Elucidation of the Molecular Mechanism Underlying Lippia citriodora(Lim.)-Induced Relaxation and Anti-Depression
Abstract
:1. Introduction
2. Results
2.1. Effect of VEE and Its Compounds on SH-SY5Y Cells’Viability
2.2. Effect of VEE on the Immobility Time of Mice
2.3. Elucidation of the Genes Regulated by VEE Treatment
2.4. Validation of Expressions of Gsn, Ttr, Camk2n1 and Itpr2
2.5. Antidepressant Effect of Low Doses of VEE and Vs
2.6. Evaluation of the Mitochondrial Activity of Cells Treated with VEE and Vs
2.7. Effect of VEE and Vs on Intracellular Calcium Levels
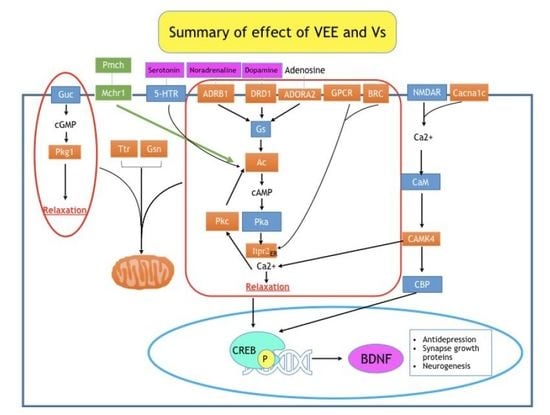
3. Discussion
4. Materials and Methods
4.1. Plant Material and Extraction Method
4.2. Chemicals
4.3. Cell Culture
4.4. Determination of Cell Viability
4.5. Animals
4.6. Tail Suspension Test
4.7. DNA Microarray Analysis
4.8. Real Time Polymerase Chain Reaction (qRT-PCR)
4.9. Quantification of Neurotransmitters and BDNF
4.10. Measurement of Mitochondrial Activity
4.11. Measurement of the Intracellular ATP Production
4.12. Measurement of Intracellular Calcium Level
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Ac | Adenylate cyclase |
| Adora2 | Adenosine A2a receptor |
| Cacna1c | Calcium channel, voltage-dependent, L type, alpha 1C subunit |
| Camk2n1 | Calcium/calmodulin-dependent protein kinase II inhibitor 1 |
| Camk4 | Calcium/calmodulin-dependent protein kinase IV |
| Dex | Dexamethasone |
| Drd1 | Dopamine receptor 1 |
| Gsn | Gelsolin |
| Hs | Hastatoside |
| Htr4 | 5 hydroxytryptamine (serotonin) receptor 4 |
| Itpr2 | Inositol 1,4,5-trisphosphate receptor type 2 |
| Mchr1 | Melanin-concentrating hormone receptor |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NA | Noradrenaline |
| Pkc | Protein kinase c |
| Pmch | Pro-melanin-concentrating hormone |
| Prkg1 | cGMP-dependent protein kinase 1 |
| Sert | Serotonin |
| TST | Tail suspension test |
| Ttr | Transthyretin |
| VEE | Verbena ethanolic extract |
| Vn | Verbenalin |
| Vs | Verbascoside |
References
- Rahmatullah, M.; Jahan, R.; Safiul Azam, F.M.; Hossan, S.; Mollik, M.A.H.; Rahman, T. Folk medicinal uses of verbenaceae family plants in Bangladesh. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Pascual, M.E.; Slowing, K.; Carretero, E.; Sánchez Mata, D.; Villar, A. Lippia: Traditional uses, chemistry and pharmacology: A review. J. Ethnopharmacol. 2001, 76, 201–214. [Google Scholar] [CrossRef]
- Carnat, A.; Carnat, A.P.; Fraisse, D.; Lamaison, J.L. The aromatic and polyphenolic composition of lemon verbena tea. Fitoterapia 1999, 70, 44–49. [Google Scholar] [CrossRef]
- Bellakhdar, J. La Pharmacopée Marocaine Traditionnelle; Ibis Press: Paris, France, 1997; ISBN 2-910728-0. [Google Scholar]
- Montari, B. Aromatic, medicinal plants and vulnerability of traditional herbal knowledge in a berber community of the high atlas mountains of Morocco. Plant Divers.Resour. 2014, 36, 388–402. [Google Scholar]
- Fitsiou, E.; Mitropoulou, G.; Spyridopoulou, K.; Vamvakias, M.; Bardouki, H.; Galanis, A.; Chlichlia, K.; Kourkoutas, Y.; Panayiotidis, M.I.; Pappa, A. Chemical composition and evaluation of the biological properties of the essential oil of the dietary phytochemical Lippia citriodora. Molecules 2018, 23, 123. [Google Scholar] [CrossRef] [PubMed]
