In Vivo Response of Growth Plate to Biodegradable Mg-Ca-Zn Alloys Depending on the Surface Modification
Abstract
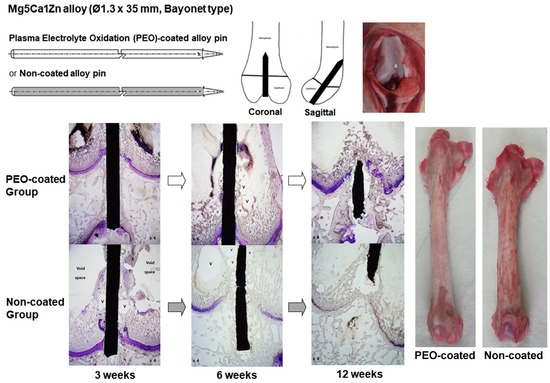
:1. Introduction
2. Results
2.1. Degradation Performance
2.2. Overall Tissue Reactions
2.3. Influence of the Mg-Ca-Zn Alloy Pin on the Growth Plate
2.4. Femoral Segment Length
2.5. The Relationship between the Measurement Parameters
3. Discussion
4. Materials and Methods
4.1. Alloys
4.2. Experimental Animals and Overall Management
4.3. Micro-CT Analyses
4.4. Histologic Analyses
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| RV | residual volume |
| PEO | plasma electrolyte oxidation |
| Ca | calcium |
| Zn | zinc |
| CT | computed tomography |
References
- Krause, A.; von der Hoh, N.; Bormann, D.; Krause, C.; Bach, F.W.; Windhagen, H.; Meyer-Lindenberg, A. Degradation behaviour and mechanical properties of magnesium implants in rabbit tibiae. J. Mater. Sci. 2010, 45, 624–632. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.J.; Windhagen, H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef] [PubMed]
- Claes, L.E. Mechanical characterization of biodegradable implants. Clin. Mater. 1992, 10, 41–46. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Y.; Geng, L. Research on Mg-Zn-Ca alloy as degradable biomaterial. In Biomaterials-Physics and Chemistry; Pignatello, R., Ed.; InTech: Rijeka, Croatia, 2011; pp. 183–204. [Google Scholar]
- Yu, K.; Chen, L.; Zhao, J.; Li, S.; Dai, Y.; Huang, Q.; Yu, Z. In vitro corrosion behavior and in vivo biodegradation of biomedical β-Ca3 (PO4) 2/Mg–Zn composites. Acta Biomater. 2012, 8, 2845–2855. [Google Scholar] [CrossRef] [PubMed]
- Kannan, M.B.; Raman, R.K. In vitro degradation and mechanical integrity of calcium-containing magnesium alloys in modified-simulated body fluid. Biomaterials 2008, 29, 2306–2314. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Nie, X.; Ma, Y. Corrosion and surface treatment of magnesium alloys. In Magnesium Alloys-Properties in Solid and Liquid States; Czerwinski, F., Ed.; InTech: Rijeka, Croatia, 2014; pp. 67–108. [Google Scholar]
- Jung, J.Y.; Kwon, S.J.; Han, H.S.; Lee, J.Y.; Ahn, J.P.; Yang, S.J.; Cho, S.Y.; Cha, P.R.; Kim, Y.C.; Seok, H.K. In vivo corrosion mechanism by elemental interdiffusion of biodegradable Mg-Ca alloy. J. BioMed Mater. Res. B Appl. Biomater. 2012, 100, 2251–2260. [Google Scholar] [CrossRef]
- Pichler, K.; Kraus, T.; Martinelli, E.; Sadoghi, P.; Musumeci, G.; Uggowitzer, P.J.; Weinberg, A.M. Cellular reactions to biodegradable magnesium alloys on human growth plate chondrocytes and osteoblasts. Int. Orthop. 2014, 38, 881–889. [Google Scholar] [CrossRef]
- Cho, S.Y.; Chae, S.W.; Choi, K.W.; Seok, H.K.; Han, H.S.; Yang, S.J.; Kim, Y.Y.; Kim, J.T.; Jung, J.Y.; Assad, M. Load-bearing capacity and biological allowable limit of biodegradable metal based on degradation rate in vivo. J. BioMed Mater. Res. B Appl. Biomater. 2012, 100, 1535–1544. [Google Scholar] [CrossRef]
- Persaud-Sharma, D.; McGoron, A. In Biodegradable Magnesium Alloys: A Review of Material Development and Applications. J. Biomim. Biomater. Tissue Eng. 2011, 12, 25–39. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, B.; Wang, Y.; Geng, L.; Jiao, X. Preparation and characterization of a new biomedical Mg–Zn–Ca alloy. Mater. Design. 2012, 34, 58–64. [Google Scholar] [CrossRef]
