar] [CrossRef]
- Zhang, Y.; Kornhauser, J.M.; Zee, P.C.; Mayo, K.E.; Takahashi, J.S.; Turek, F.W. Effects of aging on light-induced phase-shifting of circadian behavioral rhythms, fos expression and CREB phosphorylation in the hamster suprachiasmatic nucleus. Neuroscience 1996, 70, 951–961. [Google Scholar] [CrossRef]
- Touitou, Y.; Haus, E. Alterations with aging of the endocrine and neuroendocrine circadian system in humans. Chronobiol. Int. 2000, 17, 369–390. [Google Scholar] [CrossRef] [PubMed]
- Lupi, D.; Semo, M.; Foster, R.G. Impact of age and retinal degeneration on the light input to circadian brain structures. Neurobiol. Aging 2012, 33, 383–392. [Google Scholar] [CrossRef] [Green Version]
- Provencio, I.; Cooper, H.M.; Foster, R.G. Retinal projections in mice with inherited retinal degeneration: implications for circadian photoentrainment. J. Comp. Neurol. 1998, 395, 417–439. [Google Scholar] [CrossRef]
- Gordo, M.A.; Recio, J.; Sanchez-Barcelo, E.J. Decreased sleep quality in patients suffering from retinitis pigmentosa. J. Sleep Res. 2001, 10, 159–164. [Google Scholar] [CrossRef]
- Ionescu, D.; Driver, H.S.; Heon, E.; Flanagan, J.; Shapiro, C.M. Sleep and daytime sleepiness in retinitis pigmentosa patients. J. Sleep Res. 2001, 10, 329–335. [Google Scholar] [CrossRef]
- Panda, S.; Sato, T.K.; Castrucci, A.M.; Rollag, M.D.; DeGrip, W.J.; Hogenesch, J.B.; Provencio, I.; Kay, S.A. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science 2002, 298, 2213–2216. [Google Scholar] [CrossRef]
- Flynn-Evans, E.E.; Tabandeh, H.; Skene, D.J.; Lockley, S.W. Circadian rhythm disorders and melatonin production in 127 blind women with and without light perception. J. Biol. Rhythms 2014, 29, 215–224. [Google Scholar] [CrossRef]
- Van Someren, E.J. Circadian rhythms and sleep in human aging. Chronobiol. Int. 2000, 17, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Neikrug, A.B.; Ancoli-Israel, S. Sleep disorders in the older adult—A mini-review. Gerontology 2010, 56, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Gibson, E.M.; Williams, W.P., III; Kriegsfeld, L.J. Aging in the circadian system: Considerations for health, disease prevention and longevity. Exp. Gerontol. 2009, 44, 51–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drouyer, E.; Dkhissi-Benyahya, O.; Chiquet, C.; WoldeMussie, E.; Ruiz, G.; Wheeler, L.A.; Denis, P.; Cooper, H.M. Glaucoma alters the circadian timing system. PLoS ONE 2008, 3, e3931. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ayuso, D.; Salinas-Navarro, M.; Agudo, M.; Cuenca, N.; Pinilla, I.; Vidal-Sanz, M.; Villegas-Perez, M.P. Retinal ganglion cell numbers and delayed retinal ganglion cell death in the P23H rat retina. Exp. Eye Res. 2010, 91, 800–810. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ayuso, D.; Di Pierdomenico, J.; Esquiva, G.; Nadal-Nicolas, F.M.; Pinilla, I.; Cuenca, N.; Vidal-Sanz, M.; Agudo-Barriuso, M.; Villegas-Perez, M.P. Inherited photoreceptor degeneration causes the death of melanopsin-positive retinal ganglion cells and increases their coexpression of brn3a. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4592–4604. [Google Scholar] [CrossRef] [PubMed]
- Esquiva, G.; Lax, P.; Perez-Santonja, J.J.; Garcia-Fernandez, J.M.; Cuenca, N. Loss of melanopsin-expressing ganglion cell subtypes and dendritic degeneration in the aging human retina. Front. Aging Neurosci. 2017, 9, 79. [Google Scholar] [CrossRef] [PubMed]
- Videnovic, A.; Leurgans, S.; Fan, W.; Jaglin, J.; Shannon, K.M. Daytime somnolence and nocturnal sleep disturbances in Huntington disease. Parkinsonism Relat. Disord. 2009, 15, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Maywood, E.S.; O’Neill, J.; Wong, G.K.; Reddy, A.B.; Hastings, M.H. Circadian timing in health and disease. Prog. Brain Res. 2006, 153, 253–269. [Google Scholar] [PubMed]
