Biological Response to Macroporous Chitosan-Agarose Bone Scaffolds Comprising Mg- and Zn-Doped Nano-Hydroxyapatite
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Calcium Phosphate Ceramics (NanoHA)
2.2. Evaluation of Ions Concentration in Scaffolds Extracts
2.3. In Vitro Cell Culture Experiments
2.3.1. Cytotoxicity Assessment
2.3.2. Cell Adhesion and Spreading
2.3.3. Cell Proliferation
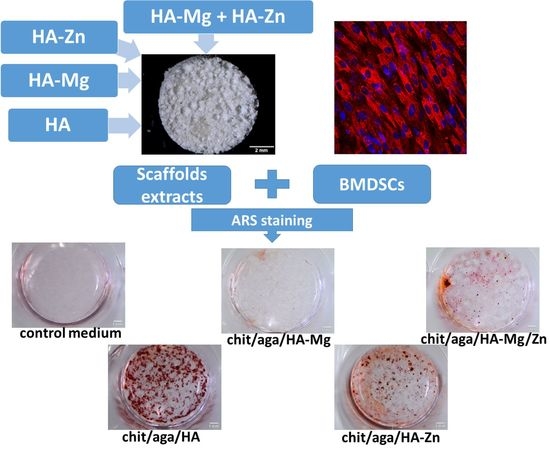
2.3.4. Evaluation of Osteogenic Differentiation
3. Materials and Methods
3.1. Synthesis and Characterization of NanoHA
3.2. Fabrication of the Scaffolds
3.3. Preparation of Scaffolds Extracts
3.4. In Vitro Cell Culture Experiments
3.4.1. Cytotoxicity Assessment
3.4.2. Cell Adhesion, Spreading, and Proliferation Assessment
3.4.3. Evaluation of Osteogenic Differentiation
3.5. Statistical Analysis
4. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADSCs | Adipose tissue-derived mesenchymal stem cells |
| bALP | Bone alkaline phosphatase |
| BMDSCs | Human bone marrow-derived stem cells |
| BSA | Bovine serum albumin |
| CLSM | Confocal laser scanning microscope |
| Col I | Type I collagen |
| DT | Doubling time |
| ECM | Extracellular matrix |
| ELISA | Enzyme-linked immunosorbent assay |
| FBS | Fetal bovine serum |
| FT-IR | Fourier-transform infrared spectroscopy |
| HA | Hydroxyapatite |
| ICP-OES | Inductively coupled plasma spectrometry |
| IF | Immunofluorescence |
| IGF-1 | Insulin-like growth factor 1 |
| MC3T3-E1 | Mouse calvarial preosteoblast cell line |
| MSCs | Mesenchymal stem cells |
| OC | Osteocalcin |
| PS | Polystyrene |
| PXRD | Powder X-ray diffraction |
| Runx-2 | Runt-related transcription factor 2 |
| TEM | Transmission electron microscope |
| VEGF | Vascular endothelial growth factor |
References
- Velasco, M.A.; Narváez-Tovar, C.A.; Garzón-Alvarado, D.A. Design, materials, and mechanobiology of biodegradable scaffolds for bone tissue engineering. BioMed Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Przekora, A. Current trends in fabrication of biomaterials for bone and cartilage regeneration: Materials modifications and biophysical stimulations. Int. J. Mol. Sci. 2019, 20, 435. [Google Scholar] [CrossRef] [PubMed]
- Evis, Z.; Webster, T.J. Nanosize hydroxyapatite: Doping with various ions. Adv. Appl. Ceram. 2011, 110, 311–321. [Google Scholar] [CrossRef]
- O’Neill, E.; Awale, G.; Daneshmandi, L.; Umerah, O.; Lo, K.W.H. The roles of ions on bone regeneration. Drug Discov. Today 2018, 23, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.; Naito, Y.; Karlsson, J.; He, W.; Miyamoto, I.; Xue, Y.; Andersson, M.; Mustafa, K.; Wennerberg, A.; Jimbo, R.; et al. Local release of magnesium from mesoporous TiO2 coatings stimulates the peri-implant expression of osteogenic markers and improves osteoconductivity in vivo. Acta Biomater. 2014, 10, 5193–5201. [Google Scholar] [CrossRef]
- Yoshizawa, S.; Brown, A.; Barchowsky, A.; Sfeir, C. Magnesium ion stimulation of bone marrow stromal cells enhances osteogenic activity, simulating the effect of magnesium alloy degradation. Acta Biomater. 2014, 10, 2834–2842. [Google Scholar] [CrossRef] [PubMed]
