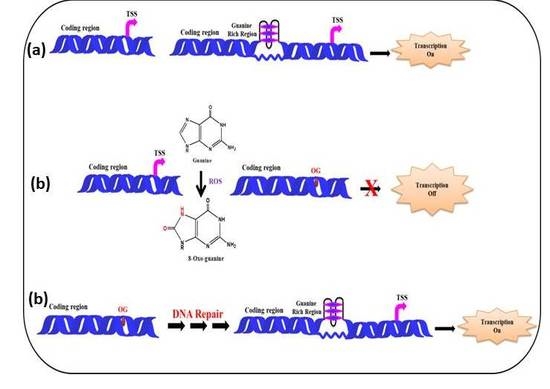
Oxidative Stress: Role and Response of Short Guanine Tracts at Genomic Locations
Abstract
:1. Introduction
2. Role of ROS in Normal Cells
3. ROS as Trigger of Oxidative Stress
4. Oxidation Mechanism of Guanine Base via ROS
5. G-Quartet Formation and Role of ROS
5.1. (i) Telomeric G-Quadruplex and Role of ROS
5.2. (ii) G-Quadruplexes in Promoter Region and Effect of ROS
6. Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Wallner, S.; Hermetter, A.; Mayer, B.; Wascher, T.C. The alpha-amino group of l-arginine mediates its antioxidant effect. Eur. J. Clin. Investig. 2001, 31, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Wang, B.; Gao, G.; Cao, W.; Zhang, Y. L-Arginine supplementation improves antioxidant defenses through L-arginine/nitric oxide pathways in exercised rats. J. Appl. Physiol. 2013, 115, 1146–1155. [Google Scholar] [CrossRef] [PubMed]
- Sestili, P.; Martinelli, C.; Colombo, E.; Barbieri, E.; Potenza, L.; Sartini, S.; Fimognari, C. Creatine as an antioxidant. Amino Acids 2011, 40, 1385–1396. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Zinc is an antioxidant and anti-inflammatory agent: Its role in human health. Front. Nutr. 2014, 1, 14. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C.; Cunningham-Bussel, A. Beyond oxidative stress: An immunologist’s guide to reactive oxygen species. Nat. Rev. Immunol. 2013, 13, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Melis, J.P.M.; Steeg, H.V.; Luijten, M. Oxidative DNA damage and nucleotide excision repair. Antioxid. Redox Signal. 2013, 18, 2409–2419. [Google Scholar] [CrossRef] [PubMed]
- Sheinman, M.; Hermsen, R. Effects of DNA oxidation on the evolution of genomes. bioRxiv 2017. [CrossRef]
- Pedro, J.; Angeli, F.; Miyamoto, S.; Schulze, A. Ferroptosis: The Greasy Side of Cell Death. Chem. Res. Toxicol. 2019, 32, 362–369. [Google Scholar]
- Alam, Z.I.; Jenner, A.; Daniel, S.E.; Lees, A.J.; Cairns, N.; Marsden, C.D.; Jenner, P.; Halliwell, B. Oxidative DNA damage in the parkinsonian brain: An apparent selective increase in 8-hydroxyguanine levels in substantia nigra. J. Neurochem. 1997, 69, 1196–1203. [Google Scholar] [CrossRef]
- Poulsen, H.E.; Prieme, H.; Loft, S. Role of oxidative DNA damage in cancer initiation and promotion. Eur. J. Cancer Prev. 1998, 7, 9–16. [Google Scholar]
- Gabbita, S.P.; Lovell, M.A.; Markesbery, W.R. Increased nuclear DNA oxidation in the brain in Alz-heimer’s disease. J. Neurochem. 1998, 71, 2034–2040. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Arlt, S.; Beisiegel, U.; Kontush, A. Lipid per-oxidation in neurodegeneration: New insights into Alz-heimer’s disease. Curr. Opin. Lipidol. 2002, 13, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Boesten, D.M.P.H.J.; de Vos-Houben, J.M.J.; Timmermans, L.; den Hartog, G.J.M.; Bast, A.; Hageman, G.J. Accelerated Aging during Chronic Oxidative Stress: A Role for PARP-1. Oxidative Med. Cell. Longev. 2013, 2013, 680414. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Sasso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Clark, D.W.; Phang, T.; Edwards, M.G.; Geraci, M.W.; Gillespie, M.N. Promoter G-quadruplex sequences are targets for base oxidation and strand cleavage during hypoxia-induced transcription. Free Radic Biol. Med. 2012, 53, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Fedeles, B.I. G-quadruplex–forming promoter sequences enable transcriptional activation in response to oxidative stress. Proc. Natl. Acad. Sci. USA 2017, 114, 2788–2790. [Google Scholar] [CrossRef]