- Menza, M.; Dobkin, R.D.; Marin, H.; Bienfait, K. Sleep disturbances in Parkinson’s disease. Mov. Disord. 2010, 25 (Suppl. 1), S117–S122. [Google Scholar] [CrossRef]
- Musiek, E.S.; Xiong, D.D.; Holtzman, D.M. Sleep, circadian rhythms, and the pathogenesis of Alzheimer disease. Exp. Mol. Med. 2015, 47, e148. [Google Scholar] [CrossRef] [PubMed]
- Ortuno-Lizaran, I.; Esquiva, G.; Beach, T.G.; Serrano, G.E.; Adler, C.H.; Lax, P.; Cuenca, N. Degeneration of human photosensitive retinal ganglion cells may explain sleep and circadian rhythms disorders in Parkinson’s disease. Acta Neuropathol. Commun. 2018, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- Videnovic, A.; Lazar, A.S.; Barker, R.A.; Overeem, S. ‘The clocks that time us’—Circadian rhythms in neurodegenerative disorders. Nat. Rev. Neurol. 2014, 10, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Musiek, E.S.; Holtzman, D.M. Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science 2016, 354, 1004–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berson, D.M.; Dunn, F.A.; Takao, M. Phototransduction by retinal ganglion cells that set the circadian clock. Science 2002, 295, 1070–1073. [Google Scholar] [CrossRef] [PubMed]
- Hannibal, J.; Fahrenkrug, J. Melanopsin: A novel photopigment involved in the photoentrainment of the brain’s biological clock? Ann. Med. 2002, 34, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Provencio, I.; Rollag, M.D.; Castrucci, A.M. Photoreceptive net in the mammalian retina. This mesh of cells may explain how some blind mice can still tell day from night. Nature 2002, 415, 493. [Google Scholar] [PubMed]
- Schmidt, T.M.; Chen, S.K.; Hattar, S. Intrinsically photosensitive retinal ganglion cells: Many subtypes, diverse functions. Trends Neurosci. 2011, 34, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.; Hankins, M.W.; Foster, R.G.; Peirson, S.N. Melanopsin phototransduction: Slowly emerging from the dark. Prog. Brain Res. 2012, 199, 19–40. [Google Scholar] [PubMed]
- Hughes, S.; Jagannath, A.; Rodgers, J.; Hankins, M.W.; Peirson, S.N.; Foster, R.G. Signalling by melanopsin (OPN4) expressing photosensitive retinal ganglion cells. Eye 2016, 30, 247–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berson, D.M.; Castrucci, A.M.; Provencio, I. Morphology and mosaics of melanopsin-expressing retinal ganglion cell types in mice. J. Comp. Neurol. 2010, 518, 2405–2422. [Google Scholar] [CrossRef] [PubMed]
- Estevez, M.E.; Fogerson, P.M.; Ilardi, M.C.; Borghuis, B.G.; Chan, E.; Weng, S.J.; Auferkorte, O.N.; Demb, J.B.; Berson, D.M. Form and function of the M4 cell, an intrinsically photosensitive retinal ganglion cell type contributing to geniculocortical vision. J. Neurosci. 2012, 32, 13608–13620. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Hill, D.D.; Wong, K.Y. Intrinsic physiological properties of the five types of mouse ganglion-cell photoreceptors. J. Neurophysiol. 2013, 109, 1876–1889. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Stafford, B.K.; Godin, A.L.; King, W.M.; Wong, K.Y. Photoresponse diversity among the five types of intrinsically photosensitive retinal ganglion cells. J. Physiol. 2014, 592, 1619–1636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reifler, A.N.; Chervenak, A.P.; Dolikian, M.E.; Benenati, B.A.; Meyers, B.S.; Demertzis, Z.D.; Lynch, A.M.; Li, B.Y.; Wachter, R.D.; Abufarha, F.S.; et al. The rat retina has five types of ganglion-cell photoreceptors. Exp. Eye Res. 2015, 130, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Hannibal, J.; Christiansen, A.T.; Heegaard, S.; Fahrenkrug, J.; Kiilgaard, J.F. Melanopsin expressing human retinal ganglion cells: Subtypes, distribution, and intraretinal connectivity. J. Comp. Neurol. 2017, 525, 1934–1961. [Google Scholar] [CrossRef] [PubMed]