- Zreiqat, H.; Howlett, C.R.; Zannettino, A.; Evans, P.; Schulze-Tanzil, G.; Knabe, C.; Shakibaei, M. Mechanisms of magnesium-stimulated adhesion of osteoblastic cells to commonly used orthopaedic implants. J. Biomed. Mater. Res. 2002, 62, 175–184. [Google Scholar] [CrossRef]
- Kwun, I.; Cho, Y.; Lomeda, R.R.; Shin, H.; Choi, J.; Kang, Y.; Beattie, J.H. Zinc deficiency suppresses matrix mineralization and retards osteogenesis transiently with catch-up possibly through Runx 2 modulation. Bone 2010, 46, 732–741. [Google Scholar] [CrossRef]
- Seo, H.J.; Cho, Y.E.; Kim, T.; Shin, H.I.; Kwun, I.-S. Zinc may increase bone formation through stimulating cell proliferation, alkaline phosphatase activity and collagen synthesis in osteoblastic MC3T3-E1 cells. Nutr. Res. Pract. 2010, 4, 356–361. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Weitzmann, M.N. Zinc stimulates osteoblastogenesis and suppresses osteoclastogenesis by antagonizing NF-ĸB activation. Mol. Cell. Chem. 2011, 355, 179–186. [Google Scholar] [CrossRef]
- Thein-Han, W.W.; Misra, R.D.K. Biomimetic chitosan–nanohydroxyapatite composite scaffolds for bone tissue engineering. Acta Biomater. 2008, 5, 1182–1197. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Liu, R.; Huang, Q. Preparation and characterization of nano-hydroxyapatite/polymer composite scaffolds. J. Mater. Sci. Mater. Med. 2008, 19, 3429–3435. [Google Scholar] [CrossRef] [PubMed]
- Iwai, S.; Shimizu, H.; Suzawa, Y.; Akashi, M.; Yura, Y. Hydroxyapatite agarose composite gels as a biochemical material for the repair of alveolar bone defects due to cleft lip and palate. J. Oral Maxillofac. Surg. Med. Pathol. 2015, 27, 637–644. [Google Scholar] [CrossRef]
- Iwai, S.; Shimizu, H.; Takeshita, A.; Akashi, M.; Yura, Y. Translational research of HAp/agarose composite gel (HAp gel) used as a bone regenerative biomechanical material in jawbone defects. Int. J. Oral Maxillofac. Surg. 2013, 42, 1180–1181. [Google Scholar] [CrossRef]
- Hu, J.; Zhu, Y.; Tong, H.; Shen, X.; Chen, L.; Ran, J. A detailed study of homogeneous agarose/hydroxyapatite nanocomposites for load-bearing bone tissue. Int. J. Biol. Macromol. 2016, 82, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Merlin Rajesh Lal, L.P.; Suraishkumar, G.K.; Nair, P.D. Chitosan-agarose scaffolds supports chondrogenesis of Human Wharton’s Jelly mesenchymal stem cells. J. Biomed. Mater. Res. Part A 2017, 105, 1845–1855. [Google Scholar] [CrossRef] [PubMed]
- Bazin, D.; Chappard, C.; Combes, C.; Carpentier, X.; Rouzière, S.; André, G.; Matzen, G.; Allix, M.; Thiaudière, D.; Reguer, S.; et al. Diffraction techniques and vibrational spectroscopy opportunities to characterise bones. Osteoporos. Int. 2009, 20, 1065–1075. [Google Scholar] [CrossRef] [Green Version]
- Haider, A.; Haider, S.; Han, S.S.; Kang, I.K. Recent advances in the synthesis, functionalization and biomedical applications of hydroxyapatite: A review. RSC Adv. 2017, 7, 7442–7458. [Google Scholar] [CrossRef]
- Shepherd, J.H.; Shepherd, D.V.; Best, S.M. Substituted hydroxyapatites for bone repair. J. Mater. Sci. Mater. Med. 2012, 23, 2335–2347. [Google Scholar] [CrossRef]
- Markovic, M.; Fowler, B.; Tung, M. Preparation and comprehensive characterization of a calcium hydroxyapatite reference material. J. Res. Natl. Inst. Stand. Technol. 2004, 109, 553–568. [Google Scholar] [CrossRef]