- Lee, S.C.; Zhang, J.; Strom, J.; Yang, D.; Dinh, T.N.; Kappeler, K.; Chen, Q.M. G-Quadruplex in the NRF2 mRNA 5’ Untranslated Region Regulates De Novo NRF2 Protein Translation under Oxidative Stress. Mol. Cell. Biol. 2016, 37, e00122-16. [Google Scholar] [CrossRef]
- Bochman, M.L.; Paeschke, K.; Zakian, V.A. DNA secondary structures: Stability and function of G-quadruplex structures. Nat. Rev. Genet. 2012, 13, 770–780. [Google Scholar] [CrossRef]
- Chambers, V.S.; Marsico, G.; Boutell, J.M.; Di Antonio, M.; Smith, G.P.; Balasubramanian, S. High-throughput sequencing of DNA G-quadruplex structures in the human genome. Nat. Biotechnol. 2015, 33, 877–881. [Google Scholar] [CrossRef] [Green Version]
- Simonsson, T.; Pecinka, P.; Kubista, M. DNA Tetraplex Formation in the Control Region of C-Myc. Nucleic Acids Res. 1998, 26, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui-Jain, A.; Grand, C.L.; Bearss, D.J.; Hurley, L.H. Direct Evidence for a G-Quadruplex In a Promoter Region and Its Targeting with a Small Molecule to Repress C-MYC Transcription. Proc. Natl. Acad. Sci. USA 2002, 99, 11593–11598. [Google Scholar] [CrossRef] [PubMed]
- Rankin, S.; Reszka, A.P.; Huppert, J.; Zloh, M.; Parkinson, G.N.; Todd, A.K.; Ladame, S.; Balasubramanian, S.; Neidle, S. Putative DNA Quadruplex Formation within the Human C-Kit Oncogene. J. Am. Chem. Soc. 2005, 127, 10584–10589. [Google Scholar] [CrossRef] [PubMed]
- Cogoi, S.; Xodo, L.E. G-quadruplex formation within the promoter of the KRAS proto-oncogene and its effect on transcription. Nucleic Acids Res. 2006, 34, 2536–2549. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Guo, K.; Rusche, J.J.; Hurley, L.H. Facilitation of a Structural Transition in the Polypurine/Polypyrimidine Tract within the Proximal Promoter Region of the Human VEGF Gene by the Presence of Potassium and G-Quadruplex-Interactive Agents. Nucleic Acids Res. 2005, 33, 6070–6080. [Google Scholar] [CrossRef]
- Sun, D.; Wei-Jun, L.; Guo, K.; Rusche, J.J.; Ebbinghaus, S.; Gokhale, V.; Hurley, L.H. The Proximal Promoter Region of the Human Vascular Endothelial Growth Factor Gene Has A G-Quadruplex Structure that can be targeted by G-Quadruplex–Interactive Agents. Mol. Cancer Ther. 2008, 7, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Agrawal, P.; Brown, R.V.; Hatzakis, E.; Hurley, L.; Yang, D. The Major G-Quadruplex Formed in the Human Platelet-Derived Growth Factor Receptor Β (PDGFR-Β) Promoter Adopts a Novel Broken-Strand Structure in K+ Solution. J. Am. Chem. Soc. 2012, 134, 13220–13223. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.; Lin, C.; Mathad, R.I.; Carver, M.; Yang, D. The major G-quadruplex formed in the human BCL-2 proximal promoter adopts a parallel structure with a 13-nt loop in K+ solution. J. Am. Chem. Soc. 2014, 136, 1750–1753. [Google Scholar] [CrossRef]
- Palumbo, S.L.; Memmott, R.M.; Uribe, D.J.; Krotova-Khan, Y.; Hurley, L.H.; Ebbinghaus, S.W. A Novel G-Quadruplex-Forming GGA Repeat Region in the C-Myb Promoter is a Critical Regulator of Promoter Activity. Nucleic Acids Res. 2008, 36, 1755–1769. [Google Scholar] [CrossRef]
- Tong, X.; Lan, W.; Zhang, X.; Wu, H.; Liu, M.; Cao, C. Solution structure of all parallel G-quadruplex formed by the oncogene RET promoter sequence. Nucleic Acids Res. 2011, 39, 6753–6763. [Google Scholar] [CrossRef]