- Ragone, M.I.; Sella, M.; Conforti, P.; Volonté, M.G.; Consolini, A.E. The spasmolytic effect of Aloysia citriodora, Palau (South American cedrón) is partially due to its vitexin but not isovitexin on rat duodenums. J. Ethnopharmacol. 2007, 113, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Akanmu, M.A.; Honda, K.; Inoué, S. Hypnotic effects of total aqueous extracts of Vervain hastata (Verbenaceae) in rats. Psychiatry Clin. Neurosci. 2002, 56, 309–310. [Google Scholar] [CrossRef]
- Herranz-López, M.; Barrajón-Catalán, E.; Segura-Carretero, A.; Menéndez, J.A.; Joven, J.; Micol, V. Lemon verbena (Lippia citriodora) polyphenols alleviate obesity-related disturbances in hypertrophic adipocytes through AMPK-dependent mechanisms. Phytomedicine 2015, 22, 605–614. [Google Scholar] [CrossRef]
- Carnat, A.P.; Carnat, A.; Fraisse, D.; Lamaison, J.L. The aromatic and polyphenolic composition of lemon balm (Melissa officinalis L. subsp. officinalis) tea. Pharm. Acta Helv. 1998, 72, 301–305. [Google Scholar] [CrossRef]
- Arthur, H.; Joubert, E.; De Beer, D.; Malherbe, C.J.; Witthuhn, R.C. Phenylethanoid glycosides as major antioxidants in Lippia multiflora herbal infusion and their stability during steam pasteurisation of plant material. Food Chem. 2011, 127, 581–588. [Google Scholar] [CrossRef]
- Vertuani, S.; Beghelli, E.; Scalambra, E.; Malisardi, G.; Copetti, S.; Toso, R.D.; Baldisserotto, A.; Manfredini, S. Activity and stability studies of verbascoside, a novel antioxidant, in dermo-cosmetic and pharmaceutical topical formulations. Molecules 2011, 16, 7068–7080. [Google Scholar] [CrossRef] [PubMed]
- Speranza, L.; Franceschelli, S.; Pesce, M.; Reale, M.; Menghini, L.; Vinciguerra, I.; De Lutiis, M.A.; Felaco, M.; Grilli, A. Antiinflammatory effects in THP-1 cells treated with verbascoside. Phytother. Res. 2010, 24, 1398–1404. [Google Scholar] [CrossRef] [PubMed]
- Díaz, A.M.; Abad, M.J.; Fernández, L.; Silván, A.M.; De Santos, J.; Bermejo, P. Phenylpropanoid glycosides from Scrophularia scorodonia: In vitro anti-inflammatory activity. Life Sci. 2004, 74, 2515–2526. [Google Scholar] [CrossRef] [PubMed]
- Guillermo Avila, J.; De Liverant, J.G.; Martínez, A.; Martínez, G.; Muñoz, J.L.; Arciniegas, A.; Romo De Vivar, A. Mode of action of Buddleja cordata verbascoside against Staphylococcus aureus. J. Ethnopharmacol. 1999, 66, 75–78. [Google Scholar] [CrossRef]
- Sheng, G.-Q.; Zhang, J.-R.; Pu, X.-P.; Ma, J.; Li, C.-L. Protective effect of verbascoside on 1-methyl-4-phenylpyridinium ion-induced neurotoxicity in PC12 cells. Eur. J. Pharm. 2002, 451, 119–124. [Google Scholar] [CrossRef]
- Razavi, B.M.; Zargarani, N.; Hosseinzadeh, H. Anti-anxiety and hypnotic effects of ethanolic and aqueous extracts of Lippia citriodora leaves and verbascoside in mice. Avicenna J. Phytomed. 2017, 7, 353–365. [Google Scholar] [PubMed]
- Makino, Y.; Kondo, S.; Nishimura, Y.; Tsukamoto, Y.; Huang, Z.L.; Urade, Y. Hastatoside and verbenalin are sleep-promoting components in verbena officinalis. Sleep Biol. Rhythm. 2009, 7, 211–217. [Google Scholar] [CrossRef]
- Nakamura, T.; Okuyama, E.; Tsukada, A.; Yamazaki, M.; Satake, M.; Nishibe, S.; Takeshi, D.; Moriya, A.; Maruno, M.; Nishimura, H. Acteoside as the analgesic priniple of cedron (Lippia triphylla), a Peruvian medicinal plant. Chem. Pharm. Bull. 1997, 45, 499–504. [Google Scholar] [CrossRef]