- Stabio, M.E.; Sabbah, S.; Quattrochi, L.E.; Ilardi, M.C.; Fogerson, P.M.; Leyrer, M.L.; Kim, M.T.; Kim, I.; Schiel, M.; Renna, J.M.; et al. The M5 cell: A color-opponent intrinsically photosensitive retinal ganglion cell. Neuron 2018, 97, 251. [Google Scholar] [CrossRef] [PubMed]
- Quattrochi, L.E.; Stabio, M.E.; Kim, I.; Ilardi, M.C.; Michelle Fogerson, P.; Leyrer, M.L.; Berson, D.M. The M6 cell: A small-field bistratified photosensitive retinal ganglion cell. J. Comp. Neurol. 2019, 527, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.M.; Do, M.T.; Dacey, D.; Lucas, R.; Hattar, S.; Matynia, A. Melanopsin-positive intrinsically photosensitive retinal ganglion cells: From form to function. J. Neurosci. 2011, 31, 16094–16101. [Google Scholar] [CrossRef]
- Schmidt, T.M.; Kofuji, P. Structure and function of bistratified intrinsically photosensitive retinal ganglion cells in the mouse. J. Comp. Neurol. 2011, 519, 1492–1504. [Google Scholar] [CrossRef] [PubMed]
- Esquiva, G.; Lax, P.; Cuenca, N. Impairment of intrinsically photosensitive retinal ganglion cells associated with late stages of retinal degeneration. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4605–4618. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Romero, C.; Jimenez-Lopez, M.; Garcia-Ayuso, D.; Salinas-Navarro, M.; Nadal-Nicolas, F.M.; Agudo-Barriuso, M.; Villegas-Perez, M.P.; Aviles-Trigueros, M.; Vidal-Sanz, M. Number and spatial distribution of intrinsically photosensitive retinal ganglion cells in the adult albino rat. Exp. Eye Res. 2013, 108, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Lax, P.; Esquiva, G.; Fuentes-Broto, L.; Segura, F.; Sanchez-Cano, A.; Cuenca, N.; Pinilla, I. Age-related changes in photosensitive melanopsin-expressing retinal ganglion cells correlate with circadian rhythm impairments in sighted and blind rats. Chronobiol. Int. 2016, 33, 374–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, H.W.; Ren, X.Z.; Peterson, B.B.; Marshak, D.W.; Yau, K.W.; Gamlin, P.D.; Dacey, D.M. Melanopsin-expressing ganglion cells on macaque and human retinas form two morphologically distinct populations. J. Comp. Neurol. 2016, 524, 2845–2872. [Google Scholar] [CrossRef] [PubMed]
- Nasir-Ahmad, S.; Lee, S.C.S.; Martin, P.R.; Grunert, U. Melanopsin-expressing ganglion cells in human retina: Morphology, distribution, and synaptic connections. J. Comp. Neurol. 2019, 527, 312–327. [Google Scholar] [CrossRef] [PubMed]
- Nadal-Nicolas, F.M.; Salinas-Navarro, M.; Jimenez-Lopez, M.; Sobrado-Calvo, P.; Villegas-Perez, M.P.; Vidal-Sanz, M.; Agudo-Barriuso, M. Displaced retinal ganglion cells in albino and pigmented rats. Front. Neuroanat. 2014, 8, 99. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.M.; Alam, N.M.; Chen, S.; Kofuji, P.; Li, W.; Prusky, G.T.; Hattar, S. A role for melanopsin in alpha retinal ganglion cells and contrast detection. Neuron 2014, 82, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Jusuf, P.R.; Lee, S.C.; Hannibal, J.; Grunert, U. Characterization and synaptic connectivity of melanopsin-containing ganglion cells in the primate retina. Eur. J. Neurosci. 2007, 26, 2906–2921. [Google Scholar] [CrossRef] [PubMed]
- Spear, P.D. Neural bases of visual deficits during aging. Vis. Res. 1993, 33, 2589–2609. [Google Scholar] [CrossRef]
- Nadal-Nicolas, F.M.; Vidal-Sanz, M.; Agudo-Barriuso, M. The senescent vision: Dysfunction or neuronal loss? Aging-Us 2019, 11, 15–17. [Google Scholar] [CrossRef]