- Mitchell, T.; Ramos-Montoya, A.; Di Antonio, M.; Murat, P.; Ohnmacht, S.; Micco, M.; Jurmeister, S.; Fryer, L.; Balasubramanian, S.; Neidle, S.; et al. Downregulation of androgen receptor transcription by promoter G-quadruplex stabilization as a potential alternative treatment for castrate-resistant prostate cancer. Biochemistry 2013, 52, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.V.; Gaerig, V.C.; Simmons, T.; Brooks, T.A. Helping Eve overcome ADAM: G-quadruplexes in the ADAM-15 promoter as new molecular targets for breast cancer therapeutics. Molecules 2013, 18, 15019–15034. [Google Scholar] [CrossRef] [PubMed]
- Chaires, J.B.; Trent, J.O.; Gray, R.D.; Dean, W.L.; Buscaglia, R.; Thomas, S.D.; Miller, D.M. An improved model for the hTERT promoter quadruplex. PLoS ONE 2014, 9, e115580. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zhao, D.; Dong, L.; Pan, S.; Hao, F.; Guan, Y. A novel G-quadruplex motif in the Human MET promoter region. Biosci. Rep. 2017, 37, BSR20171128. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Choi, E.S.; Hwang, K.; Kim, J.; Sampath, S.; Venkitaraman, A.R.; Lee, H. The Breast Cancer Susceptibility Gene BRCA2 is Required for the Maintenance of Telomere Homeostasis. J. Biol. Chem. 2012, 287, 5091–5101. [Google Scholar] [CrossRef] [PubMed]
- Grigg, J.C.; Shumayrikh, N.; Sen, D. G-quadruplex structures formed by expanded hexanucleotide repeat RNA and DNA from the neurodegenerative disease-linked C9orf72 gene efficiently sequester and activate heme. PLoS ONE 2014, 9, e106449. [Google Scholar] [CrossRef] [PubMed]
- Simone, R.; Fratta, P.; Neidle, S.; Parkinson, G.N.; Isaacs, A.M. G-Quadruplexes: Emerging Roles in Neurodegenerative Diseases and the Non-Coding Transcriptome. Fed. Eur. Biochem. Soc. 2015, 589, 1653–1668. [Google Scholar] [CrossRef]
- Zhou, B.; Geng, Y.; Liu, C.; Miao, H.; Ren, Y.; Xu, N.; Shi, X.; You, Y.; Lee, T.; Zhu, G. Characterizations of Distinct Parallel and Antiparallel G-Quadruplexes Formed by Two-Repeat ALS and FTD Related GGGGCC Sequence. Sci. Rep. 2018, 8, 2366. [Google Scholar] [CrossRef] [PubMed]
- Darnell, J.C.; Jensen, K.B.; Jin, P.; Brown, V.; Warren, S.T.; Darnell, R.B. Fragile X Mental Retardation Protein Targets G Quartet mRNAs Important for Neuronal Function. Cell 2001, 107, 489–499. [Google Scholar] [CrossRef]
- Balkwill, G.D.; Kamila, D.; Garner, T.P.; Hodgman, C.; Flint, A.P.; Searle, M.S. Repression of Translation of Human Estrogen Receptor R by G-Quadruplex Formation. Biochemistry 2009, 48, 11487–11495. [Google Scholar] [CrossRef]
- Bugaut, A.; Balasubramanian, S. 5′-UTR RNA G-quadruplexes: Translation regulation and targeting. Nucleic Acids Res. 2012, 40, 4727–4741. [Google Scholar] [CrossRef] [PubMed]
- Vorlícková, M.; Tomasko, M.; Sagi, A.J.; Bednarova, K.; Sagi, J. 8-Oxoguanine in a quadruplex of the human telomere DNA sequence. FEBS J. 2012, 279, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Rinker, L.; Peng, J.; Chilian, W.M. Reactive Oxygen Species: The Good and the Bad, Reactive Oxygen Species (ROS) in Living Cells, Cristiana Filip and Elena Albu; IntechOpen: London, UK, 2017. [Google Scholar]
- Kohchi, C.; Inagawa, H.; Nishizawa, T.; Soma, G. Innate immunity. Anticancer Res. 2009, 29, 817–821. [Google Scholar] [PubMed]
- Li, Q.; Verma, I.M. NF-kappaB regulation in the immune system. Nat. Rev. Immunol. 2002, 2, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Thannickal, V.J.; Fanburg, B.L. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell Mol. Physiol. 2000, 279, L1005–L1028. [Google Scholar] [CrossRef] [Green Version]
- Tulard, A.; Hoffschir, F.; de Boisferon, F.H.; Luccioni, C.; Bravard, A. Persistent oxidative stress after ionizing radiation is involved in inherited radiosensitivity. Free Radic. Biol. Med. 2003, 35, 68–77. [Google Scholar] [CrossRef]
