Variations in Surgical Procedures for Inducing Hind Limb Ischemia in Mice and the Impact of These Variations on Neovascularization Assessment
Abstract
:1. Introduction
2. Mouse Model for Hind Limb Ischemia
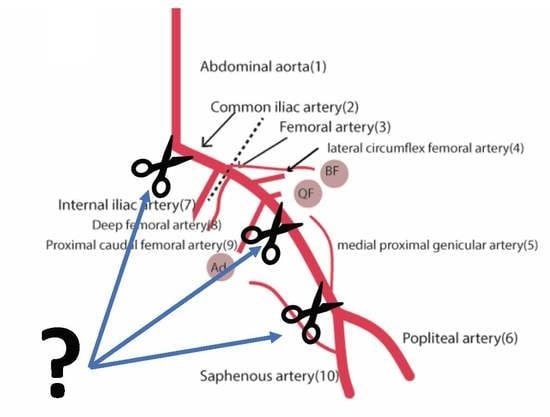
2.1. Vascular Anatomy of the Mouse
2.2. Technical Aspects of Inducing Mouse Limb Ischemia
2.3. Variants of Surgical Procedure
2.3.1. Single Electrocoagulation or Ligation of Femoral Artery
2.3.2. Single Electrocoagulation of Iliac Artery
2.3.3. Double Electrocoagulation of Both Femoral Artery and Iliac Artery
2.3.4. Total Excision of the Femoral Artery
2.3.5. Ameroid Constrictors
3. Analysis of Blood Flow Perfusion and Neovascularization/End-Points
3.1. Laser Doppler Perfusion Imaging
3.2. Immunohistochemical Analysis
3.3. Other Methods for Assessment of Collateral Formation and Limb Perfusion
3.4. Methods to Further Differentiate the Results of HLI and Neovascularisation
3.4.1. Matrigel Plug Assay
3.4.2. Pre-existing Collateral Density
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J. Vasc. Surg. 2007, 45, 5–67. [Google Scholar] [CrossRef] [PubMed]
- Van Oostrom, M.C.; van Oostrom, O.; Quax, P.H.; Verhaar, M.C.; Hoefer, I.E. Insights into mechanisms behind arteriogenesis: What does the future hold? J. Leukoc. Biol. 2008, 84, 1379–1391. [Google Scholar] [CrossRef] [PubMed]
- Heil, M.; Eitenmuller, I.; Schmitz-Rixen, T.; Schaper, W. Arteriogenesis versus angiogenesis: Similarities and differences. J. Cell. Mol. Med. 2006, 10, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Annex, B.H. Therapeutic angiogenesis for critical limb ischaemia. Nat. Rev. Cardiol. 2013, 10, 387–396. [Google Scholar] [CrossRef]
- Murohara, T.; Asahara, T.; Silver, M.; Bauters, C.; Masuda, H.; Kalka, C.; Kearney, M.; Chen, D.; Symes, J.F.; Fishman, M.C.; et al. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J. Clin. Investig. 1998, 101, 2567–2578. [Google Scholar] [CrossRef]
- Padgett, M.E.; McCord, T.J.; McClung, J.M.; Kontos, C.D. Methods for Acute and Subacute Murine Hindlimb Ischemia. J. Vis. Exp. JoVE 2016. [Google Scholar] [CrossRef]
- Nossent, A.Y.; Bastiaansen, A.J.; Peters, E.A.; de Vries, M.R.; Aref, Z.; Welten, S.M.; de Jager, S.C.; van der Pouw Kraan, T.C.; Quax, P.H. CCR7-CCL19/CCL21 Axis is Essential for Effective Arteriogenesis in a Murine Model of Hindlimb Ischemia. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, H.; Dai, X.; Sealock, R.; Faber, J.E. Genetic architecture underlying variation in extent and remodeling of the collateral circulation. Circ. Res. 2010, 107, 558–568. [Google Scholar] [CrossRef]
- Bastiaansen, A.J.; Karper, J.C.; Wezel, A.; de Boer, H.C.; Welten, S.M.; de Jong, R.C.; Peters, E.A.; de Vries, M.R.; van Oeveren-Rietdijk, A.M.; van Zonneveld, A.J.; et al. TLR4 accessory molecule RP105 (CD180) regulates monocyte-driven arteriogenesis in a murine hind limb ischemia model. PLoS ONE 2014, 9, e99882. [Google Scholar] [CrossRef]
- Hellingman, A.A.; Bastiaansen, A.J.; de Vries, M.R.; Seghers, L.; Lijkwan, M.A.; Lowik, C.W.; Hamming, J.F.; Quax, P.H. Variations in surgical procedures for hind limb ischaemia mouse models result in differences in collateral formation. Eur. J. Vasc. Endovasc. Surg 2010, 40, 796–803. [Google Scholar] [CrossRef]
