Setting Fire to ESA and EMA Resistance: New Targeted Treatment Options in Lower Risk Myelodysplastic Syndromes
Abstract
:1. Introduction
2. Current Standards to Treat Anemia in Low-Risk MDS
2.1. Erythropoiesis-Stimulating Agents (ESAs)
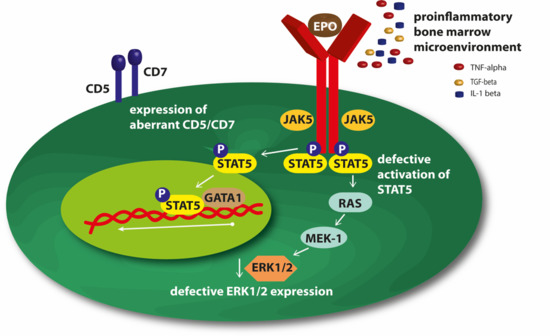
Mechanisms of Resistance to ESAs
2.2. Erythroid Maturation Agents (EMAs)
2.3. Immune Modulatory Drugs (ImiDs)
Lenalidomide
3. Novel Targeted Strategies to Treat Anemia in Low-Risk MDS
3.1. Roxadustat
3.2. Imetelstat
3.3. Immunosuppressive Therapy (IST)
4. Conclusions
Conflicts of Interest
References
- Platzbecker, U. Treatment of MDS. Blood 2019, 133, 1096–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steensma, D.P. Myelodysplastic syndromes current treatment algorithm 2018. Blood Cancer J. 2018, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Hellstrom-Lindberg, E. Efficacy of erythropoietin in the myelodysplastic syndromes: A meta-analysis of 205 patients from 17 studies. Br. J. Haematol. 1995, 89, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Fenaux, P.; Santini, V.; Spiriti, M.A.A.; Giagounidis, A.; Schlag, R.; Radinoff, A.; Gercheva-Kyuchukova, L.; Anagnostopoulos, A.; Oliva, E.N.; Symeonidis, A.; et al. A phase 3 randomized, placebo-controlled study assessing the efficacy and safety of epoetin-alpha in anemic patients with low-risk MDS. Leukemia 2018. [Google Scholar] [CrossRef] [PubMed]
- Ganan-Gomez, I.; Wei, Y.; Starczynowski, D.T.; Colla, S.; Yang, H.; Cabrero-Calvo, M.; Bohannan, Z.S.; Verma, A.; Steidl, U.; Garcia-Manero, G. Deregulation of innate immune and inflammatory signaling in myelodysplastic syndromes. Leukemia 2015, 29, 1458–1469. [Google Scholar] [CrossRef] [PubMed]
- Platzbecker, U.; Symeonidis, A.; Oliva, E.N.; Goede, J.S.; Delforge, M.; Mayer, J.; Slama, B.; Badre, S.; Gasal, E.; Mehta, B.; et al. A phase 3 randomized placebo-controlled trial of darbepoetin alfa in patients with anemia and lower-risk myelodysplastic syndromes. Leukemia 2017, 31, 1944–1950. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Hamel, J.F.; Toma, A.; Kelaidi, C.; Thepot, S.; Campelo, M.D.; Santini, V.; Sekeres, M.A.; Balleari, E.; Kaivers, J.; et al. Outcome of Lower-Risk Patients With Myelodysplastic Syndromes Without 5q Deletion After Failure of Erythropoiesis-Stimulating Agents. J. Clin. Oncol. 2017, 35, 1591–1597. [Google Scholar] [CrossRef] [PubMed]
- Hellstrom-Lindberg, E.; Gulbrandsen, N.; Lindberg, G.; Ahlgren, T.; Dahl, I.M.; Dybedal, I.; Grimfors, G.; Hesse-Sundin, E.; Hjorth, M.; Kanter-Lewensohn, L.; et al. A validated decision model for treating the anaemia of myelodysplastic syndromes with erythropoietin + granulocyte colony-stimulating factor: Significant effects on quality of life. Br. J. Haematol. 2003, 120, 1037–1046. [Google Scholar] [CrossRef]