- Katz, M.L.; Robison, W.G., Jr. Evidence of cell loss from the rat retina during senescence. Exp. Eye Res. 1986, 42, 293–304. [Google Scholar] [CrossRef]
- Weisse, I. Changes in the aging rat retina. Ophthalmic Res. 1995, 27 (Suppl. 1), 154–163. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, P.; Nag, T.C.; Wadhwa, S. Age-related decrease in rod bipolar cell density of the human retina: An immunohistochemical study. J. Biosci. 2007, 32, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Eliasieh, K.; Liets, L.C.; Chalupa, L.M. Cellular reorganization in the human retina during normal aging. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2824–2830. [Google Scholar] [CrossRef] [PubMed]
- Samuel, M.A.; Zhang, Y.; Meister, M.; Sanes, J.R. Age-related alterations in neurons of the mouse retina. J. Neurosci. 2011, 31, 16033–16044. [Google Scholar] [CrossRef] [PubMed]
- Nadal-Nicolas, F.M.; Vidal-Sanz, M.; Agudo-Barriuso, M. The aging rat retina: From function to anatomy. Neurobiol. Aging 2018, 61, 146–168. [Google Scholar] [CrossRef] [PubMed]
- Noailles, A.; Kutsyr, O.; Maneu, V.; Ortuno-Lizaran, I.; Campello, L.; de Juan, E.; Gomez-Vicente, V.; Cuenca, N.; Lax, P. The absence of toll-like receptor 4 mildly affects the structure and function in the adult mouse retina. Front. Cell. Neurosci. 2019, 13, 59. [Google Scholar] [CrossRef] [PubMed]
- Baba, K.; Tosini, G. Aging alters circadian rhythms in the mouse eye. J. Biol. Rhythms 2018, 33, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Steponenaite, A.; Biello, S.M.; Lall, G.S. Aging clocks: Disrupted circadian rhythms. Aging-Us 2018, 10, 3065–3066. [Google Scholar] [CrossRef] [PubMed]
- Hood, S.; Amir, S. The aging clock: Circadian rhythms and later life. J. Clin. Investig. 2017, 127, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Herbst, K.; Sander, B.; Lund-Andersen, H.; Broendsted, A.E.; Kessel, L.; Hansen, M.S.; Kawasaki, A. Intrinsically photosensitive retinal ganglion cell function in relation to age: A pupillometric study in humans with special reference to the age-related optic properties of the lens. BMC Ophthalmol. 2012, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Brainard, G.C.; Zee, P.C.; Pinto, L.H.; Takahashi, J.S.; Turek, F.W. Effects of aging on lens transmittance and retinal input to the suprachiasmatic nucleus in golden hamsters. Neurosci. Lett. 1998, 258, 167–170. [Google Scholar] [CrossRef]
- Lockley, S.W.; Arendt, J.; Skene, D.J. Visual impairment and circadian rhythm disorders. Dialogues Clin. Neurosci. 2007, 9, 301–314. [Google Scholar] [PubMed]
- de Zavalia, N.; Plano, S.A.; Fernandez, D.C.; Lanzani, M.F.; Salido, E.; Belforte, N.; Sarmiento, M.I.; Golombek, D.A.; Rosenstein, R.E. Effect of experimental glaucoma on the non-image forming visual system. J. Neurochem. 2011, 117, 904–914. [Google Scholar] [CrossRef] [PubMed]
- Lahouaoui, H.; Coutanson, C.; Cooper, H.M.; Bennis, M.; Dkhissi-Benyahya, O. Clock genes and behavioral responses to light are altered in a mouse model of diabetic retinopathy. PLoS ONE 2014, 9, e101584. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Fleitas, M.F.; Bordone, M.; Rosenstein, R.E.; Dorfman, D. Effect of retinal ischemia on the non-image forming visual system. Chronobiol. Int. 2015, 32, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Jean-Louis, G.; Zizi, F.; Lazzaro, D.R.; Wolintz, A.H. Circadian rhythm dysfunction in glaucoma: A hypothesis. J. Circadian Rhythms 2008, 6, 1. [Google Scholar] [CrossRef]