- Hu, W.; Ma, J.; Wang, J.; Zhang, S. Fine structure study on low concentration zinc substituted hydroxyapatite nanoparticles. Mater. Sci. Eng. C 2012, 32, 2404–2410. [Google Scholar] [CrossRef]
- Ren, F.; Xin, R.; Ge, X.; Leng, Y. Characterization and structural analysis of zinc-substituted hydroxyapatites. Acta Biomater. 2009, 5, 3141–3149. [Google Scholar] [CrossRef] [PubMed]
- He, L.Y.; Zhang, X.M.; Liu, B.; Tian, Y.; Ma, W.H. Effect of magnesium ion on human osteoblast activity. Braz. J. Med. Biol. Res. 2016, 49, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Yusa, K.; Yamamoto, O.; Fukuda, M.; Koyota, S.; Koizumi, Y.; Sugiyama, T. In vitro prominent bone regeneration by release zinc ion from Zn-modified implant. Biochem. Biophys. Res. Commun. 2011, 412, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, S.; Brown, A.; Barchowsky, A.; Sfeir, C. Role of magnesium ions on osteogenic response in bone marrow stromal cells. Connect. Tissue Res. 2014, 55, 155–159. [Google Scholar] [CrossRef] [PubMed]
- An, S.; Gong, Q.; Huang, Y. Promotive effect of zinc ions on the vitality, migration, and osteogenic differentiation of human dental pulp cells. Biol. Trace Elem. Res. 2017, 175, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Thian, E.S.; Konishi, T.; Kawanobe, Y.; Lim, P.N.; Choong, C.; Ho, B.; Aizawa, M. Zinc-substituted hydroxyapatite: A biomaterial with enhanced bioactivity and antibacterial properties. J. Mater. Sci. Mater. Med. 2013, 24, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.Y.; Kim, H.J.; Choi, B.Y.; Sohn, M.; Chung, T.N.; Suh, S.W. Zinc promotes adipose-derived mesenchymal stem cell proliferation and differentiation towards a neuronal fate. Stem Cells Int. 2018, 2018, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Zainol, I.; Idrus, R.H. Incorporation of zinc oxide nanoparticles into chitosan-collagen 3D porous scaffolds: Effect on morphology, mechanical properties and cytocompatibility of 3D porous scaffolds. Int. J. Biol. Macromol. 2017, 104, 1020–1029. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Li, X.; Tan, Y.; Fan, H.; Zhang, X. The material and biological characteristics of osteoinductive calcium phosphate ceramics. Regen. Biomater. 2018, 5, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Przekora, A. The summary of the most important cell-biomaterial interactions that need to be considered during in vitro biocompatibility testing of bone scaffolds for tissue engineering applications. Mater. Sci. Eng. C 2019, 97, 1036–1051. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Cao, H.; Qiao, Y.; Meng, F.; Zhu, H.; Liu, X. Osteogenic activity and antibacterial effect of zinc ion implanted titanium. Colloids Surf. B Biointerfaces 2014, 117, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.H.; Wang, P.; Wang, L.; Bao, C.; Chen, Q.; Weir, M.D.; Chow, L.C.; Zhao, L.; Zhou, X.; Reynolds, M.A.; et al. Calcium phosphate cements for bone engineering and their biological properties. Bone Res. 2017, 5, 17056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, M.; Chi, G.; Xu, J.; Tan, Y.; Xu, J.; Lv, S.; Xu, Z.; Xia, Y.; Li, L.; Li, Y.; et al. Extracellular matrix stiffness controls osteogenic differentiation of mesenchymal stem cells mediated by integrin α5. Stem Cell Res. Ther. 2018, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xing, Y.; Li, J.; Zhang, Z.; Luan, H.; Chu, Z.; Gong, H.; Fan, Y. Osteogenesis-related behavior of MC3T3-E1 cells on substrates with tunable stiffness. Biomed Res. Int. 2018, 2018, 4025083. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef]