- Casanova, E.; García-Mina, J.M.; Calvo, M.I. Antioxidant and antifungal activity of Verbena officinalis L. leaves. Plant Food Hum. Nutr. 2008, 63, 93–97. [Google Scholar] [CrossRef]
- Deepak, M.; Handa, S.S. Quantitative determination of the major constituents of Verbena officinalis using high performance thin layer chromatography and high pressure liquid chromatography. Phytochem. Anal. 2000, 11, 351–355. [Google Scholar] [CrossRef]
- Funes, L.; Laporta, O.; Cerdán-Calero, M.; Micol, V. Effects of verbascoside, a phenylpropanoid glycoside from lemon verbena, on phospholipid model membranes. Chem. Phys. Lipids 2010, 163, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Funes, L.; Carrera-Quintanar, L.; Cerdán-Calero, M.; Ferrer, M.D.; Drobnic, F.; Pons, A.; Roche, E.; Micol, V. Effect of lemon verbena supplementation on muscular damage markers, proinflammatory cytokines release and neutrophils’ oxidative stress in chronic exercise. Eur. J. Appl. Physiol. 2011, 111, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Rehecho, S.; Hidalgo, O.; García-Iñiguez de Cirano, M.; Navarro, I.; Astiasarán, I.; Ansorena, D.; Cavero, R.Y.; Calvo, M.I. Chemical composition, mineral content and antioxidant activity of Verbena officinalis L. LWT Food Sci. Technol. 2011, 44, 875–882. [Google Scholar] [CrossRef]
- Santulli, G.; Marks, A.R. Essential roles of intracellular calcium release channels in muscle, brain, metabolism, and aging. Curr. Mol. Pharm. 2015, 8, 206–222. [Google Scholar] [CrossRef]
- Simms, B.A.; Zamponi, G.W. Neuronal voltage-gated calcium channels: Structure, function, and dysfunction. Neuron 2014, 82, 24–45. [Google Scholar] [CrossRef] [PubMed]
- Jornayvaz, F.R.; Shulman, G.I.G. Regulation of mitochondrial biogenesis. Essays Biochem. 2010, 47, 69–84. [Google Scholar] [CrossRef] [Green Version]
- Tang, M.; Wang, G.; Lu, P.; Karas, R.H.; Aronovitz, M.; Heximer, S.P.; Kaltenbronn, K.M.; Blumer, K.J.; Siderovski, D.P.; Zhu, Y.; et al. Regulator of G-protein signaling-2 mediates vascular smooth muscle relaxation and blood pressure. Nat. Med. 2003, 9, 1506–1512. [Google Scholar] [CrossRef]
- Ohtsuki, T.; Ishiguro, H.; Detera-Wadleigh, S.D.; Toyota, T.; Shimizu, H.; Yamada, K.; Yoshitsugu, K.; Hattori, E.; Yoshikawa, T.; Arinami, T. Association between serotonin 4 receptor gene polymorphisms and bipolar disorder in Japanese case-control samples and the NIMH Genetics Initiative Bipolar Pedigrees. Mol. Psychiatry 2002, 7, 954–961. [Google Scholar] [CrossRef] [Green Version]
- Chiu, F.L.; Lin, J.T.; Chuang, C.Y.; Chien, T.; Chen, C.M.; Chen, K.H.; Hsiao, H.Y.; Lin, Y.S.; Chern, Y.; Kuo, H.C. Elucidating the role of the A2Aadenosine receptor in neurodegeneration using neurons derived from Huntington’s disease iPSCs. Hum. Mol. Genet. 2015, 24, 6066–6079. [Google Scholar] [CrossRef]
- Horgusluoglu-Moloch, E.; Nho, K.; Risacher, S.L.; Kim, S.; Foroud, T.; Shaw, L.M.; Trojanowski, J.Q.; Aisen, P.S.; Petersen, R.C.; Jack, C.R.; et al. Targeted neurogenesis pathway-based gene analysis identifies ADORA2A associated with hippocampal volume in mild cognitive impairment and Alzheimer’s disease. Neurobiol. Aging 2017, 60, 92–103. [Google Scholar] [CrossRef]
