Brazilian Green Propolis Rescues Oxidative Stress-Induced Mislocalization of Claudin-1 in Human Keratinocyte-Derived HaCaT Cells
Abstract
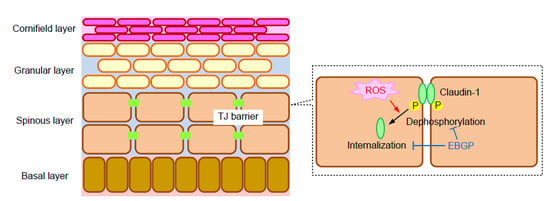
:1. Introduction
2. Results
2.1. Effect of UVB Radiation on the Production of ROS
2.2. Effect of H2O2 on the Cell Localization of TJ Protein
2.3. Effects of EBGP on the UVB- and H2O2-Induced Destruction of the TJ Barrier
2.4. Effect of CLDN1 Expression on the TJ Barrier
2.5. Effects of Endocytosis Inhibitors on the Localization of CLDN1
2.6. Effects of Phosphorylation Mimic Mutants of CLDN1 on the TJ Barrier
2.7. Phosphorylation of CLDN1 by PKC
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. UVB Irradiation
4.4. Production of Reactive Oxygen Species
4.5. Confocal Microscopy
4.6. SDS-Polyacrylamide Gel Electrophoresis and Immunoblotting
4.7. Measurement of Paracellular Permeability
4.8. Isolation of Total RNA and Quantitative Real-Time Polymerase Chain Reaction
4.9. PKC and Serine/Threonine Protein Phosphatase Activity Assays
4.10. Vector Construction and Transfection
4.11. Statistics
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CLDN | Claudin |
| Ct | Threshold cycle |
| FBS | Fetal bovine serum |
| EBGP | Ethanol extract of Brazilian green propolis |
| H2DCFDA | 2′,7′-Dichlorodihydrofluorescein diacetate |
| H2O2 | Hydrogen peroxide |
| LY | Lucifer yellow |
| MβCD | Methyl-β-cyclodextrin |
| MDC | Monodansylcadaverine |
| p-Ser | Phosphoserine |
| p-Thr | Phosphothreonine |
| PCR | Polymerase chain reaction |
| PDZ | PSD95/Dlg/ZO-1 |
| PMA | Phorbol 12-myristate 13-acetate |
| pNPP | Paranitrophenylphosphate |
| PPs | Protein phosphatases |
| ROS | Reactive oxygen species |
| SDS-PAGE | SDS-polyacrylamide gel electrophoresis |
| siRNA | Small interfering RNA |
| TER | Transepithelial electrical resistance |
| TJ | Tight junction |
| UV | Ultraviolet |
| WST-1 | 2-(4-Iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium |
| WT | Wild-type |
References
- Coolen, N.A.; Schouten, K.C.; Middelkoop, E.; Ulrich, M.M. Comparison between human fetal and adult skin. Arch. Dermatol. Res. 2010, 302, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Young, A.R.; Chadwick, C.A.; Harrison, G.I.; Nikaido, O.; Ramsden, J.; Potten, C.S. The similarity of action spectra for thymine dimers in human epidermis and erythema suggests that DNA is the chromophore for erythema. J. Investig. Dermatol. 1998, 111, 982–988. [Google Scholar] [CrossRef] [PubMed]
- De Gruijl, F.R. Action spectrum for photocarcinogenesis. Recent Results Cancer Res. 1995, 139, 21–30. [Google Scholar] [PubMed]
- Moon, S.E.; Youn, J.I.; Kim, J.A. The effect of ultraviolet-B exposure scheduling on the photodamage of hairless mouse skin. Photodermatol. Photoimmunol. Photomed. 2000, 16, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Pustisek, N.; Situm, M. UV-radiation, apoptosis and skin. Coll. Antropol. 2011, 35 (Suppl. 2), 339–341. [Google Scholar]
- Ichihashi, M.; Ueda, M.; Budiyanto, A.; Bito, T.; Oka, M.; Fukunaga, M.; Tsuru, K.; Horikawa, T. UV-induced skin damage. Toxicology 2003, 189, 21–39. [Google Scholar] [CrossRef]
- Mineta, K.; Yamamoto, Y.; Yamazaki, Y.; Tanaka, H.; Tada, Y.; Saito, K.; Tamura, A.; Igarashi, M.; Endo, T.; Takeuchi, K.; et al. Predicted expansion of the claudin multigene family. FEBS Lett. 2011, 585, 606–612. [Google Scholar] [CrossRef] [Green Version]
- Turksen, K.; Troy, T.C. Barriers built on claudins. J. Cell Sci. 2004, 117, 2435–2447. [Google Scholar] [CrossRef] [Green Version]
- Basler, K.; Bergmann, S.; Heisig, M.; Naegel, A.; Zorn-Kruppa, M.; Brandner, J.M. The role of tight junctions in skin barrier function and dermal absorption. J. Control. Release 2016, 242, 105–118. [Google Scholar] [CrossRef]
- Sugawara, T.; Iwamoto, N.; Akashi, M.; Kojima, T.; Hisatsune, J.; Sugai, M.; Furuse, M. Tight junction dysfunction in the stratum granulosum leads to aberrant stratum corneum barrier function in claudin-1-deficient mice. J. Dermatol. Sci. 2013, 70, 12–18. [Google Scholar] [CrossRef]