- Perez-Rico, C.; de la Villa, P.; Arribas-Gomez, I.; Blanco, R. Evaluation of functional integrity of the retinohypothalamic tract in advanced glaucoma using multifocal electroretinography and light-induced melatonin suppression. Exp. Eye Res. 2010, 91, 578–583. [Google Scholar] [CrossRef]
- Fernandez, D.C.; Sande, P.H.; de Zavalia, N.; Belforte, N.; Dorfman, D.; Casiraghi, L.P.; Golombek, D.; Rosenstein, R.E. Effect of experimental diabetic retinopathy on the non-image-forming visual system. Chronobiol. Int. 2013, 30, 583–597. [Google Scholar] [CrossRef]
- Muller, L.P.D.; Sargoy, A.; Rodriguez, A.R.; Brecha, N.C. Melanopsin ganglion cells are the most resistant retinal ganglion cell type to axonal injury in the rat retina. PLoS ONE 2014, 9, e93274. [Google Scholar]
- Vidal-Sanz, M.; Galindo-Romero, C.; Valiente-Soriano, F.J.; Nadal-Nicolas, F.M.; Ortin-Martinez, A.; Rovere, G.; Salinas-Navarro, M.; Lucas-Ruiz, F.; Sanchez-Migallon, M.C.; Sobrado-Calvo, P.; et al. Shared and differential retinal responses against optic nerve injury and ocular hypertension. Front. Neurosci. 2017, 11, 235. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Ren, C.; Sollars, P.J.; Pickard, G.E.; So, K.F. The injury resistant ability of melanopsin-expressing intrinsically photosensitive retinal ganglion cells. Neuroscience 2015, 284, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Georg, B.; Ghelli, A.; Giordano, C.; Ross-Cisneros, F.N.; Sadun, A.A.; Carelli, V.; Hannibal, J.; La Morgia, C. Melanopsin-expressing retinal ganglion cells are resistant to cell injury, but not always. Mitochondrion 2017, 36, 77–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valiente-Soriano, F.J.; Nadal-Nicolas, F.M.; Salinas-Navarro, M.; Jimenez-Lopez, M.; Bernal-Garro, J.M.; Villegas-Perez, M.P.; Agudo-Barriuso, M.; Vidal-Sanz, M. BDNF rescues RGCs but not intrinsically photosensitive RGCs in ocular hypertensive albino rat retinas. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1924–1936. [Google Scholar] [CrossRef] [PubMed]
- Rovere, G.; Nadal-Nicolas, F.M.; Wang, J.; Bernal-Garro, J.M.; Garcia-Carrillo, N.; Villegas-Perez, M.P.; Agudo-Barriuso, M.; Vidal-Sanz, M. Melanopsin-containing or non-melanopsin-containing retinal ganglion cells response to acute ocular hypertension with or without brain-derived neurotrophic factor neuroprotection. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6652–6661. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Sanz, M.; Valiente-Soriano, F.J.; Ortin-Martinez, A.; Nadal-Nicolas, F.M.; Jimenez-Lopez, M.; Salinas-Navarro, M.; Alarcon-Martinez, L.; Garcia-Ayuso, D.; Aviles-Trigueros, M.; Agudo-Barriuso, M.; et al. Retinal neurodegeneration in experimental glaucoma. Prog. Brain Res. 2015, 220, 1–35. [Google Scholar] [PubMed]
- Obara, E.A.; Hannibal, J.; Heegaard, S.; Fahrenkrug, J. Loss of melanopsin-expressing retinal ganglion cells in severely staged glaucoma patients. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4661–4667. [Google Scholar] [CrossRef]
- Obara, E.A.; Hannibal, J.; Heegaard, S.; Fahrenkrug, J. Loss of melanopsin-expressing retinal ganglion cells in patients with diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2187–2192. [Google Scholar] [CrossRef]
- Cugini, P.; Cruciani, F.; De Rosa, R.; Pellegrino, A.M.; Fontana, S.; Coda, S.; De Francesco, G.P.; Mastromatteo, A.; Antonelli, B.; Vingolo, E.M.; et al. Alterations of blood pressure and heart rate circadian rhythmic structure in non-blind patients affected by retinitis pigmentosa. J. Hum. Hypertens. 2001, 15, 577–581. [Google Scholar] [CrossRef] [Green Version]
- Lax, P.; Otalora, B.B.; Esquiva, G.; Rol Mde, L.; Madrid, J.A.; Cuenca, N. Circadian dysfunction in P23H rhodopsin transgenic rats: Effects of exogenous melatonin. J. Pineal Res. 2011, 50, 183–191. [Google Scholar] [CrossRef]