- Westers, T.M.; Alhan, C.; Chamuleau, M.E.; van der Vorst, M.J.; Eeltink, C.; Ossenkoppele, G.J.; van de Loosdrecht, A.A. Aberrant immunophenotype of blasts in myelodysplastic syndromes is a clinically relevant biomarker in predicting response to growth factor treatment. Blood 2010, 115, 1779–1784. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Grabar, S.; Kelaidi, C.; Beyne-Rauzy, O.; Picard, F.; Bardet, V.; Coiteux, V.; Leroux, G.; Lepelley, P.; Daniel, M.T.; et al. Predictive factors of response and survival in myelodysplastic syndrome treated with erythropoietin and G-CSF: The GFM experience. Blood 2008, 111, 574–582. [Google Scholar] [CrossRef]
- Santini, V.; Schemenau, J.; Levis, A.; Balleari, E.; Sapena, R.; Adès, L.; Guerci, A.; Beyne-Rauzy, O.; Gourin, M.-P.; Cheze, S.; et al. Can the revised IPSS predict response to erythropoietic-stimulating agents in patients with classical IPSS low or intermediate-1 MDS? Blood 2013, 122, 2286–2288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hellstrom-Lindberg, E.; Negrin, R.; Stein, R.; Krantz, S.; Lindberg, G.; Vardiman, J.; Ost, A.; Greenberg, P. Erythroid response to treatment with G-CSF plus erythropoietin for the anaemia of patients with myelodysplastic syndromes: Proposal for a predictive model. Br. J. Haematol. 1997, 99, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Merchav, S.; Nielsen, O.J.; Rosenbaum, H.; Sharon, R.; Brenner, B.; Tatarsky, I.; Scigalla, P.; Wieczorek, L. In vitro studies of erythropoietin-dependent regulation of erythropoiesis in myelodysplastic syndromes. Leukemia 1990, 4, 771–774. [Google Scholar] [PubMed]
- Mittelman, M.; Gardyn, J.; Carmel, M.; Malovani, H.; Barak, Y.; Nir, U. Analysis of the erythropoietin receptor gene in patients with myeloproliferative and myelodysplastic syndromes. Leuk. Res. 1996, 20, 459–466. [Google Scholar] [CrossRef]
- Backx, B.; Broeders, L.; Hoefsloot, L.H.; Wognum, B.; Lowenberg, B. Erythropoiesis in myelodysplastic syndrome: Expression of receptors for erythropoietin and kit ligand. Leukemia 1996, 10, 466–472. [Google Scholar] [PubMed]
- Hoefsloot, L.H.; van Amelsvoort, M.P.; Broeders, L.C.; van der Plas, D.C.; van Lom, K.; Hoogerbrugge, H.; Touw, I.P.; Lowenberg, B. Erythropoietin-induced activation of STAT5 is impaired in the myelodysplastic syndrome. Blood 1997, 89, 1690–1700. [Google Scholar] [PubMed]
- Frisan, E.; Pawlikowska, P.; Pierre-Eugene, C.; Viallon, V.; Gibault, L.; Park, S.; Mayeux, P.; Dreyfus, F.; Porteu, F.; Fontenay, M. p-ERK1/2 is a predictive factor of response to erythropoiesis-stimulating agents in low/int-1 myelodysplastic syndromes. Haematologica 2010, 95, 1964–1968. [Google Scholar] [CrossRef] [Green Version]
- Oelschlaegel, U.; Alexander Rohnert, M.; Mohr, B.; Sockel, K.; Herold, S.; Ehninger, G.; Bornhauser, M.; Thiede, C.; Platzbecker, U. Clonal architecture of del(5q) myelodysplastic syndromes: Aberrant CD5 or CD7 expression within the myeloid progenitor compartment defines a subset with high clonal burden. Leukemia 2016, 30, 517–520. [Google Scholar] [CrossRef]
- Rigolin, G.M.; Porta, M.D.; Bigoni, R.; Cavazzini, F.; Ciccone, M.; Bardi, A.; Cuneo, A.; Castoldi, G. rHuEpo administration in patients with low-risk myelodysplastic syndromes: Evaluation of erythroid precursors’ response by fluorescence in situ hybridization on May-Grunwald-Giemsa-stained bone marrow samples. Br. J. Haematol. 2002, 119, 652–659. [Google Scholar] [CrossRef]