- Little, J.B. Ionizing Radiation. In Holland-Frei Cancer Medicine, 5th ed.; Bast, R.C., Jr., Croce, C.M., Hait, W.N., Hong, W.K., Kufe, D.W., Piccart-Gebart, M., Pollock, R.E., Weichselbaum, R.R., Wang, H., Holland, J.F., Eds.; BC Decker: Hamilton, ON, Canada, 2000; Chapter 14. [Google Scholar]
- Klaunig, J.E.; Kamendulis, L.M. The Role of Oxidative Stress in Carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 239–267. [Google Scholar] [CrossRef] [PubMed]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA Damage: Mechanisms, Mutation, and Disease. J. Fed. Am. Soc. Exp. Biol. 2003, 17, 1195–1214. [Google Scholar] [CrossRef]
- Ohtsubo, T.; Ohya, Y.; Nakamura, Y.; Kansui, Y.; Furuichi, M.; Matsumura, K.; Fujii, K.; Iida, M.; Nakabeppu, Y. Accumulation of 8-oxo-deoxyguanosine in Cardiovascular Tissues with the Development of Hypertension. DNA Repair 2007, 6, 760–769. [Google Scholar] [CrossRef]
- Mariarosaria, D.; Eleonora, P.; Eugenia, D. Mechanism of Oxidative DNA Damage Repair and Relevance to Human Pathology. Mutat. Res. 2008, 659, 4–14. [Google Scholar]
- Maynard, S.; Schurman, S.H.; Harboe, C.; de Souza-Pinto, N.C.; Bohr, V.A. Base Excision Repair of Oxidative DNA Damage and Association with Cancer and Aging. Carcinogenesis 2009, 30, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, L.; Bialkowski, K.; Risom, L.; Løhr, M.; Loft, S.; Møller, P. Aging and Defense Against Generation of 8-oxo-7,8-dihydro-2′deoxyguanosine in DNA. Free Radic. Biol. Med. 2009, 47, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Sitkovsky, M.; Lukashev, D. Regulation of immune cells by local-tissue oxygen tension: HIF1 [alpha] and adenosine receptors. Nat. Rev. Immunol. 2005, 5, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Krystona, T.B.; Georgieva, A.B.; Pissisb, P.; Georgakilasa, A.G. Role of oxidative stress and DNA damage in human carcinogenesis. Mutat. Res. 2011, 711, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Eiberger, W.; Volkmer, B.; Amouroux, R.; Dhérin, C.; Radicella, J.P.; Epe, B. Oxidative stress impairs the repair of oxidative DNA base modifications in human skin fibroblasts and melanoma cells. DNA Repair 2008, 7, 912–921. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.M.; Buettner, G.R.; Aust, S.D. Transition Metals as Catalysts of “autoxidation” Reactions. Free Radic. Biol. Med. 1990, 8, 95–108. [Google Scholar] [CrossRef]
- Klaunig, J.E.; Kamendulis, L.M.; Hocevar, B.A. Oxidative Stress and Oxidative Damage in Carcinogenesis. Toxicol. Pathol. 2010, 38, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Lund, L.G.; Aust, A.E. Iron mobilization from asbestos by chelators and ascorbic acid. Arch. Biochem. Biophys. 1990, 270, 60–64. [Google Scholar] [CrossRef]
- Imlay, J.A.; Chin, S.M.; Linn, S. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science 1988, 240, 640–642. [Google Scholar] [CrossRef]
- Inoue, S.; Kawanishi, S. Hydroxyl radical production and human DNA damage induced by ferric nitrilotriacetate and hydrogen peroxide. Cancer Res. 1987, 47, 6522–6527. [Google Scholar]
- Celander, D.W.; Cech, T.R. Iron (II)-ethylenediaminetetraacetic acid catalyzed cleavage of RNA and DNA oligonucleotides: Similar reactivity toward single- and double-stranded forms. Biochemistry 1990, 29, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Grisham, M.B. Reactive Metabolites of Oxygen and Nitrogen in Biology and Medicine, Georgetown. Landes 1992, 43, 47–48. [Google Scholar]