- Lax, P.; Kutsyr, O.; Esquiva, G.; Altavilla, C.; Maneu, V.; Cuenca, N. Cannabinoid-mediated retinal rescue correlates with improved circadian parameters in retinal dystrophic rats. Exp. Eye Res. 2018, 180, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Dryja, T.P.; McGee, T.L.; Reichel, E.; Hahn, L.B.; Cowley, G.S.; Yandell, D.W.; Sandberg, M.A.; Berson, E.L. A point mutation of the rhodopsin gene in one form of retinitis pigmentosa. Nature 1990, 343, 364–366. [Google Scholar] [CrossRef]
- Machida, S.; Kondo, M.; Jamison, J.A.; Khan, N.W.; Kononen, L.T.; Sugawara, T.; Bush, R.A.; Sieving, P.A. P23H rhodopsin transgenic rat: Correlation of retinal function with histopathology. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3200–3209. [Google Scholar]
- Strettoi, E.; Pignatelli, V. Modifications of retinal neurons in a mouse model of retinitis pigmentosa. Proc. Natl. Acad. Sci. USA 2000, 97, 11020–11025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marc, R.E.; Jones, B.W.; Watt, C.B.; Strettoi, E. Neural remodeling in retinal degeneration. Prog. Retin. Eye Res. 2003, 22, 607–655. [Google Scholar] [CrossRef]
- Cuenca, N.; Pinilla, I.; Sauve, Y.; Lu, B.; Wang, S.; Lund, R.D. Regressive and reactive changes in the connectivity patterns of rod and cone pathways of P23H transgenic rat retina. Neuroscience 2004, 127, 301–317. [Google Scholar] [CrossRef]
- Puthussery, T.; Taylor, W.R. Functional changes in inner retinal neurons in animal models of photoreceptor degeneration. Adv. Exp. Med. Biol. 2010, 664, 525–532. [Google Scholar]
- Phillips, M.J.; Otteson, D.C.; Sherry, D.M. Progression of neuronal and synaptic remodeling in the rd10 mouse model of retinitis pigmentosa. J. Comp. Neurol. 2010, 518, 2071–2089. [Google Scholar] [CrossRef]
- Cuenca, N.; Fernandez-Sanchez, L.; Campello, L.; Maneu, V.; De la Villa, P.; Lax, P.; Pinilla, I. Cellular responses following retinal injuries and therapeutic approaches for neurodegenerative diseases. Prog. Retin. Eye Res. 2014, 43, 17–75. [Google Scholar] [CrossRef]
- Jones, B.W.; Pfeiffer, R.L.; Ferrell, W.D.; Watt, C.B.; Marmor, M.; Marc, R.E. Retinal remodeling in human retinitis pigmentosa. Exp. Eye Res. 2016, 150, 149–165. [Google Scholar] [CrossRef] [Green Version]
- Kolomiets, B.; Dubus, E.; Simonutti, M.; Rosolen, S.; Sahel, J.A.; Picaud, S. Late histological and functional changes in the P23H rat retina after photoreceptor loss. Neurobiol. Dis. 2010, 38, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Robinson, G.A.; Madison, R.D. Axotomized mouse retinal ganglion cells containing melanopsin show enhanced survival, but not enhanced axon regrowth into a peripheral nerve graft. Vis. Res. 2004, 44, 2667–2674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.S.; Chen, B.Y.; Tay, D.K.; Chan, H.H.; Pu, M.L.; So, K.F. Melanopsin-expressing retinal ganglion cells are more injury-resistant in a chronic ocular hypertension model. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2951–2958. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Yau, S.Y.; Chen, B.Y.; Tay, D.K.; Lee, V.W.; Pu, M.L.; Chan, H.H.; So, K.F. Enhanced survival of melanopsin-expressing retinal ganglion cells after injury is associated with the PI3 K/Akt pathway. Cell. Mol. Neurobiol. 2008, 28, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Migallon, M.C.; Valiente-Soriano, F.J.; Nadal-Nicolas, F.M.; Di Pierdomenico, J.; Vidal-Sanz, M.; Agudo-Barriuso, M. Survival of melanopsin expressing retinal ganglion cells long term after optic nerve trauma in mice. Exp. Eye Res. 2018, 174, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Fahn, S. Description of Parkinson’s disease as a clinical syndrome. Parkinson’s Dis. Life Cycle Dopamine Neuron 2003, 991, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; Massano, J. An updated review of Parkinson’s disease genetics and clinicopathological correlations. Acta Neurol. Scand. 2017, 135, 273–284. [Google Scholar] [CrossRef]