- Zermati, Y.; Fichelson, S.; Valensi, F.; Freyssinier, J.M.; Rouyer-Fessard, P.; Cramer, E.; Guichard, J.; Varet, B.; Hermine, O. Transforming growth factor inhibits erythropoiesis by blocking proliferation and accelerating differentiation of erythroid progenitors. Exp. Hematol. 2000, 28, 885–894. [Google Scholar] [CrossRef]
- Akhurst, R.J.; Hata, A. Targeting the TGFbeta signalling pathway in disease. Nat. Rev. Drug Discov. 2012, 11, 790–811. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, K.; Nakatani, M.; Hitachi, K.; Uezumi, A.; Sunada, Y.; Ageta, H.; Inokuchi, K. Activin signaling as an emerging target for therapeutic interventions. Cell Commun. Signal. 2009, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Platzbecker, U.; Germing, U.; Gotze, K.S.; Kiewe, P.; Mayer, K.; Chromik, J.; Radsak, M.; Wolff, T.; Zhang, X.; Laadem, A.; et al. Luspatercept for the treatment of anaemia in patients with lower-risk myelodysplastic syndromes (PACE-MDS): A multicentre, open-label phase 2 dose-finding study with long-term extension study. Lancet Oncol. 2017, 18, 1338–1347. [Google Scholar] [CrossRef]
- Platzbecker, U. The Medalist Trial: Results of a Phase 3, Randomized, Double-Blind, Placebo-Controlled Study of Luspatercept to Treat Anemia in Patients with Very Low-, Low-, or Intermediate-Risk Myelodysplastic Syndromes (MDS) with Ring Sideroblasts (RS) Who Require Red Blood Cell (RBC) Transfusions. In Proceedings of the ASH 2018, San Diego, SD, USA, November 2018. [Google Scholar]
- List, A.; Dewald, G.; Bennett, J.; Giagounidis, A.; Raza, A.; Feldman, E.; Powell, B.; Greenberg, P.; Thomas, D.; Stone, R.; et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N. Engl. J. Med. 2006, 355, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Fenaux, P.; Giagounidis, A.; Selleslag, D.; Beyne-Rauzy, O.; Mufti, G.; Mittelman, M.; Muus, P.; Te Boekhorst, P.; Sanz, G.; Del Canizo, C.; et al. A randomized phase 3 study of lenalidomide versus placebo in RBC transfusion-dependent patients with Low-/Intermediate-1-risk myelodysplastic syndromes with del5q. Blood 2011, 118, 3765–3776. [Google Scholar] [CrossRef] [PubMed]
- Mossner, M.; Jann, J.C.; Nowak, D.; Platzbecker, U.; Giagounidis, A.; Gotze, K.; Letsch, A.; Haase, D.; Shirneshan, K.; Braulke, F.; et al. Prevalence, clonal dynamics and clinical impact of TP53 mutations in patients with myelodysplastic syndrome with isolated deletion (5q) treated with lenalidomide: Results from a prospective multicenter study of the german MDS study group (GMDS). Leukemia 2016, 30, 1956–1959. [Google Scholar] [CrossRef] [PubMed]
- Santini, V.; Almeida, A.; Giagounidis, A.; Gropper, S.; Jonasova, A.; Vey, N.; Mufti, G.J.; Buckstein, R.; Mittelman, M.; Platzbecker, U.; et al. Randomized Phase III Study of Lenalidomide Versus Placebo in RBC Transfusion-Dependent Patients With Lower-Risk Non-del(5q) Myelodysplastic Syndromes and Ineligible for or Refractory to Erythropoiesis-Stimulating Agents. J. Clin. Oncol. 2016, 34, 2988–2996. [Google Scholar] [CrossRef]