- Ehrlich, A.T.; Furuyashiki, T.; Kitaoka, S.; Kakizuka, A.; Narumiya, S. Prostaglandin E receptor EP1 forms a complex with dopamine D1 receptor and directs D1-induced cAMP production to adenylyl cyclase 7 through mobilizing G(βγ) subunits in human embryonic kidney 293T cells. Mol. Pharm. 2013, 84, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Antequera, D.; Vargas, T.; Ugalde, C.; Spuch, C.; Molina, J.A.; Ferrer, I.; Bermejo-Pareja, F.; Carro, E. Cytoplasmic gelsolin increases mitochondrial activity and reduces Aβ burden in a mouse model of Alzheimer’s disease. Neurobiol. Dis. 2009, 36, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Oka, S.; Leon, J.; Sakumi, K.; Ide, T.; Kang, D.; LaFerla, F.M.; Nakabeppu, Y. Human mitochondrial transcriptional factor A breaks the mitochondria-mediated vicious cycle in Alzheimer’s disease. Sci. Rep. 2016, 6, 37889. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liu, Z.; Guo, S.; Wu, L.; Li, M.; Chen, R.; Xu, H.; Cai, S.; Chen, H.; Li, W.; et al. The tumor suppressive role of CAMK2N1 in castration-resistant prostate cancer. Oncotarget 2014, 5, 3611. [Google Scholar] [CrossRef] [PubMed]
- MacNeil, D.J. The role of melanin-concentrating hormone and its receptors in energy homeostasis. Front. Endocrinol. 2013, 4, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Borowsky, B.; Durkin, M.M.; Ogozalek, K.; Marzabadi, M.R.; DeLeon, J.; Heurich, R.; Lichtblau, H.; Shaposhnik, Z.; Daniewska, I.; Blackburn, T.P.; et al. Antidepressant, anxiolytic and anorectic effects of a melanin-concentrating hormone-1 receptor antagonist. Nat. Med. 2002, 8, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Shen, Z.; Strack, A.M.; Marsh, D.J.; Shearman, L.P. Enhanced running wheel activity of both Mch1r- and Pmch-deficient mice. Regul. Pept. 2005, 124, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Buxbaum, J.N. Transthyretin and the brain re-visited: Is neuronal synthesis of transthyretin protective in Alzheimer’s disease? Mol. Neurodegener. 2011, 6, 79. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Sun, L.D.; Atkins, C.M.; Soderling, T.R.; Wilson, M.A.; Tonegawa, S. An important role of neural activity-dependent CaMKIV signaling in the consolidation of long-term memory. Cell 2001, 106, 771–783. [Google Scholar] [CrossRef]
- Kawabe, J.; Iwami, G.; Ebina, T.; Ohno, S.; Katada, T.; Ueda, Y.; Homcy, C.J.; Ishikawa, Y. Differential activation of adenylyl cyclase by protein kinase C isoenzymes. J. Biol. Chem. 1994, 269, 16554–16558. [Google Scholar]
- Iwamoto, T.; Umemura, S.; Toya, Y.; Uchibori, T.; Kogi, K.; Takagi, N.; Ishii, M. Identification of A2a receptor-cAMP system in Human Aortic Endothelial Cells. Biochem. Biophys. Res. Commun. 1994, 199, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Tresguerres, M.; Levin, L.R.; Buck, J. Intracellular cAMP signaling by soluble adenylyl cyclase. KidneyInt. 2011, 79, 1277–1288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pissios, P.; Maratos-Flier, E. Melanin-concentrating hormone: From fish skin to skinny mammals. Trends Endocrinol. Metab. 2003, 14, 243–248. [Google Scholar] [CrossRef]
- Viale, A.; Ortola, C.; Hervieu, G.; Furuta, M.; Barbero, P.; Steiner, D.F.; Seidah, N.G.; Nahon, J.L. Cellular localization and role of prohormone convertases in the processing of pro-melanin concentrating hormone in mammals. J. Biol. Chem. 1999, 274, 6536–6545. [Google Scholar] [CrossRef] [PubMed]