- Van Weel, V.; Toes, R.E.; Seghers, L.; Deckers, M.M.; de Vries, M.R.; Eilers, P.H.; Sipkens, J.; Schepers, A.; Eefting, D.; van Hinsbergh, V.W.; et al. Natural killer cells and CD4+ T-cells modulate collateral artery development. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2310–2318. [Google Scholar] [CrossRef]
- Van Weel, V.; de Vries, M.; Voshol, P.J.; Verloop, R.E.; Eilers, P.H.; van Hinsbergh, V.W.; van Bockel, J.H.; Quax, P.H. Hypercholesterolemia reduces collateral artery growth more dominantly than hyperglycemia or insulin resistance in mice. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1383–1390. [Google Scholar] [CrossRef]
- Helisch, A.; Wagner, S.; Khan, N.; Drinane, M.; Wolfram, S.; Heil, M.; Ziegelhoeffer, T.; Brandt, U.; Pearlman, J.D.; Swartz, H.M.; et al. Impact of mouse strain differences in innate hindlimb collateral vasculature. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 520–526. [Google Scholar] [CrossRef]
- Chalothorn, D.; Faber, J.E. Strain-dependent variation in collateral circulatory function in mouse hindlimb. Physiol. Genom. 2010, 42, 469–479. [Google Scholar] [CrossRef]
- Bonheur, J.A.; Albadawi, H.; Patton, G.M.; Watkins, M.T. A noninvasive murine model of hind limb ischemia-reperfusion injury. J. Surg. Res. 2004, 116, 55–63. [Google Scholar] [CrossRef]
- Tran, T.P.; Tu, H.; Pipinos, I.I.; Muelleman, R.L.; Albadawi, H.; Li, Y.L. Tourniquet-induced acute ischemia-reperfusion injury in mouse skeletal muscles: Involvement of superoxide. Eur. J. Pharmacol. 2011, 650, 328–334. [Google Scholar] [CrossRef]
- Drysch, M.; Wallner, C.; Schmidt, S.V.; Reinkemeier, F.; Wagner, J.M.; Lehnhardt, M.; Behr, B. An optimized low-pressure tourniquet murine hind limb ischemia reperfusion model: Inducing acute ischemia reperfusion injury in C57BL/6 wild type mice. PLoS ONE 2019, 14, e0210961. [Google Scholar] [CrossRef]
- Hellingman, A.A.; Zwaginga, J.J.; van Beem, R.T.; Hamming, J.F.; Fibbe, W.E.; Quax, P.H.; Geutskens, S.B. T-cell-pre-stimulated monocytes promote neovascularisation in a murine hind limb ischaemia model. Eur. J. Vasc. Endovasc. Surg 2011, 41, 418–428. [Google Scholar] [CrossRef]
- Couffinhal, T.; Silver, M.; Zheng, L.P.; Kearney, M.; Witzenbichler, B.; Isner, J.M. Mouse model of angiogenesis. Am. J. Pathol. 1998, 152, 1667–1679. [Google Scholar]
- Kochi, T.; Imai, Y.; Takeda, A.; Watanabe, Y.; Mori, S.; Tachi, M.; Kodama, T. Characterization of the arterial anatomy of the murine hindlimb: Functional role in the design and understanding of ischemia models. PLoS ONE 2013, 8, e84047. [Google Scholar] [CrossRef]
- Limbourg, A.; Korff, T.; Napp, L.C.; Schaper, W.; Drexler, H.; Limbourg, F.P. Evaluation of postnatal arteriogenesis and angiogenesis in a mouse model of hind-limb ischemia. Nat. Protoc. 2009, 4, 1737–1746. [Google Scholar] [CrossRef]
- Niiyama, H.; Huang, N.F.; Rollins, M.D.; Cooke, J.P. Murine model of hindlimb ischemia. J. Vis. Exp: JoVE 2009. [Google Scholar] [CrossRef]
- Greco, A.; Ragucci, M.; Liuzzi, R.; Gargiulo, S.; Gramanzini, M.; Coda, A.R.; Albanese, S.; Mancini, M.; Salvatore, M.; Brunetti, A. Repeatability, reproducibility and standardisation of a laser Doppler imaging technique for the evaluation of normal mouse hindlimb perfusion. Sensors 2012, 13, 500–515. [Google Scholar] [CrossRef]
- Medgett, I.C.; Ruffolo, R.R., Jr. Alpha adrenoceptor-mediated vasoconstriction in rat hindlimb: Innervated alpha-2 adrenoceptors in the saphenous arterial bed. J. Pharmacol. Exp. Ther. 1988, 246, 249–254. [Google Scholar]