- Besarab, A.; Provenzano, R.; Hertel, J.; Zabaneh, R.; Klaus, S.J.; Lee, T.; Leong, R.; Hemmerich, S.; Yu, K.H.; Neff, T.B. Randomized placebo-controlled dose-ranging and pharmacodynamics study of roxadustat (FG-4592) to treat anemia in nondialysis-dependent chronic kidney disease (NDD-CKD) patients. Nephrol. Dial. Transpl. 2015, 30, 1665–1673. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, X.; Zhang, Y.; Ding, G.; Zhu, C.; Huang, S.; Jia, Z.; Zhang, A. Hypoxia-inducible factor prolyl hydroxylase inhibitor roxadustat (FG-4592) protects against cisplatin-induced acute kidney injury. Clin. Sci. 2018, 132, 825–838. [Google Scholar] [CrossRef]
- Beck, J.; Henschel, C.; Chou, J.; Lin, A.; Del Balzo, U. Evaluation of the Carcinogenic Potential of Roxadustat (FG-4592), a Small Molecule Inhibitor of Hypoxia-Inducible Factor Prolyl Hydroxylase in CD-1 Mice and Sprague Dawley Rats. Int. J. Toxicol. 2017, 36, 427–439. [Google Scholar] [CrossRef] [Green Version]
- Steensma, D.P.; Platzbecker, U.; Van Eygen, K.; Raza, A.; Santini, V.; Germing, U.; Font, P.; Samarina, I.; Díez-Campelo, M.; Thepot, S.; et al. Imetelstat Treatment Leads to Durable Transfusion Independence (TI) in RBC Transfusion-Dependent (TD), Non-Del(5q) Lower Risk MDS Relapsed/Refractory to Erythropoiesis-Stimulating Agent (ESA) Who Are Lenalidomide (LEN) and HMA Naive. Blood 2018, 132, 463. [Google Scholar] [CrossRef]
- Park, H.S.; Choi, J.; See, C.J.; Kim, J.A.; Park, S.N.; Im, K.; Kim, S.M.; Lee, D.S.; Hwang, S.M. Dysregulation of Telomere Lengths and Telomerase Activity in Myelodysplastic Syndrome. Ann. Lab. Med. 2017, 37, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Fenaux, P.; Raza, A.; Vellenga, E.; Platzbecker, U.; Santini, V.; Samarina, I.; Van Eygen, K.; Díez-Campelo, M.; Patnaik, M.M.; Sherman, L.; et al. Efficacy and Safety of Imetelstat in RBC Transfusion-Dependent (TD) IPSS Low/Int-1 MDS Relapsed/Refractory to Erythropoiesis-Stimulating Agents (ESA) (IMerge). Blood 2017, 130, 4256. [Google Scholar]
- Bulycheva, E.; Rauner, M.; Medyouf, H.; Theurl, I.; Bornhauser, M.; Hofbauer, L.C.; Platzbecker, U. Myelodysplasia is in the niche: Novel concepts and emerging therapies. Leukemia 2015, 29, 259–268. [Google Scholar] [CrossRef]
- Sperling, A.S.; Gibson, C.J.; Ebert, B.L. The genetics of myelodysplastic syndrome: From clonal haematopoiesis to secondary leukaemia. Nat. Rev. Cancer 2017, 17, 5–19. [Google Scholar] [CrossRef]
- Medyouf, H. The microenvironment in human myeloid malignancies: Emerging concepts and therapeutic implications. Blood 2017, 129, 1617–1626. [Google Scholar] [CrossRef]
- Parikh, A.R.; Olnes, M.J.; Barrett, A.J. Immunomodulatory treatment of myelodysplastic syndromes: Antithymocyte globulin, cyclosporine, and alemtuzumab. Semin. Hematol. 2012, 49, 304–311. [Google Scholar] [CrossRef]