- Briley, M.; Chantal, M. The importance of norepinephrine in depression. Neuropsychiatr. Dis. Treat. 2011, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Belmaker, R.; Agam, G. Major depressive disorder. N. Engl. J. Med. 2008, 358, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Nestler, E.J. The molecular neurobiology of depression. Nature 2008, 455, 894–902. [Google Scholar] [CrossRef]
- Jawaid, T.; Imam, S.A.; Kamal, M. Antidepressant activity of methanolic extract of Verbena Officinalis Linn. plant in mice. Asian J. Pharm. Clin. Res. 2015, 8, 308–310. [Google Scholar]
- Makram, S.; Alaoui, K.; Benabboyha, T.; Faridi, B.; Cherrah, Y.; Zellou, A. Extraction et activité psychotrope de l’huile essentielle de la verveine odorante Lippia citriodora. Phytotherapie 2015, 13, 163–167. [Google Scholar] [CrossRef]
- Calvo, M.I. Anti-inflammatory and analgesic activity of the topical preparation of Verbena officinalis L. J. Ethnopharmacol. 2006, 3, 380–382. [Google Scholar] [CrossRef]
- Kuo, I.Y.; Ehrlich, B.E. Signaling in muscle contraction. Cold Spring Harb. Perspect. Biol. 2015, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Di Villa Bianca, R.D.E.; Sorrentino, R.; Roviezzo, F.; Imbimbo, C.; Palmieri, A.; De Dominicis, G.; Montorsi, F.; Cirino, G.; Mirone, V. Peripheral relaxant activity of apomorphine and of a D1 selective receptor agonist on human corpus cavernosum strips. Int. J. Impot. Res. 2005, 17, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, M.K.; Dusek, J.A.; Chang, B.-H.; Joseph, M.G.; Denninger, J.W.; Fricchione, G.L.; Benson, H.; Libermann, T.A. Relaxation response induces temporal transcriptome changes in energy metabolism, insulin secretion and inflammatory pathways. PLoS ONE 2013, 8, e62817. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, S.; Iwasaki, H.; Inagami, T.; Numaguchi, K.; Yamakawa, T.; Motley, E.D.; Owada, K.M.; Fumiaki, M.; Hirata, Y. Vascular smooth muscle cells. Hypertension 1999, 33, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Kovac, J.R.; Preiksaitis, H.G.; Sims, S.M. Functional and molecular analysis of L-type calcium channels in human esophagus and lower esophageal sphincter smooth muscle. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 289, G998–G1006. [Google Scholar] [CrossRef] [PubMed]
- Arduíno, D.M.; Esteves, A.R.; Cardoso, S.M.; Oliveira, C.R. Endoplasmic reticulum and mitochondria interplay mediates apoptotic cell death: Relevance to Parkinson’s disease. Neurochem. Int. 2009, 55, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Schapira, A.H.V.; Olanow, C.W.; Greenamyre, J.T.; Bezard, E. Slowing of neurodegeneration in Parkinson’s disease and Huntington’s disease: Future therapeutic perspectives. Lancet 2014, 384, 545–555. [Google Scholar] [CrossRef]
- Zatti, G.; Ghidoni, R.; Barbiero, L.; Binetti, G.; Pozzan, T.; Fasolato, C.; Pizzo, P. The presenilin 2 M239I mutation associated with familial Alzheimer’s disease reduces Ca2+release from intracellular stores. Neurobiol. Dis. 2004, 15, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Lessard, C.B.; Lussier, M.P.; Cayouette, S.; Bourque, G.; Boulay, G. The overexpression of presenilin2 and Alzheimer’s-disease-linked presenilin2 variants influences TRPC6-enhanced Ca2+entry into HEK293 cells. Cell. Signal. 2005, 17, 437–445. [Google Scholar] [CrossRef]