- Hadj-Rabia, S.; Baala, L.; Vabres, P.; Hamel-Teillac, D.; Jacquemin, E.; Fabre, M.; Lyonnet, S.; De Prost, Y.; Munnich, A.; Hadchouel, M.; et al. Claudin-1 gene mutations in neonatal sclerosing cholangitis associated with ichthyosis: A tight junction disease. Gastroenterology 2004, 127, 1386–1390. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.P.; Li, S.; Chen, Y.M.; Li, H.B. Natural Polyphenols for Prevention and Treatment of Cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef] [PubMed]
- Sawicka, D.; Car, H.; Borawska, M.H.; Niklinski, J. The anticancer activity of propolis. Folia Histochem. Cytobiol. 2012, 50, 25–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toreti, V.C.; Sato, H.H.; Pastore, G.M.; Park, Y.K. Recent progress of propolis for its biological and chemical compositions and its botanical origin. Evid. Based Complement. Alternat. Med. 2013, 2013, 697390. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, Y.M.; Marquele-Oliveira, F.; Vicentini, F.T.; Furtado, N.A.; Sousa, J.P.; Lucisano-Valim, Y.M.; Fonseca, M.J. Evaluation of the Potential of Brazilian Propolis against UV-Induced Oxidative Stress. Evid. Based Complement. Alternat. Med. 2011, 2011, 863917. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.B.; Yoo, B.S. Propolis Inhibits UVA-Induced Apoptosis of Human Keratinocyte HaCaT Cells by Scavenging ROS. Toxicol. Res. 2016, 32, 345–351. [Google Scholar] [CrossRef] [Green Version]
- De Almeida, E.B.; Cordeiro Cardoso, J.; Karla de Lima, A.; de Oliveira, N.L.; de Pontes-Filho, N.T.; Oliveira Lima, S.; Leal Souza, I.C.; de Albuquerque-Junior, R.L. The incorporation of Brazilian propolis into collagen-based dressing films improves dermal burn healing. J. Ethnopharmacol. 2013, 147, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Q.; Geng, S. Alltrans retinoic acid alters the expression of the tight junction proteins Claudin1 and 4 and epidermal barrier functionassociated genes in the epidermis. Int. J. Mol. Med. 2019, 43, 1789–1805. [Google Scholar] [PubMed]
- D’Souza, T.; Agarwal, R.; Morin, P.J. Phosphorylation of claudin-3 at threonine 192 by cAMP-dependent protein kinase regulates tight junction barrier function in ovarian cancer cells. J. Biol. Chem. 2005, 280, 26233–26240. [Google Scholar] [CrossRef]
- Ikari, A.; Matsumoto, S.; Harada, H.; Takagi, K.; Hayashi, H.; Suzuki, Y.; Degawa, M.; Miwa, M. Phosphorylation of paracellin-1 at Ser217 by protein kinase A is essential for localization in tight junctions. J. Cell Sci. 2006, 119, 1781–1789. [Google Scholar] [CrossRef] [Green Version]
- Sjo, A.; Magnusson, K.E.; Peterson, K.H. Protein kinase C activation has distinct effects on the localization, phosphorylation and detergent solubility of the claudin protein family in tight and leaky epithelial cells. J. Membr. Biol. 2010, 236, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, W.; Shabbiri, K.; Ijaz, B.; Asad, S.; Sarwar, M.T.; Gull, S.; Kausar, H.; Fouzia, K.; Shahid, I.; Hassan, S. Claudin-1 required for HCV virus entry has high potential for phosphorylation and O-glycosylation. Virol. J. 2011, 8, 229. [Google Scholar] [CrossRef] [PubMed]
- Nunbhakdi-Craig, V.; Machleidt, T.; Ogris, E.; Bellotto, D.; White, C.L.; Sontag, E. Protein phosphatase 2A associates with and regulates atypical PKC and the epithelial tight junction complex. J. Cell Biol. 2002, 158, 967–978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alhasaniah, A.; Sherratt, M.J.; O’Neill, C.A. The Impact of Ultraviolet Radiation on Barrier Function in Human Skin: Molecular Mechanisms and Topical Therapeutics. Curr. Med. Chem. 2018, 25, 5503–5511. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Ma, Y.; Wu, S.; Chen, T.; He, Y.; Sun, J.; Jiao, R.; Jiang, X.; Huang, Y.; Deng, L.; et al. Protective Effect of Cyanidin-3-O-Glucoside against Ultraviolet B Radiation-Induced Cell Damage in Human HaCaT Keratinocytes. Front. Pharmacol. 2016, 7, 301. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Kim, Y.M.; Chang, K.C. Hemin Reduces HMGB1 Release by UVB in an AMPK/HO-1-dependent Pathway in Human Keratinocytes HaCaT Cells. Arch. Med. Res. 2017, 48, 423–431. [Google Scholar] [CrossRef] [PubMed]