- Stahl, M.; DeVeaux, M.; de Witte, T.; Neukirchen, J.; Sekeres, M.A.; Brunner, A.M.; Roboz, G.J.; Steensma, D.P.; Bhatt, V.R.; Platzbecker, U.; et al. The use of immunosuppressive therapy in MDS: Clinical outcomes and their predictors in a large international patient cohort. Blood Adv. 2018, 2, 1765–1772. [Google Scholar] [CrossRef]
- Arranz, L.; Arriero, M.D.M.; Villatoro, A. Interleukin-1beta as emerging therapeutic target in hematological malignancies and potentially in their complications. Blood Rev. 2017, 31, 306–317. [Google Scholar] [CrossRef]
- Carey, A.; Edwards, D.K.t.; Eide, C.A.; Newell, L.; Traer, E.; Medeiros, B.C.; Pollyea, D.A.; Deininger, M.W.; Collins, R.H.; Tyner, J.W.; et al. Identification of Interleukin-1 by Functional Screening as a Key Mediator of Cellular Expansion and Disease Progression in Acute Myeloid Leukemia. Cell Rep. 2017, 18, 3204–3218. [Google Scholar] [CrossRef]
- Cluzeau, T.; McGraw, K.L.; Irvine, B.; Masala, E.; Ades, L.; Basiorka, A.A.; Maciejewski, J.; Auberger, P.; Wei, S.; Fenaux, P.; et al. Pro-inflammatory proteins S100A9 and tumor necrosis factor-alpha suppress erythropoietin elaboration in myelodysplastic syndromes. Haematologica 2017, 102, 2015–2020. [Google Scholar] [CrossRef]

| Therapeutic Compound | Phase | Included Patients | Ongoing/Recent Trial Number | Efficacy | Reference |
|---|---|---|---|---|---|
| EPO-α | III | LR-MDS patients with Hb 10 g/dL, serum erythropoietin <500 mU/mL | NCT01381809 | HI-E: 45.9% vs. 4.4% (placebo) | [5] |
| Luspatercept | III | RBC-transfusion depended, RS+ LR-MDS patients | NCT02631070 | HI-E: 52.9% vs. 11.8% (placebo) | [5] |
| Lenalidomide | III | RBC-transfusion depended LR-MDS patients with del5q | NCT00179621 | RBC-TI: 42.6–56.1% vs. 5.9% (placebo) | [5] |
| Roxadustat | III | LR-MDS patients with low RBD-transfusion burden (LTB) | NCT03263091 | ongoing | ongoing |
| Imetelstat | II/III | RBC-transfusion depended LR-MDS patients relapsed/refractory to ESA | NCT02598661 | HI-E: 71%, RBC-TI: 37% | [5], ongoing |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubasch, A.S.; Platzbecker, U. Setting Fire to ESA and EMA Resistance: New Targeted Treatment Options in Lower Risk Myelodysplastic Syndromes. Int. J. Mol. Sci. 2019, 20, 3853. https://doi.org/10.3390/ijms20163853
Kubasch AS, Platzbecker U. Setting Fire to ESA and EMA Resistance: New Targeted Treatment Options in Lower Risk Myelodysplastic Syndromes. International Journal of Molecular Sciences. 2019; 20(16):3853. https://doi.org/10.3390/ijms20163853
Chicago/Turabian StyleKubasch, Anne Sophie, and Uwe Platzbecker. 2019. "Setting Fire to ESA and EMA Resistance: New Targeted Treatment Options in Lower Risk Myelodysplastic Syndromes" International Journal of Molecular Sciences 20, no. 16: 3853. https://doi.org/10.3390/ijms20163853
APA StyleKubasch, A. S., & Platzbecker, U. (2019). Setting Fire to ESA and EMA Resistance: New Targeted Treatment Options in Lower Risk Myelodysplastic Syndromes. International Journal of Molecular Sciences, 20(16), 3853. https://doi.org/10.3390/ijms20163853