- Postuma, R.B.; Poewe, W.; Litvan, I.; Lewis, S.; Lang, A.E.; Halliday, G.; Goetz, C.G.; Chan, P.; Slow, E.; Seppi, K.; et al. Validation of the MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2018, 33, 1601–1608. [Google Scholar] [CrossRef]
- Kozlowski, P.B.; Glazman, S.; Bodis-Wollner, I. Localization of alpha-synuclein in the retina in Parkinson Disease (PD). Ann. Neurol. 2013, 74, S52–S53. [Google Scholar]
- Lee, J.Y.; Ahn, J.; Kim, T.W.; Jeon, B.S. Optical coherence tomography in Parkinson’s disease: Is the retina a biomarker? J. Parkinsons Dis. 2014, 4, 197–204. [Google Scholar] [PubMed]
- Weil, R.S.; Schrag, A.E.; Warren, J.D.; Crutch, S.J.; Lees, A.J.; Morris, H.R. Visual dysfunction in Parkinson’s disease. Brain 2016, 139, 2827–2843. [Google Scholar] [CrossRef] [PubMed]
- Ortuno-Lizaran, I.; Beach, T.G.; Serrano, G.E.; Walker, D.G.; Adler, C.H.; Cuenca, N. Phosphorylated alpha-synuclein in the retina is a biomarker of Parkinson’s disease pathology severity. Mov. Disord. 2018, 33, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Giza, E.; Fotiou, D.; Bostantjopoulou, S.; Katsarou, Z.; Karlovasitou, A. Pupil light reflex in Parkinson’s disease: Evaluation with pupillometry. Int. J. Neurosci. 2011, 121, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.A.; McInnis, H.; Brien, D.C.; Pari, G.; Munoz, D.P. Disruption of pupil size modulation correlates with voluntary motor preparation deficits in Parkinson’s disease. Neuropsychologia 2016, 80, 176–184. [Google Scholar] [CrossRef]
- Joyce, D.S.; Feigl, B.; Kerr, G.; Roeder, L.; Zele, A.J. Melanopsin-mediated pupil function is impaired in Parkinson’s disease. Sci. Rep. 2018, 8, 7796. [Google Scholar] [CrossRef]
- Neikrug, A.B.; Maglione, J.E.; Liu, L.; Natarajan, L.; Avanzino, J.A.; Corey-Bloom, J.; Palmer, B.W.; Loredo, J.S.; Ancoli-Israel, S. Effects of sleep disorders on the non-motor symptoms of Parkinson disease. J. Clin. Sleep Med. 2013, 9, 1119–1129. [Google Scholar] [CrossRef]
- Videnovic, A.; Golombek, D. Circadian and sleep disorders in Parkinson’s disease. Exp. Neurol. 2013, 243, 45–56. [Google Scholar] [CrossRef]
- Gros, P.; Videnovic, A. Sleep and circadian rhythm disorders in Parkinson’s disease. Curr. Sleep Med. Rep. 2017, 3, 222–234. [Google Scholar] [CrossRef]
- Gan-Or, Z.; Alcalay, R.N.; Rouleau, G.A.; Postuma, R.B. Sleep disorders and Parkinson disease; lessons from genetics. Sleep Med. Rev. 2018, 41, 101–112. [Google Scholar] [CrossRef]
- Ben, V.; Bruguerolle, B. Effects of bilateral striatal 6-OHDA lesions on circadian rhythms in the rat: A radiotelemetric study. Life Sci. 2000, 67, 1549–1558. [Google Scholar] [CrossRef]
- Almirall, H.; Bautista, V.; Sanchez-Bahillo, A.; Trinidad-Herrero, M. Ultradian and circadian body temperature and activity rhythms in chronic MPTP treated monkeys. Neurophysiol. Clin. 2001, 31, 161–170. [Google Scholar] [CrossRef]
- Kudo, T.; Loh, D.H.; Truong, D.; Wu, Y.; Colwell, C.S. Circadian dysfunction in a mouse model of Parkinson’s disease. Exp. Neurol. 2011, 232, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Lax, P.; Esquiva, G.; Esteve-Rudd, J.; Otalora, B.B.; Madrid, J.A.; Cuenca, N. Circadian dysfunction in a rotenone-induced parkinsonian rodent model. Chronobiol. Int. 2012, 29, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Normando, E.M.; Shah, P.A.; De Groef, L.; Cordeiro, M.F. Oculo-visual abnormalities in Parkinson’s disease: Possible value as biomarkers. Mov. Disord. 2018, 33, 1390–1406. [Google Scholar] [CrossRef] [PubMed]