- Tarasov, A.I.; Griffiths, E.J.; Rutter, G.A. Regulation of ATP production by mitochondrial Ca2+. Cell Calcium 2012, 52, 28–35. [Google Scholar] [CrossRef]
- Gillis, J.M. Inhibition of mitochondrial calcium uptake slows down relaxation in mitochondria-rich skeletal muscles. J. Muscle Res. Cell Motil. 1997, 18, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Di Benedetto, G.; Scalzotto, E.; Mongillo, M.; Pozzan, T. Mitochondrial Ca2+ uptake induces cyclic AMP generation in the matrix and modulates organelle ATP levels. Cell Metab. 2013, 17, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, G.M.; Hatterer, J.A.; Herbert, J.; Chen, X.; Roose, S.P.; Attia, E.; Mann, J.J.; Marangell, L.B.; Goetz, R.R.; Gorman, J.M. Low levels of transthyretin in the CSF of depressed patients. Am. J. Psychiatry 1999, 156, 710–714. [Google Scholar] [PubMed]
- Tsuzuki, K.; Fukatsu, R.; Yamaguchi, H.; Tateno, M.; Imai, K.; Fujii, N.; Yamauchi, T. Transthyretin binds amyloid beta peptides, Abeta1-42 and Abeta1-40 to form complex in the autopsied human kidney—Possible role of transthyretin for abeta sequestration. Neurosci. Lett. 2000, 281, 171–174. [Google Scholar] [CrossRef]
- Buxbaum, J.N.; Ye, Z.; Reixach, N.; Friske, L.; Levy, C.; Das, P.; Golde, T.; Masliah, E.; Roberts, A.R.; Bartfai, T. Transthyretin protects Alzheimer’s mice from the behavioral and biochemical effects of A toxicity. Proc. Natl. Acad. Sci. USA 2008, 105, 2681–2686. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, X.; Ladiwala, A.R.A.; Du, D.; Yadav, J.K.; Tessier, P.M.; Wright, P.E.; Kelly, J.W.; Buxbaum, J.N. Mechanisms of Transthyretin Inhibition of -Amyloid Aggregation In Vitro. J. Neurosci. 2013, 33, 19423–19433. [Google Scholar] [CrossRef] [PubMed]
- Stein, T.D.; Johnson, J.A. Lack of neurodegeneration in transgenic mice overexpressing mutant amyloid precursor protein is associated with increased levels of transthyretin and the activation of cell survival pathways. J. Neurosci. 2002, 22, 7380–7388. [Google Scholar] [CrossRef] [PubMed]
- Hori, H.; Kunugi, H. Dopamine agonist-responsive depression. Psychogeriatrics 2013, 13, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Kim, Y.K. The roles of BDNF in the pathophysiology of major depression and in antidepressant treatment. Psychiatry Investig. 2010, 7, 231–235. [Google Scholar] [CrossRef]
- Duman, R.S.; Monteggia, L.M. A neurotrophic model for stress-related mood disorders. Biol. Psychiatry 2006, 59, 1116–1127. [Google Scholar] [CrossRef]
- Mizuno, K.; Giese, K.P. Hippocampus-dependent memory formation: Do memory type-specific mechanisms exist? J. Pharm. Sci. 2005, 98, 191–197. [Google Scholar] [CrossRef]
- Denton, R.M.; Mccormack, J.G.; Edgell, N.J. Role of calcium ions in the regulation of intramitochondrial metabolism. Biochem. J. 1980, 190, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Hansford, R.G.; Castro, F. Effect of micromolar concentrations of free calcium ions on the reduction of heart mitochondrial NAD(P) by 2-oxoglutarate. Biochem. J. 2015, 198, 525–533. [Google Scholar] [CrossRef]