- Stabile, E.; Burnett, M.S.; Watkins, C.; Kinnaird, T.; Bachis, A.; la Sala, A.; Miller, J.M.; Shou, M.; Epstein, S.E.; Fuchs, S. Impaired arteriogenic response to acute hindlimb ischemia in CD4-knockout mice. Circulation 2003, 108, 205–210. [Google Scholar] [CrossRef]
- Westvik, T.S.; Fitzgerald, T.N.; Muto, A.; Maloney, S.P.; Pimiento, J.M.; Fancher, T.T.; Magri, D.; Westvik, H.H.; Nishibe, T.; Velazquez, O.C.; et al. Limb ischemia after iliac ligation in aged mice stimulates angiogenesis without arteriogenesis. J. Vasc. Surg. 2009, 49, 464–473. [Google Scholar] [CrossRef] [Green Version]
- Hellingman, A.A.; van der Vlugt, L.E.; Lijkwan, M.A.; Bastiaansen, A.J.; Sparwasser, T.; Smits, H.H.; Hamming, J.F.; Quax, P.H. A limited role for regulatory T cells in post-ischemic neovascularization. J. Cell Mol. Med. 2012, 16, 328–336. [Google Scholar] [CrossRef]
- Yang, Y.; Tang, G.; Yan, J.; Park, B.; Hoffman, A.; Tie, G.; Wang, R.; Messina, L.M. Cellular and molecular mechanism regulating blood flow recovery in acute versus gradual femoral artery occlusion are distinct in the mouse. J. Vasc. Surg. 2008, 48, 1546–1558. [Google Scholar] [CrossRef] [Green Version]
- Simons, K.H.; Aref, Z.; Peters, H.A.B.; Welten, S.P.; Nossent, A.Y.; Jukema, J.W.; Hamming, J.F.; Arens, R.; de Vries, M.R.; Quax, P.H.A. The role of CD27-CD70-mediated T cell co-stimulation in vasculogenesis, arteriogenesis and angiogenesis. Int. J. Cardiol. 2018, 260, 184–190. [Google Scholar] [CrossRef] [Green Version]
- Pusztaszeri, M.P.; Seelentag, W.; Bosman, F.T. Immunohistochemical expression of endothelial markers CD31, CD34, von Willebrand factor, and Fli-1 in normal human tissues. J. Histochem. Cytochem. J. Histochem. Soc. 2006, 54, 385–395. [Google Scholar] [CrossRef]
- Van Weel, V.; Deckers, M.M.; Grimbergen, J.M.; van Leuven, K.J.; Lardenoye, J.H.; Schlingemann, R.O.; van Nieuw Amerongen, G.P.; van Bockel, J.H.; van Hinsbergh, V.W.; Quax, P.H. Vascular endothelial growth factor overexpression in ischemic skeletal muscle enhances myoglobin expression in vivo. Circ. Res. 2004, 95, 58–66. [Google Scholar] [CrossRef]
- Nebuloni, L.; Kuhn, G.A.; Vogel, J.; Muller, R. A novel in vivo vascular imaging approach for hierarchical quantification of vasculature using contrast enhanced micro-computed tomography. PLoS ONE 2014, 9, e86562. [Google Scholar] [CrossRef]
- Simons, M. Chapter 14. Assessment of arteriogenesis. Methods Enzymol. 2008, 445, 331–342. [Google Scholar] [CrossRef]
- Simons, M.; Alitalo, K.; Annex, B.H.; Augustin, H.G.; Beam, C.; Berk, B.C.; Byzova, T.; Carmeliet, P.; Chilian, W.; Cooke, J.P.; et al. State-of-the-Art Methods for Evaluation of Angiogenesis and Tissue Vascularization: A Scientific Statement from the American Heart Association. Circ. Res. 2015, 116, e99–e132. [Google Scholar] [CrossRef]
- Liu, X.; Terry, T.; Pan, S.; Yang, Z.; Willerson, J.T.; Dixon, R.A.; Liu, Q. Osmotic drug delivery to ischemic hindlimbs and perfusion of vasculature with microfil for micro-computed tomography imaging. J. Vis. Exp. JoVE 2013. [Google Scholar] [CrossRef]
- Wehrli, F.W.; Shimakawa, A.; Gullberg, G.T.; MacFall, J.R. Time-of-flight MR flow imaging: selective saturation recovery with gradient refocusing. Radiology 1986, 160, 781–785. [Google Scholar] [CrossRef]
- Wagner, S.; Helisch, A.; Bachmann, G.; Schaper, W. Time-of-flight quantitative measurements of blood flow in mouse hindlimbs. J. Magn. Reson. Imaging JMRI 2004, 19, 468–474. [Google Scholar] [CrossRef]