- Julien, M.; Khoshniat, S.; Lacreusette, A.; Gatius, M.; Bozec, A.; Wagner, E.F.; Wittrant, Y.; Masson, M.; Weiss, P.; Beck, L.; et al. Phosphate-dependent regulation of MGP in osteoblasts: Role of ERK1/2 and Fra-1. J. Bone Miner. Res. 2009, 24, 1856–1868. [Google Scholar] [CrossRef]
- Kim, H.; Han, H.; Lee, K.; Lee, D.; Lee, J.W.; Jeon, H.; Cho, S.; Roh, H.; Kim, Y.; Seok, H.; et al. Comprehensive study on the roles of released ions from biodegradable Mg—5 wt% Ca—1 wt% Zn alloy in bone regeneration. J. Tissue Eng. Regen. Med. 2017, 11, 2710–2724. [Google Scholar] [CrossRef]
- Kolmas, J.; Pajor, K.; Pajchel, L.; Przekora, A.; Ginalska, G.; Oledzka, E.; Sobczak, M. Fabrication and physicochemical characterization of porous composite microgranules with selenium oxyanions and risedronate sodium for potential applications in bone tumors. Int. J. Nanomed 2017, 12, 5633. [Google Scholar] [CrossRef]
- Przekora, A.; Ginalska, G. Enhanced differentiation of osteoblastic cells on novel chitosan/B-1,3-glucan/bioceramic scaffolds for bone tissue regeneration. Biomed. Mater. 2015, 10, 015009. [Google Scholar] [CrossRef]









| Nanopowder | HA | HA-Mg | HA-Zn | |
|---|---|---|---|---|
| Unit cell parameters (nm) * | a | 0.943 | 0.9425 | 0.9420 |
| c | 0.6872 | 0.6853 | 0.6818 | |
| Crystal size (nm) | 65 ± 5 | 58 ± 4 | 64 ± 6 |
| Conc. (µg/mL) | Control Culture Medium | Chit/Aga/HA | Chit/Aga/HA-Mg | Chit/Aga/HA-Zn | Chit/Aga/HA-Mg/Zn |
|---|---|---|---|---|---|
| Mg2+ | 29.12 ± 3.14 | 16.90 ± 0.83 *#$ | 41.04 ± 1.09 * | 21.36 ± 1.50 *#$ | 36.16 ± 2.67 * |
| Zn2+ | 0.16 ± 0.06 | 0.11 ± 0.02 $& | 0.12 ± 0.03 | 4.42 ± 0.25 *#$ | 0.64 ± 0.13 *# |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazimierczak, P.; Kolmas, J.; Przekora, A. Biological Response to Macroporous Chitosan-Agarose Bone Scaffolds Comprising Mg- and Zn-Doped Nano-Hydroxyapatite. Int. J. Mol. Sci. 2019, 20, 3835. https://doi.org/10.3390/ijms20153835
Kazimierczak P, Kolmas J, Przekora A. Biological Response to Macroporous Chitosan-Agarose Bone Scaffolds Comprising Mg- and Zn-Doped Nano-Hydroxyapatite. International Journal of Molecular Sciences. 2019; 20(15):3835. https://doi.org/10.3390/ijms20153835
Chicago/Turabian StyleKazimierczak, Paulina, Joanna Kolmas, and Agata Przekora. 2019. "Biological Response to Macroporous Chitosan-Agarose Bone Scaffolds Comprising Mg- and Zn-Doped Nano-Hydroxyapatite" International Journal of Molecular Sciences 20, no. 15: 3835. https://doi.org/10.3390/ijms20153835
APA StyleKazimierczak, P., Kolmas, J., & Przekora, A. (2019). Biological Response to Macroporous Chitosan-Agarose Bone Scaffolds Comprising Mg- and Zn-Doped Nano-Hydroxyapatite. International Journal of Molecular Sciences, 20(15), 3835. https://doi.org/10.3390/ijms20153835