- Hänzi, A.C.; Gerber, I.; Schinhammer, M.; Löffler, J.F.; Uggowitzer, P.J. On the in vitro and in vivo degradation performance and biological response of new biodegradable Mg–Y–Zn alloys. Acta Biomater. 2010, 6, 1824–1833. [Google Scholar] [CrossRef]
- Kraus, T.; Fischerauer, S.; Treichler, S.; Martinelli, E.; Eichler, J.; Myrissa, A.; Zotsch, S.; Uggowitzer, P.J.; Loffler, J.F.; Weinberg, A.M. The influence of biodegradable magnesium implants on the growth plate. Acta Biomater. 2018, 66, 109–117. [Google Scholar] [CrossRef]
- Banks, W.A.; Kastin, A.J. Aluminum-Induced Neurotoxicity—Alterations in Membrane-Function at the Blood-Brain-Barrier. Neurosci. Biobehav. R 1989, 13, 47–53. [Google Scholar] [CrossRef]
- Darbre, P.D. Metalloestrogens: An emerging class of inorganic xenoestrogens with potential to add to the oestrogenic burden of the human breast. J. Appl. Toxicol. 2006, 26, 191–197. [Google Scholar] [CrossRef]
- Cho, S.Y.; Chae, S.W.; Choi, K.W.; Seok, H.K.; Kim, Y.C.; Jung, J.Y.; Yang, S.J.; Kwon, G.J.; Kim, J.T.; Assad, M. Biocompatibility and strength retention of biodegradable Mg-Ca-Zn alloy bone implants. J. BioMed Mater. Res. B Appl. Biomater. 2013, 101, 201–212. [Google Scholar] [CrossRef]
- Glasson, S.S.; Chambers, M.G.; Van Den Berg, W.B.; Little, C.B. The OARSI histopathology initiative—recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthr. Cartil. 2010, 18 (Suppl. 3), S17–S23. [Google Scholar] [CrossRef]
- Darband, G.B.; Aliofkhazraei, M.; Hamghalam, P.; Valizade, N. Plasma electrolytic oxidation of magnesium and its alloys: Mechanism, properties and applications. J. Magnes. Alloys 2017, 5, 74–132. [Google Scholar] [CrossRef]
- Cai, J.S.; Cao, F.H.; Chang, L.R.; Zheng, J.J.; Zhang, J.Q.; Cao, C.A. The preparation and corrosion behaviors of MAO coating on AZ91D with rare earth conversion precursor film. Appl. Surf. Sci. 2011, 257, 3804–3811. [Google Scholar] [CrossRef]
- Pan, Y.; Che, C.; Wang, D.; Lin, Z. Preparation and bioactivity of micro-arc oxidized calcium phosphate coatings. Mater. Chem. Phys. 2013, 141, 842–849. [Google Scholar] [CrossRef]
- Vu, T.N.; Veys-Renaux, D.; Rocca, E. Potential bioactivity of coatings formed on AZ91D magnesium alloy by plasma electrolytic anodizing. J. BioMed Mater. Res. B Appl. Biomater. 2012, 100, 1846–1853. [Google Scholar] [CrossRef]
- Pan, Y.; Che, C.; Wang, D.; Zhao, T. Effects of phosphates on microstructure and bioactivity of micro-arc oxidized calcium phosphate coatings on Mg–Zn–Zr magnesium alloy. Coll. Sulf. B Biointerfaces 2013, 9, 1–9. [Google Scholar] [CrossRef]
- Surmeneva, M.A.; Tyurin, A.I.; Mukhametkaliyev, T.M.; Pirozhkova, T.S.; Shuvarin, I.A.; Syrtanov, M.S.; Surmenev, R.A. Enhancement of the mechanical properties of AZ31 magnesium alloy via nanostructured hydroxyapatite thin films fabricatedviaradio-frequency magnetron sputtering. J. Mech. Behav. BioMed Mater. 2015, 46, 127–136. [Google Scholar] [CrossRef]
- Hasler, C.C.; Foster, B.K. Secondary tethers after physeal bar resection: A common source of failure? Clin. Orthop. Relat. 2002, 405, 242–249. [Google Scholar] [CrossRef]