- Wagner, S.; Helisch, A.; Ziegelhoeffer, T.; Bachmann, G.; Schaper, W. Magnetic resonance angiography of collateral vessels in a murine femoral artery ligation model. NMR Biomed. 2004, 17, 21–27. [Google Scholar] [CrossRef]
- Hendrikx, G.; Vries, M.H.; Bauwens, M.; De Saint-Hubert, M.; Wagenaar, A.; Guillaume, J.; Boonen, L.; Post, M.J.; Mottaghy, F.M. Comparison of LDPI to SPECT perfusion imaging using (99m)Tc-sestamibi and (99m)Tc-pyrophosphate in a murine ischemic hind limb model of neovascularization. EJNMMI Res. 2016, 6, 44. [Google Scholar] [CrossRef]
- Zhang, H.; Prabhakar, P.; Sealock, R.; Faber, J.E. Wide genetic variation in the native pial collateral circulation is a major determinant of variation in severity of stroke. J. Cereb. Blood Flow Metab. J. Int. Soc. Cereb. Blood Flow Metab. 2010, 30, 923–934. [Google Scholar] [CrossRef]
- Bastiaansen, A.J.; Ewing, M.M.; de Boer, H.C.; Tineke, C.P.K.; de Vries, M.R.; Peters, E.A.; Welten, S.M.; Arens, R.; Moore, S.M.; Faber, J.E.; et al. Lysine acetyltransferase PCAF is a key regulator of arteriogenesis. Arterioscler. Thromb. Vasc. Biol/ 2013, 33, 1902–1910. [Google Scholar] [CrossRef]
- De Groot, D.; Pasterkamp, G.; Hoefer, I.E. Cardiovascular risk factors and collateral artery formation. Eur. J. Clin. Investig. 2009, 39, 1036–1047. [Google Scholar] [CrossRef]
- Kolluru, G.K.; Bir, S.C.; Kevil, C.G. Endothelial dysfunction and diabetes: effects on angiogenesis, vascular remodeling, and wound healing. Int. J. Vasc. Med. 2012, 2012, 918267. [Google Scholar] [CrossRef]
- Van Weel, V.; Seghers, L.; de Vries, M.R.; Kuiper, E.J.; Schlingemann, R.O.; Bajema, I.M.; Lindeman, J.H.; Delis-van Diemen, P.M.; van Hinsbergh, V.W.; van Bockel, J.H.; et al. Expression of vascular endothelial growth factor, stromal cell-derived factor-1, and CXCR4 in human limb muscle with acute and chronic ischemia. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1426–1432. [Google Scholar] [CrossRef]
- Van Weel, V.; van Tongeren, R.B.; van Hinsbergh, V.W.; van Bockel, J.H.; Quax, P.H. Vascular growth in ischemic limbs: a review of mechanisms and possible therapeutic stimulation. Ann. Vasc. Surg. 2008, 22, 582–597. [Google Scholar] [CrossRef]
- Sealock, R.; Zhang, H.; Lucitti, J.L.; Moore, S.M.; Faber, J.E. Congenic fine-mapping identifies a major causal locus for variation in the native collateral circulation and ischemic injury in brain and lower extremity. Circ. Res. 2014, 114, 660–671. [Google Scholar] [CrossRef]
- Dokun, A.O.; Keum, S.; Hazarika, S.; Li, Y.; Lamonte, G.M.; Wheeler, F.; Marchuk, D.A.; Annex, B.H. A quantitative trait locus (LSq-1) on mouse chromosome 7 is linked to the absence of tissue loss after surgical hindlimb ischemia. Circulation 2008, 117, 1207–1215. [Google Scholar] [CrossRef]
- Schmidt, C.A.; Amorese, A.J.; Ryan, T.E.; Goldberg, E.J.; Tarpey, M.D.; Green, T.D.; Karnekar, R.R.; Yamaguchi, D.J.; Spangenburg, E.E.; McClung, J.M. Strain-Dependent Variation in Acute Ischemic Muscle Injury. Am. J. Pathol. 2018, 188, 1246–1262. [Google Scholar] [CrossRef] [Green Version]

| Surgical Method Used to Induce HLI | Time Course of Blood Reperfusion (Days) | Ischemia Rate | Suitable to Studying Arteriogenesis? | Which Collateral Group Can Be Studied? | Suitable to Studying Angiogenesis in Calf Muscle? |
|---|---|---|---|---|---|