- Crompton, M. The regulation of mitochondrial calcium transport in heart. Curr. Top. Membr. Transp. 1985, 25, 231–276. [Google Scholar]
- Jouaville, L.S.; Pinton, P.; Bastianutto, C.; Rutter, G.A.; Rizzuto, R. Regulation of mitochondrial ATP synthesis by calcium: Evidence for a long-term metabolic priming. Proc. Natl. Acad. Sci. USA 1999, 96, 13807–13812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steru, L.; Chermat, R.; Thierry, B.; Simon, P. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology 1985, 85, 367–370. [Google Scholar] [CrossRef]
- Matsukawa, T.; Motojima, H.; Sato, Y.; Takahashi, S.; Villareal, M.O.; Isoda, H. Upregulation of skeletal muscle PGC-1α through the elevation of cyclic AMP levels by Cyanidin-3-glucoside enhances exercise performance. Sci. Rep. 2017, 7, 44799. [Google Scholar] [CrossRef]







| Gene ID | Gene Name | Verbena Ratio | Bupropion Ratio | Function |
|---|---|---|---|---|
| Gsn | Gelsolin | 5.26 | 1.54 | Amyloid beta peptides aggregation [33,39] |
| Ttr | Transthyretin | 3.72 | 3.91 | |
| Camk2n1 | Calcium/calmodulin-dependent protein kinase 2 inhibitor 1 | 2.19 | 1.03 | Tumor suppressor [35] |
| CaMK4 | calcium/calmodulin-dependent protein kinase IV | 1.46 | 1.20 | Long-term memory [40] |
| Cacna1c | Calcium channel, voltage-dependent, L type, alpha 1C subunit | 1.45 | 1.07 | Cytosolic calcium content [26] |
| Pkc | Protein kinase c | 1.45 | 0.98 | Adenylate cyclase activation [32,41] |
| Drd1 | Dopamine receptor 1 | 1.43 | 1.07 | |
| Adora2 | Adenosine A2a receptor | 1.34 | 1.1 | Cyclic-Adenosine monophosphate (cAMP) production [42] |
| Htr4 | 5 hydroxytryptamine (serotonin) receptor 4 | 1.34 | 1.25 | Modulation of neurotransmitter release [29] |
| Itpr2 | Inositol 1,4,5-trisphosphate receptor type 2 | 1.30 | 1.22 | Intracellular calcium release [25] |
| Ac | Adenylate cyclase | 1.28 | 0.85 | Production of cAMP [43] |
| Prkg1 | cGMP-dependent protein kinase 1 | 1.25 | 1.32 | Induction of relaxation [28] |
| Mchr1 | melanin-concentrating hormone receptor | 0.55 | 1.01 | Inhibition of cAMP accumulation [44] |
| Pmch | pro-melanin-concentrating hormone | 0.12 | 0.12 | Melanin-concentrating hormone activity [45] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabti, M.; Sasaki, K.; Gadhi, C.; Isoda, H. Elucidation of the Molecular Mechanism Underlying Lippia citriodora(Lim.)-Induced Relaxation and Anti-Depression. Int. J. Mol. Sci. 2019, 20, 3556. https://doi.org/10.3390/ijms20143556
Sabti M, Sasaki K, Gadhi C, Isoda H. Elucidation of the Molecular Mechanism Underlying Lippia citriodora(Lim.)-Induced Relaxation and Anti-Depression. International Journal of Molecular Sciences. 2019; 20(14):3556. https://doi.org/10.3390/ijms20143556
Chicago/Turabian StyleSabti, Mouad, Kazunori Sasaki, Chemseddoha Gadhi, and Hiroko Isoda. 2019. "Elucidation of the Molecular Mechanism Underlying Lippia citriodora(Lim.)-Induced Relaxation and Anti-Depression" International Journal of Molecular Sciences 20, no. 14: 3556. https://doi.org/10.3390/ijms20143556
APA StyleSabti, M., Sasaki, K., Gadhi, C., & Isoda, H. (2019). Elucidation of the Molecular Mechanism Underlying Lippia citriodora(Lim.)-Induced Relaxation and Anti-Depression. International Journal of Molecular Sciences, 20(14), 3556. https://doi.org/10.3390/ijms20143556