- Cheon, J.E.; Kim, I.O.; Choi, I.H.; Kim, C.J.; Cho, T.J.; Kim, W.S.; Yoo, W.J.; Yeon, K.M. Magnetic resonance imaging of remaining physis in partial physeal resection with graft interposition in a rabbit model: A comparison with physeal resection alone. Investig. Radiol. 2005, 40, 235–242. [Google Scholar] [CrossRef]
- Mäkelä, E.A.; Vainionpää, S.; Vihtonen, K.; Mero, M.; Rokkanen, P. The effect of trauma to the lower femoral epiphyseal plate. An experimental study in rabbits. J. Bone Jt. Surg. Br. 1988, 70, 187–191. [Google Scholar] [CrossRef]
- Janarv, P.M.; Wikström, B.; Hirsch, G. The influence of transphyseal drilling and tendon grafting on bone growth: An experimental study in the rabbit. J. Pediatr. Orthop. 1998, 18, 149–154. [Google Scholar] [CrossRef]
- Masoud, I.; Shapiro, F.; Kent, R.; Moses, A. A longitudinal study of the growth of the New Zealand white rabbit: Cumulative and biweekly incremental growth rates for body length, body weight, femoral length, and tibial length. J. Orthop. Res. 1986, 4, 221–231. [Google Scholar] [CrossRef]









| Follow-Up Period (Weeks) | 6 | 12 | ||||
|---|---|---|---|---|---|---|
| PEO-Coated Group | Non-Coated Group | p Value ** | PEO-Coated Group | Non-Coated Group | p Value ** | |
| No. of animals | 6 | 6 | 6 | 6 | ||
| Residual volume (RV) (mm3) | 42.5 (40.1 to 44.5) * | 33.6 (30.9 to 37.6) * | 0.010 | 36.1 (30.3 to 40.6) * | 28.4 (23.5 to 33.2) * | 0.026 |
| Void volume (VV) (mm3) | 2.4 (1.1 to 4.3) * | 19.4 (13.9 to 34.2) * | 0.010 | 19.4 (8.6 to 33.7) * | 42.1 (15.5 to 67.3) * | 0.132 |
| Degradation percentage (%) | 5.3 (0.8 to 10.6) * | 25.2 (31.2 to 16.2) * | 0.010 | 19.6 (0.6 to 32.5) * | 36.7 (26.1 to 47.5) * | 0.026 |
| Degradation rate † (mm/year) | 0.145 (0.021 to 0.292) * | 0.690 (0.445 to 0.856) * | 0.010 | 0.269 (0.131 to 0.446) * | 0.503 (0.357 to 0.651) * | 0.026 |
| Follow-Up Period (weeks) | Group | Rabbits | Physeal Height (µm) | Chondrocyte | Cell Cloning † | Physeal Discontinuity * | Femoral Segment Length | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Orientation | Density † | Percentage (%) | Width (µm) | Interposed Substance | Length (mm) (Ipsilat./Contralat.) | Ratio Compared to Control | |||||
| 3 | PEO-coated | 1 | 302.8 | orderly | 1 | 2 | - | - | - | 78/78 | 1 |
| 2 | 499.9 | orderly | 1 | 0 | - | - | - | 79/79 | 1 | ||
| 3 | 751.2 | orderly | 3 | 4 | - | - | - | 79/79 | 1 | ||
| 4 | 497.4 | orderly | 1 | 0 | - | - | - | 76/76 | 1 | ||
| 5 | 504.3 | orderly | 0 | 1 | - | - | - | 80/80 | 1 | ||
| 6 | 620.8 | orderly | 2 | 1 | - | - | - | 78/78 | 1 | ||
| Non-coated | 1 | 549.4 | orderly | 1 | 4 | - | - | - | 78/78 | 1 | |
| 2 | 631.6 | orderly | 0 | 4 | - | - | - | 79/79 | 1 | ||
| 3 | 666.1 | orderly | 1 | 0 | - | - | - | 74/74 | 1 | ||
| 4 | 630.4 | orderly | 1 | 1 | - | - | - | 76/76 | 1 | ||
| 5 | 303.3 | orderly | 1 | 4 | 1.3 | 492 | Void area (Hydrogen gas) | 77/78 | 0.99 | ||
| 6 | 270.7 | orderly | 0 | 2 | - | - | - | 79/79 | 1 | ||
| Control | (average) | 466.4 | orderly | 0 to 1 | 0 to 1 | 0.8 | 325.3 | Bone bridge | - | - | |
| 6 | PEO-coated | 1 | 403.3 | orderly | 1 | 1 | 0.9 | 511 | Bone bridge | 96/96 | 1 |