- Halliwell, B.; Aruoma, O.I. DNA damage by oxygen-derived species. Its mechanism and measurement in mammalian systems. FEBS Lett. 1991, 281, 9–19. [Google Scholar] [CrossRef]
- Goldberg, I.H. Free radical mechanism in neocarzinostatin-induced DNA damage. Free Radic Biol. Med. 1987, 3, 41–54. [Google Scholar] [CrossRef]
- Aust, A.E.; Eveleigh, J.F. Mechanism of DNA Oxidation (44449). Exp. Biol. Med. 1999, 222, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Szabó, C.; Ohshima, H. DNA Damage Induced by Peroxynitrite: Subsequent Biological Effects. Nitric Oxide 1997, 1, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, S.; Hiraku, Y.; Oikawa, S. Mechanism of guanine-specific DNA damage by oxidative stress and its role in carcinogenesis and aging. Mutat. Res. 2001, 488, 65–76. [Google Scholar] [CrossRef]
- Niles, J.C.; Wishnok, J.S.; Tannenbaum, S.R. Peroxynitrite-induced oxidation and nitration products of guanine and 8-oxoguanine: Structures and mechanisms of product formation. Nitric Oxide 2006, 14, 109–121. [Google Scholar] [CrossRef]
- Tretyakova, N.Y.; Wishnok, J.S.; Tannenbaum, S.R. Peroxynitrite-Induced Secondary Oxidative Lesions at Guanine Nucleobases: Chemical Stability and Recognition by the Fpg DNA Repair Enzyme. Chem. Res. Toxicol. 2000, 13, 658–664. [Google Scholar] [CrossRef]
- Kaushik, M.; Kaushik, S.; Bansal, A.; Saxena, S.; Kukreti, S. Structural diversity and specific recognition of four stranded G-quadruplex DNA. Curr. Mol. Med. 2011, 11, 744–769. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, S. A triple stranded G-quadruplex formation in the promoter region of human myosin β (Myh7) gene. J. Biomol. Struct. Dyn. 2018, 36, 2773–2786. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, D.; Arachchilage, G.M.; Basu, S. Metal Cations in G-Quadruplex Folding and Stability. Front. Chem. 2016, 4, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siebenmorgen, T.; Zacharias, M. Origin of Ion Specificity of Telomeric DNA G-Quadruplexes Investigated by Free-Energy Simulations. Biophys. J. 2017, 112, 2280–2290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiziria, E.; Gorgoshidze, M.; Gogichaishvili, S.; Sokhadze, V.; Khachidze, D.; Kiladze, M.; Lomidze, E.; Barbakadze, S.; Tvauri, G.; Monaselidze, J. Influence of K+ Ions on Thermodynamic Stability of DNA G-Quadruplex. Bull. Georgian Natl. Acad. Sci. 2017, 11, 42–46. [Google Scholar]
- Rhodes, D.; Lipps, H.J. G-quadruplexes and their regulatory roles in biology. Nucleic Acids Res. 2015, 43, 8627–8637. [Google Scholar] [CrossRef] [Green Version]
- Bedrat, A.; Lacroix, L.; Mergny, J.L. Re-evaluation of G-quadruplex propensity with G4Hunter. Nucleic Acids Res. 2016, 44, 1746–1759. [Google Scholar] [CrossRef]
- Kouzine, F.; Wojtowicz, D.; Baranello, L.; Yamane, A.; Nelson, S.; Resch, W.; Kieffer-Kwon, K.R.; Benham, C.J.; Casellas, R.; Przytycka, T.M.; et al. Permanganate/S1 nuclease footprinting reveals non-B DNA structures with regulatory potential across a mammalian genome. Cell Syst. 2017, 4, 344–356. [Google Scholar] [CrossRef]
- Rigo, R.; Palumbo, M.; Sissi, C. G-quadruplexes in human promoters: A challenge for therapeutic applications. Biochim. Biophys. Acta 2017, 1861, 1399–1413. [Google Scholar] [CrossRef]
- Biffi, G.; Tannahill, D.; McCafferty, J.; Balasubramanian, S. Quantitative visualization of DNA G-quadruplex structures in human cells. Nat. Chem. 2013, 5, 182–186. [Google Scholar] [CrossRef]
- Buket, O.; Clement, L.; DanZhou, Y. DNA G-quadruplex and its potential as anticancer drug target. Sci. China Chem. 2014, 57, 1605–1614. [Google Scholar] [CrossRef]