- Esteve-Rudd, J.; Fernandez-Sanchez, L.; Lax, P.; De Juan, E.; Martin-Nieto, J.; Cuenca, N. Rotenone induces degeneration of photoreceptors and impairs the dopaminergic system in the rat retina. Neurobiol. Dis. 2011, 44, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Beach, T.G.; Carew, J.; Serrano, G.; Adler, C.H.; Shill, H.A.; Sue, L.I.; Sabbagh, M.N.; Akiyama, H.; Cuenca, N.; Arizona Parkinson’s Disease Consortium. Phosphorylated alpha-synuclein-immunoreactive retinal neuronal elements in Parkinson’s disease subjects. Neurosci. Lett. 2014, 571, 34–38. [Google Scholar] [CrossRef]
- Vuong, H.E.; Hardi, C.N.; Barnes, S.; Brecha, N.C. Parallel inhibition of dopamine amacrine cells and intrinsically photosensitive retinal ganglion cells in a non-image-forming visual circuit of the mouse retina. J. Neurosci. 2015, 35, 15955–15970. [Google Scholar] [CrossRef]
- Viney, T.J.; Balint, K.; Hillier, D.; Siegert, S.; Boldogkoi, Z.; Enquist, L.W.; Meister, M.; Cepko, C.L.; Roska, B. Local retinal circuits of melanopsin-containing ganglion cells identified by transsynaptic viral tracing. Curr. Biol. 2007, 17, 981–988. [Google Scholar] [CrossRef]
- Sakamoto, K.; Liu, C.; Kasamatsu, M.; Pozdeyev, N.V.; Iuvone, P.M.; Tosini, G. Dopamine regulates melanopsin mRNA expression in intrinsically photosensitive retinal ganglion cells. Eur. J. Neurosci. 2005, 22, 3129–3136. [Google Scholar] [CrossRef]
- Van Hook, M.J.; Wong, K.Y.; Berson, D.M. Dopaminergic modulation of ganglion-cell photoreceptors in rat. Eur. J. Neurosci. 2012, 35, 507–518. [Google Scholar] [CrossRef] [PubMed]
- La Morgia, C.; Ross-Cisneros, F.N.; Koronyo, Y.; Hannibal, J.; Gallassi, R.; Cantalupo, G.; Sambati, L.; Pan, B.X.; Tozer, K.R.; Barboni, P.; et al. Melanopsin retinal ganglion cell loss in alzheimer disease. Ann. Neurol. 2016, 79, 90–109. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.Q.; Li, L.J.; Yu, H.Y.; Liu, M.; Zhao, W. Melanopsin retinal ganglion cell loss and circadian dysfunction in Alzheimer’s disease. Mol. Med. Rep. 2016, 13, 3397–3400. [Google Scholar] [CrossRef] [PubMed]
- La Morgia, C.; Ross-Cisneros, F.N.; Sadun, A.A.; Carelli, V. Retinal ganglion cells and circadian rhythms in Alzheimer’s disease, Parkinson’s disease, and beyond. Front. Neurol. 2017, 8, 162. [Google Scholar] [CrossRef] [PubMed]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lax, P.; Ortuño-Lizarán, I.; Maneu, V.; Vidal-Sanz, M.; Cuenca, N. Photosensitive Melanopsin-Containing Retinal Ganglion Cells in Health and Disease: Implications for Circadian Rhythms. Int. J. Mol. Sci. 2019, 20, 3164. https://doi.org/10.3390/ijms20133164
Lax P, Ortuño-Lizarán I, Maneu V, Vidal-Sanz M, Cuenca N. Photosensitive Melanopsin-Containing Retinal Ganglion Cells in Health and Disease: Implications for Circadian Rhythms. International Journal of Molecular Sciences. 2019; 20(13):3164. https://doi.org/10.3390/ijms20133164
Chicago/Turabian StyleLax, Pedro, Isabel Ortuño-Lizarán, Victoria Maneu, Manuel Vidal-Sanz, and Nicolás Cuenca. 2019. "Photosensitive Melanopsin-Containing Retinal Ganglion Cells in Health and Disease: Implications for Circadian Rhythms" International Journal of Molecular Sciences 20, no. 13: 3164. https://doi.org/10.3390/ijms20133164
APA StyleLax, P., Ortuño-Lizarán, I., Maneu, V., Vidal-Sanz, M., & Cuenca, N. (2019). Photosensitive Melanopsin-Containing Retinal Ganglion Cells in Health and Disease: Implications for Circadian Rhythms. International Journal of Molecular Sciences, 20(13), 3164. https://doi.org/10.3390/ijms20133164