| Electrocoagulation of common iliac artery | 7–14 days | moderate | Yes | Medial thigh muscles (includes adductor muscles) Quadriceps femoris Biceps femoris | Yes |
| Electrocoagulation of the femoral artery, distal from origin of deep branch | 7–14 days | mild | Yes | Adductor muscles Quadriceps femoris Biceps femoris | Yes |
| Electrocoagulation of the femoral artery, proximal from origin of deep branch | 7–14 days | mild | Yes | Quadriceps femoris Biceps femoris | |
| Total excision of the femoral artery | 28 days | severe | No | Yes | |
| Double electrocoagulation of iliac and femoral artery | 28 days | moderate | Yes | Medial thigh muscles (includes adductor muscles) Quadriceps femoris Biceps femoris | Yes |
| Ameriod constrictors | mild | Unclear | No |
| Techniques | Results Obtained | Advantages | Disadvantages |
|---|---|---|---|
| Micro-CT | - Anatomical visualization of vasculature circulation - Extent of vasculature formation | - Non-invasive - Reproducible - Repeatable - 3D reconstruction | - Challenging to discriminate arteries from veins - Ionizing radiation |
| Ex vivo Micro-CT of polymer casted vasculature | - Extent of vasculature formation - Quantification of changes in the microvasculature | - Only arteries - 3D reconstruction | - Invasive - Repeated measurement not possible |
| X-ray microangiography | - Gross anatomical view of the circulation | - Overview of the vascular anatomy | - Invasive - Technically challenging - Only 2D projection images |
| Post-mortem X-ray microangiography | - Gross anatomical view of the circulation | - Overview of the vascular anatomy | - Repeated measurement not possible |
| MRA | - Detecting arterial blood flow - Determine collateral formation - Follow collateral arterial formation | - Repeated measurements possible - 3D reconstructions | - Invasive (vascular access for injecting contrast) - Long scan times |
| MRI TOF | - Visualizing flow within vessels - Quantitative evaluation of arterial blood flow - Determine collateral formation | - Non-invasive - Repeated measurements possible - 3D reconstructions | - Long scan times |
| SPECT | -Analyzing perfusion recovery | -Non-invasive -Accurate | -Special facility is needed |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aref, Z.; de Vries, M.R.; Quax, P.H.A. Variations in Surgical Procedures for Inducing Hind Limb Ischemia in Mice and the Impact of These Variations on Neovascularization Assessment. Int. J. Mol. Sci. 2019, 20, 3704. https://doi.org/10.3390/ijms20153704
Aref Z, de Vries MR, Quax PHA. Variations in Surgical Procedures for Inducing Hind Limb Ischemia in Mice and the Impact of These Variations on Neovascularization Assessment. International Journal of Molecular Sciences. 2019; 20(15):3704. https://doi.org/10.3390/ijms20153704
Chicago/Turabian StyleAref, Zeen, Margreet R. de Vries, and Paul H.A. Quax. 2019. "Variations in Surgical Procedures for Inducing Hind Limb Ischemia in Mice and the Impact of These Variations on Neovascularization Assessment" International Journal of Molecular Sciences 20, no. 15: 3704. https://doi.org/10.3390/ijms20153704
APA StyleAref, Z., de Vries, M. R., & Quax, P. H. A. (2019). Variations in Surgical Procedures for Inducing Hind Limb Ischemia in Mice and the Impact of These Variations on Neovascularization Assessment. International Journal of Molecular Sciences, 20(15), 3704. https://doi.org/10.3390/ijms20153704