- Yang, F.; Sun, X.; Wang, L.; Li, Q.; Guan, A.; Shen, G.; Tang, Y. Selective recognition of c-myc promoter G-quadruplex and down-regulation of oncogene c-myc transcription in human cancer cells by 3,8 a-disubstituted indolizinone. RSC Adv. 2017, 7, 51965–51969. [Google Scholar] [CrossRef]
- Brázda, V.; Hároníková, L.; Liao, J.C.C.; Fojta, M. DNA and RNA Quadruplex-Binding Proteins. Int. J. Mol. Sci. 2014, 15, 17493–17517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heddi, B.; Cheong, V.V.; Martadinata, H.; Phan, A.T. Insights into G-quadruplex specific recognition by the DEAH-box helicase RHAU: Solution structure of a peptide–quadruplex complex. Proc. Natl. Acad. Sci. USA 2015, 112, 9608–9613. [Google Scholar] [CrossRef] [PubMed]
- McAninch, D.S.; Heinaman, A.M.; Lang, C.N.; Moss, K.R.; Bassell, G.J.; Mihailescua, M.R.; Evans, T.L. Fragile X mental retardation protein recognizes a G quadruplex structure within the survival motor neuron domain containing 1 mRNA 5′-UTR. Mol. BioSyst. 2017, 13, 1448–1457. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hoshiyama, H.; Shay, J.W.; Wright, W.E. Quantitative telomeric overhang determination using a double-strand specific nuclease. Nucleic Acids Res. 2008, 36, e14. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, J.-Q.; Chen, Z.; Zheng, K.-W.; Chen, C.-Y.; Hao, Y.-H.; Tan, Z. G-quadruplex formation at the 30 end of telomere DNA inhibits its extension by telomerase, polymerase and unwinding by helicase. Nucleic Acids Res. 2011, 39, 6229–6237. [Google Scholar] [CrossRef] [PubMed]
- Delaney, S.; Barton, J.K. Charge Transport in DNA Duplex/Quadruplex Conjugates. Biochemistry 2003, 42, 14159–14165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harley, C.B.; Futcher, A.B.; Greider, C.W. Telomeres shorten during ageing of human fibroblasts. Nature 1990, 345, 458–460. [Google Scholar] [CrossRef]
- Petersen, S.; Saretzki, G.; von Zglinicki, T. Preferential accumulation of single-stranded regions in telomeres of human fibroblasts. Exp. Cell Res. 1998, 239, 152–160. [Google Scholar] [CrossRef]
- Oikawa, S.; Kawanishi, S. Site-specific DNA damage at GGG sequence by oxidative stress may accelerate telomere shortening. FEBS Lett. 1999, 453, 365–368. [Google Scholar] [CrossRef] [Green Version]
- Zglinicki, T.V. Oxidative stress shortens telomeres. Trends Biochem. Sci. 2002, 27, 339–344. [Google Scholar] [CrossRef]
- Stewart, S.A.; Ben-Porath, I.; Carey, V.J.; O’Connor, B.F.; Hahn, W.C.; Weinberg, R.A. Erosion of the telomeric single-strand overhang at replicative senescence. Nat. Genet. 2003, 33, 492–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawanishi, S.; Oikawa, S. Mechanism of telomere shortening by oxidative stress. Ann. NY Acad. Sci. 2004, 1019, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Epel, E.S.; Blackburn, E.H.; Lin, J.; Dhabhar, F.S.; Adler, N.E.; Morrow, J.D.; Cawthon, R.M. Accelerated telomere shortening in response to life stress. Proc. Natl. Acad. Sci. USA 2004, 101, 17312–17315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passos, J.F.; Saretzki, G.; Zglinicki, T.V. DNA damage in telomeres and mitochondria during cellular senescence: Is there a connection? Nucleic Acids Res. 2007, 35, 7505–7513. [Google Scholar] [CrossRef] [PubMed]
- Coluzzi, E.; Colamartino, M.; Cozzi, R.; Leone, S.; Meneghini, C.; O’Callaghan, N.; Sgura, A. Oxidative Stress Induces Persistent Telomeric DNA Damage Responsible for Nuclear Morphology Change in Mammalian Cells. PLoS ONE 2014, 9, e110963. [Google Scholar] [CrossRef] [PubMed]