| 2 | 0 | disorderly | 2 | 0 | 2.0 | 733 | Bone bridge | 92/92 | 1 | ||
| 3 | 428.7 | orderly | 1 | 0 | 0.8 | 250 | Bone bridge | 90/90 | 1 | ||
| 4 | 706.1 | orderly | 1 | 0 | - | - | - | 99/99 | 1 | ||
| 5 | 756.6 | orderly | 1 | 0 | - | - | - | 97/97 | 1 | ||
| 6 | 441.5 | orderly | 3 | 0 | - | - | - | 79/80 | 0.99 | ||
| Non-coated | 1 | 0 | disorderly | 4 | 4 | 15.8 | 3168 | Bone bridge | 88/92 | 0.96 | |
| 2 | 0 | disorderly | 4 | 4 | 16.5 | 3656 | Bone bridge | 87/91 | 0.96 | ||
| 3 | 0 | N/C | 4 | 4 | Premature physeal arrest | 88/93 | 0.95 | ||||
| 4 | 0 | disorderly | 1 | 1 | 2.3 | 920 | Bone bridge | 89/89 | 1 | ||
| 5 | 391.1 | orderly | 1 | 4 | - | - | - | 87/87 | 1 | ||
| 6 | 333.6 | disorderly | 2 | 4 | - | - | - | 91/91 | 1 | ||
| Control | (average) | 309.7 | orderly | 0 to 1 | 0 to 1 | 0.9 | 351.7 | Bone bridge | - | - | |
| 12 | PEO-coated | 1 | 113.5 | orderly | 0 | 4 | - | - | - | 92/92 | 1 |
| 2 | 0 | disorderly | 2 | 4 | 0.2 | 103 | Bone bridge | 95/96 | 0.99 | ||
| 3 | 0 | disorderly | 2 | 4 | 0.3 | 267 | Bone bridge | 91/93 | 0.98 | ||
| 4 | 0 | disorderly | 2 | 4 | 0.5 | 444 | Bone bridge | 94/96 | 0.98 | ||
| 5 | 0 | orderly | 2 | 2 | 0.5 | 487 | Bone bridge | 96/98 | 0.98 | ||
| 6 | 136.8 | orderly | 0 | 4 | - | - | - | 93/93 | 1 | ||
| Non-coated | 1 | 0 | N/C | 4 | 4 | Premature physeal arrest | 85/96 | 0.89 | |||
| 2 | 0 | N/C | 4 | 4 | Premature physeal arrest | 89/97 | 0.92 | ||||
| 3 | 0 | N/C | 4 | 4 | Premature physeal arrest | 85/92 | 0.92 | ||||
| 4 | 0 | disorderly | 3 | 1 | 1.8 | 467 | Bone bridge | 9.1/9.1 | 1 | ||
| 5 | 0 | disorderly | 4 | 4 | 15.9 | 3487 | Bone bridge | 9.3/9.9 | 0.94 | ||
| 6 | 0 | N/C | 4 | 4 | Premature physeal arrest | 8.8/9.5 | 0.93 | ||||
| Control | (average) | 154.1 | orderly | 0 to 1 | 0 to 1 | 0.9 | 330.5 | Bone bridge | - | - | |
| Materials | Degradation Rate (mm/yr) * |
|---|---|
| AZ31 † | 0.650 |
| AZ91D † | 0.770 |
| WE43 † | 0.868 |
| LAE442 † | 0.218 |
| Mg-1Ca † | 1.269 |
| Mg-Ca-Zn (non-coated) † | 0.348 to 0.690 |
| Mg-Ca-Zn (PEO-coated, current study) | 0.145 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, M.H.; Yoo, W.J.; Cho, T.-J.; Park, Y.K.; Lee, W.-J.; Choi, I.H. In Vivo Response of Growth Plate to Biodegradable Mg-Ca-Zn Alloys Depending on the Surface Modification. Int. J. Mol. Sci. 2019, 20, 3761. https://doi.org/10.3390/ijms20153761
Song MH, Yoo WJ, Cho T-J, Park YK, Lee W-J, Choi IH. In Vivo Response of Growth Plate to Biodegradable Mg-Ca-Zn Alloys Depending on the Surface Modification. International Journal of Molecular Sciences. 2019; 20(15):3761. https://doi.org/10.3390/ijms20153761
Chicago/Turabian StyleSong, Mi Hyun, Won Joon Yoo, Tae-Joon Cho, Yong Koo Park, Wang-Jae Lee, and In Ho Choi. 2019. "In Vivo Response of Growth Plate to Biodegradable Mg-Ca-Zn Alloys Depending on the Surface Modification" International Journal of Molecular Sciences 20, no. 15: 3761. https://doi.org/10.3390/ijms20153761
APA StyleSong, M. H., Yoo, W. J., Cho, T. -J., Park, Y. K., Lee, W. -J., & Choi, I. H. (2019). In Vivo Response of Growth Plate to Biodegradable Mg-Ca-Zn Alloys Depending on the Surface Modification. International Journal of Molecular Sciences, 20(15), 3761. https://doi.org/10.3390/ijms20153761