- El-Chami, C.; Haslam, I.S.; Steward, M.C.; O’Neill, C.A. Organic osmolytes preserve the function of the developing tight junction in ultraviolet B-irradiated rat epidermal keratinocytes. Sci. Rep. 2018, 8, 5167. [Google Scholar] [CrossRef]
- Gruber, R.; Bornchen, C.; Rose, K.; Daubmann, A.; Volksdorf, T.; Wladykowski, E.; Vidal, Y.S.S.; Peters, E.M.; Danso, M.; Bouwstra, J.A.; et al. Diverse regulation of claudin-1 and claudin-4 in atopic dermatitis. Am. J. Pathol. 2015, 185, 2777–2789. [Google Scholar] [CrossRef]
- Furuse, M.; Hata, M.; Furuse, K.; Yoshida, Y.; Haratake, A.; Sugitani, Y.; Noda, T.; Kubo, A.; Tsukita, S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: A lesson from claudin-1-deficient mice. J. Cell Biol. 2002, 156, 1099–1111. [Google Scholar] [CrossRef]
- Shiomi, R.; Shigetomi, K.; Inai, T.; Sakai, M.; Ikenouchi, J. CaMKII regulates the strength of the epithelial barrier. Sci. Rep. 2015, 5, 13262. [Google Scholar] [CrossRef] [Green Version]
- Fujii, N.; Matsuo, Y.; Matsunaga, T.; Endo, S.; Sakai, H.; Yamaguchi, M.; Yamazaki, Y.; Sugatani, J.; Ikari, A. Hypotonic stress-induced down-regulation of claudin-1 and -2 mediated by dephosphorylation and clathrin-dependent endocytosis in renal tubular epithelial cells. J. Biol. Chem. 2016, 291, 24787–24799. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.S.; Cha, B.Y.; Iida, K.; Lee, Y.S.; Yonezawa, T.; Teruya, T.; Nagai, K.; Woo, J.T. Artepillin C, as a PPARgamma ligand, enhances adipocyte differentiation and glucose uptake in 3T3-L1 cells. Biochem. Pharmacol. 2011, 81, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Rebai, O.; Amri, M. Chlorogenic Acid Prevents AMPA-Mediated Excitotoxicity in Optic Nerve Oligodendrocytes Through a PKC and Caspase-Dependent Pathways. Neurotox. Res. 2018, 34, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Avci, C.B.; Sahin, F.; Gunduz, C.; Selvi, N.; Aydin, H.H.; Oktem, G.; Topcuoglu, N.; Saydam, G. Protein phosphatase 2A (PP2A) has a potential role in CAPE-induced apoptosis of CCRF-CEM cells via effecting human telomerase reverse transcriptase activity. Hematology 2007, 12, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.K.S.; Denadai, M.; de Oliveira, C.S.; Nunes, M.L.; Narain, N. Evaluation of bioactive compounds potential and antioxidant activity of brown, green and red propolis from Brazilian northeast region. Food Res. Int. 2017, 101, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Sonoki, H.; Tanimae, A.; Endo, S.; Matsunaga, T.; Furuta, T.; Ichihara, K.; Ikari, A. Kaempherol and Luteolin Decrease Claudin-2 Expression Mediated by Inhibition of STAT3 in Lung Adenocarcinoma A549 Cells. Nutrients 2017, 9, 597. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marunaka, K.; Kobayashi, M.; Shu, S.; Matsunaga, T.; Ikari, A. Brazilian Green Propolis Rescues Oxidative Stress-Induced Mislocalization of Claudin-1 in Human Keratinocyte-Derived HaCaT Cells. Int. J. Mol. Sci. 2019, 20, 3869. https://doi.org/10.3390/ijms20163869
Marunaka K, Kobayashi M, Shu S, Matsunaga T, Ikari A. Brazilian Green Propolis Rescues Oxidative Stress-Induced Mislocalization of Claudin-1 in Human Keratinocyte-Derived HaCaT Cells. International Journal of Molecular Sciences. 2019; 20(16):3869. https://doi.org/10.3390/ijms20163869
Chicago/Turabian StyleMarunaka, Kana, Mao Kobayashi, Shokoku Shu, Toshiyuki Matsunaga, and Akira Ikari. 2019. "Brazilian Green Propolis Rescues Oxidative Stress-Induced Mislocalization of Claudin-1 in Human Keratinocyte-Derived HaCaT Cells" International Journal of Molecular Sciences 20, no. 16: 3869. https://doi.org/10.3390/ijms20163869
APA StyleMarunaka, K., Kobayashi, M., Shu, S., Matsunaga, T., & Ikari, A. (2019). Brazilian Green Propolis Rescues Oxidative Stress-Induced Mislocalization of Claudin-1 in Human Keratinocyte-Derived HaCaT Cells. International Journal of Molecular Sciences, 20(16), 3869. https://doi.org/10.3390/ijms20163869