- Jafri, M.A.; Ansari, S.A.; Alqahtani, M.H.; Shay, J.W. Roles of telomeres and telomerase in cancer, and advances in telomerase targeted therapies. Genome Med. 2016, 8, 69. [Google Scholar] [CrossRef]
- Graham, M.K.; Meeker, A. Telomeres and telomerase in prostate cancer development and therapy. Nat. Rev. Urol. 2017, 14, 607–619. [Google Scholar] [CrossRef] [Green Version]
- Opresko, P.L.; Fan, J.; Danzy, S.; Wilson, D.M.; Bohr, V.A. Oxidative damage in telomeric DNA disrupts recognition by TRF1 and TRF2. Nucleic Acids Res. 2005, 33, 1230–1239. [Google Scholar] [CrossRef]
- Lee, H.-T.; Bose, A.; Lee, C.-Y.; Opresko, P.L.; Myong, S. Molecular mechanisms by which oxidative DNA damage promotes telomerase activity. Nucleic Acids Res. 2017, 45, 11752–11765. [Google Scholar] [CrossRef] [Green Version]
- Grollman, A.P.; Moriya, M. Mutagenesis by 8-oxoguanine: An enemy within. Trends Genet. 1993, 9, 246–249. [Google Scholar] [CrossRef]
- Bielskutė, S.; Plavec, J.; Podbevšek, P. Impact of Oxidative Lesions on the Human Telomeric G-Quadruplex. J. Am. Chem. Soc. 2019, 141, 2594–2603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bozkus, F. Could serum levels of telomerase be considered as an oxidative stress marker in COPD? Telomere Telomerase 2016, 3, e1258. [Google Scholar] [CrossRef]
- Aeby, E.; Ahmed, W.; Redon, S.; Simanis, V.; Lingner, J. Peroxiredoxin 1 Protects Telomeres from Oxidative Damage and Preserves Telomeric DNA for Extension by Telomerase. Cell Rep. 2016, 17, 3107–3114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virgilio, A.; Esposito, V.; Mayol, L.; Giancola, C.; Petraccone, L.; Galeone, A. The oxidative damage to the human telomere: Effects of 5-hydroxymethyl-2′-deoxyuridine on telomeric G-quadruplex structures. Org. Biomol. Chem. 2015, 13, 7421–7429. [Google Scholar] [CrossRef] [PubMed]
- Fouquerel, E.; Lormand, J.; Bose, A.; Lee, H.-T.; Kim, G.S.; Li, J.; Sobol, R.W.; Freudenthal, B.D.; Myong, S.; Opresko, P.L. Oxidative guanine base damage regulates human telomerase activity. Nat. Struct. Mol. Biol. 2016, 23, 1092–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkar, J.; Liu, Y. The origin of oxidized guanine resolves the puzzle of oxidation induced telomere-length alterations. Nat. Struct. Mol. Biol. 2016, 23, 1070–1071. [Google Scholar] [CrossRef]
- Balasubramanian, S. Targeting G-quadruplexes in gene promoters: A novel anticancer strategy? Nat. Rev. Drug Discov. 2011, 10, 261–275. [Google Scholar] [CrossRef]
- Zhou, W.; Suntharalingam, K.; Brand, N.J.; Barton, P.J.R.; Vilar, R.; Ying, L. Possible Regulatory Roles of Promoter G-Quadruplexes in Cardiac Function-Related Genes—Human TnIc as a Model. PLoS ONE 2013, 8, e53137. [Google Scholar] [CrossRef]
- Yuan, L.; Tian, T.; Chen, Y.; Yan, S.; Xing, X.; Zhang, Z.; Zhai, Q.; Xu, L.; Wang, S.; Weng, X.; et al. Existence of G-quadruplex structures in promoter region of oncogenes confirmed by G-quadruplex DNA cross-linking strategy. Sci. Rep. 2013, 3, 01811. [Google Scholar] [CrossRef]
- Fleming, A.M.; Zhou, J.; Wallace, S.S.; Burrows, C.J. A Role for the Fifth G-Track in G-Quadruplex Forming Oncogene Promoter Sequences during Oxidative Stress: Do These “Spare Tires” Have an Evolved Function? ACS Cent. Sci. 2015, 1, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A.M.; Dinga, Y.; Burrowsa, C.J. Oxidative DNA damage is epigenetic by regulating gene transcription via base excision repair. Proc. Natl. Acad. Sci. USA 2017, 114, 2604–2609. [Google Scholar] [CrossRef] [PubMed]
- Byrd, A.K.; Zybailov, B.L.; Maddukuri, L.; Gao, J.; Marecki, J.C.; Jaiswal, M.; Bell, M.R.; Griffin, W.C.; Reed, M.R.; Chib, S.; et al. Evidence that G-quadruplex DNA Accumulates in the Cytoplasm and Participates in Stress Granule Assembly in Response to Oxidative Stress. J. Biol. Chem. 2016, 291, 18041–18057. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Wang, M.; Li, H.; Wang, M.; Luo, X.; Huang, Y.; Wang, H.-H.; Nie, Z.; Yao, S. Simultaneous Monitoring of Cell-surface Receptor and Tumor-targeted Photodynamic Therapy via TdT-initiated Poly-G-Quadruplexes. Sci. Rep. 2018, 8, 5551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cogoi, S.; Ferino, A.; Miglietta, G.; Pedersen, E.B.; Xodo, L.E. The regulatory G4 motif of the Kirsten ras (KRAS) gene is sensitive to guanine oxidation: Implications on transcription. Nucleic Acids Res. 2018, 46, 661–676. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.D. Type 2 Diabetes as a redox disease. Lancet 2014, 383, 841–843. [Google Scholar] [CrossRef]
- Bartas, M.; Brazda, V.; Karlicky, V.; Cerven, J.; Pecinka, P. Bioinformatics analyses and in vitro evidence for five and six stacked G-quadruplex forming sequences. Biochimie 2018, 150, 70–75. [Google Scholar] [CrossRef]
- Dvorkin, S.A.; Karslslotis, A.I.; da Silva, M.W. Encoding canonical DNA quadruplex structure. Sci. Adv. 2018, 4, eaat3007. [Google Scholar] [CrossRef] [Green Version]
- Kaushik, M.; Kaushik, S.; Roy, K.; Singh, A.; Mahendru, S.; Kumar, M.; Chaudhary, S.; Ahmed, S.; Kukreti, S. A bouquet of DNA structures: Emerging diversity. Biochem. Biophys. Rep. 2016, 5, 388–395. [Google Scholar] [CrossRef] [Green Version]








| S. No. | Gene | Disease | References |
|---|---|---|---|
| 1 | c-Myc | Gastrointestinal, ovarian and breast cancer tumors | [21,22] |
| 2 | c-Kit | Gastrointestinal stromal tumors (GIST) | [23] |
| 3 | KRAS | Pancreatic carcinoma | [24] |
| 4 | VEGF | Tumor angiogenesis | [25,26] |
| 5 | PDGF | Cancers and fibrotic disorders | [27] |
| 6 | BCL-2 | B-cell and T-cell lymphomas and breast prostate cervical Colorectal and non-small cell lung carcinomas | [28] |
| 7 | C-Myb | Leukemias | [29] |
| 8 | RET | Thyroid cancers | [30] |
| 9 | AR | Castrate-resistant prostate cancer | [31] |
| 10 | ADAM | Breast cancer | [32] |
| 11 | hTERT | Limitless replication and cancer | [33] |
| 12 | MET | Cancers of kidney, liver, stomach, breast, and brain | [34] |
| 13 | BRCA2 | Familial breast/ovarian cancer, telomere homeostasis | [35] |
| 14 | C9orf72 Gene | Amyotrophic lateral sclerosis (ALS) or frontotemporal dementia (FTD) | [36,37,38] |
| 15 | FMR1 Gene | Fragile X syndrome | [39] |
| 16 | ESR1 | Cancer and neoplasia | [40] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: Role and Response of Short Guanine Tracts at Genomic Locations. Int. J. Mol. Sci. 2019, 20, 4258. https://doi.org/10.3390/ijms20174258
Singh A, Kukreti R, Saso L, Kukreti S. Oxidative Stress: Role and Response of Short Guanine Tracts at Genomic Locations. International Journal of Molecular Sciences. 2019; 20(17):4258. https://doi.org/10.3390/ijms20174258
Chicago/Turabian StyleSingh, Anju, Ritushree Kukreti, Luciano Saso, and Shrikant Kukreti. 2019. "Oxidative Stress: Role and Response of Short Guanine Tracts at Genomic Locations" International Journal of Molecular Sciences 20, no. 17: 4258. https://doi.org/10.3390/ijms20174258
APA StyleSingh, A., Kukreti, R., Saso, L., & Kukreti, S. (2019). Oxidative Stress: Role and Response of Short Guanine Tracts at Genomic Locations. International Journal of Molecular Sciences, 20(17), 4258. https://doi.org/10.3390/ijms20174258