Nitric Oxide Is Involved in Heavy Ion-Induced Non-Targeted Effects in Human Fibroblasts
Abstract
:1. Introduction
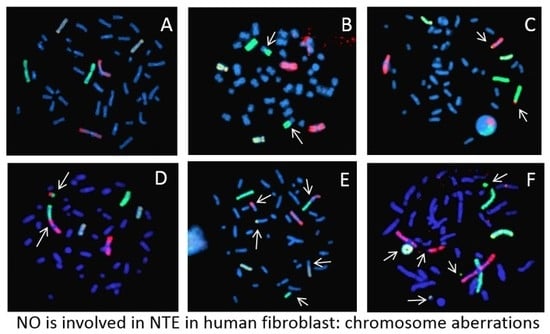
2. Results
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Irradiation
4.3. Media Transfer
4.4. Premature Chromosome Condensation (PCC)
4.5. Fluorescence In Situ Hybridization (FISH)
4.6. Classification of Chromosome Aberrations
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 18α-GA | 18α-glycyrrhetinic acid |
| ASC2-P | L-ascorbic acid-2-phosphate |
| BNL | Brookhaven National Laboratory |
| CA | Chromosome aberrations |
| CCD | Charge-coupled device |
| cPTIO | 2-(4-Carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide potassium salt |
| DAPI | 4, 6-diamidino-2-phenylindole |
| DDREF | Dose and dose-rate reduction effectiveness factor |
| DMSO | Dimethylsulfoxide |
| DSB | Double strand breaks |
| FISH | Fluorescence in situ hybridization |
| GCR | Galactic cosmic rays |
| HZE | High atomic number and energy |
| LET | Linear Energy Transfer |
| MeV/n | Mega electron Volt per nucleon |
| NSRL | NASA Space Radiation Laboratory |
| NO | Nitric Oxide |
| NTE | Non-targeted effect |
| PCC | Premature Chromosome Condensation |
| ROS | Reactive Oxygen Species |
| SE | Standard Error |
| TAMU | Texas A&M University |
References
- Cucinotta, F.A.; Durante, M. Cancer risk from exposure to galactic cosmic rays: Implications for space exploration by human beings. Lancet Oncol. 2006, 7, 431–435. [Google Scholar] [CrossRef]
- Cucinotta, F.A. Space radiation risks for astronauts on multiple International Space Station missions. PLoS ONE 2014, 9, e96099. [Google Scholar] [CrossRef] [PubMed]
- George, K.; Durante, M.; Wu, H.; Willingham, V.; Badhwar, G.; Cucinotta, F.A. Chromosome aberrations in the blood lymphocytes of astronauts after space flight. Radiat. Res. 2001, 156, 731–738. [Google Scholar] [CrossRef]
- Alpen, E.L.; Powers-Risius, P.; Curtis, S.B.; DeGuzman, R. Tumorigenic potential of high-Z, high-LET charged particle radiations. Radiat. Res. 1993, 88, 132–143. [Google Scholar] [CrossRef]
- Bonassi, S.; Norppa, H.; Ceppi, M.; Strömberg, U.; Vermeulen, R.; Znaor, A.; Cebulska-Wasilewska, A.; Fabianova, E.; Fucic, A.; Gundy, S.; et al. Chromosomal aberration frequency in lymphocytes predicts the risk of cancer: Results from a pooled cohort study of 22358 subjects in 11 countries. Carcinogenesis 2008, 29, 1178–1183. [Google Scholar] [CrossRef] [PubMed]
- Weil, M.M.; Bedford, J.S.; Bielefeldt-Ohmann, H.; Ray, A.F.; Gernik, P.C.; Ehrhart, E.J.; Fallgren, C.M.; Hailu, F.; Battaglia, C.L.; Charles, B.; et al. Incidence of acute myeloid leukemia and hepatocellular carcinoma in mice irradiated with 1 GeV/nucleon 56Fe ions. Radiat. Res. 2009, 172, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Cucinotta, F.A.; Chappell, L.J. Non-targeted effects and the dose response for heavy ion tumor formation. Mutat. Res. 2010, 687, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Durante, M.; Cucinotta, F.A. Heavy ion carcinogenesis and human space exploration. Nat. Rev. Cancer 2008, 8, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Hada, M.; Chappell, L.J.; Wang, M.; George, K.A.; Cucinotta, F.A. Induction of chromosomal aberrations at fluences of less than one HZE particle per cell nucleus. Radiat. Res. 2014, 182, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Cacao, E.; Hada, M.; Saganti, P.B.; George, K.A.; Cucinotta, F.A. Relative biological effectiveness of HZE particles for chromosomal exchanges and other surrogate cancer risk endpoints. PLoS ONE 2016, 11, e0153998. [Google Scholar] [CrossRef]
- Desoukey, O.; Ding, N.; Zhou, G. Targeted and non-targeted effects of ionizing radiation. J. Radiat. Res. Appl. Sci. 2015, 8, 247–254. [Google Scholar] [CrossRef] [Green Version]
- Azzam, E.I.; de Toledo, S.M.; Little, J.B. Direct evidence for participation of gap junction-mediated intercellular communication in the transmission of damage signals from alpha-particle irradiated to nonirradiated cells. Proc. Natl. Acad. Sci. USA 2001, 98, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Dowling-Warriner, C.; Trosko, J.E. Induction of gap junction intercellular communication, connecxin43 expression, and subsequent differentiation in human fetal neuronal cells by simulation of the cyclic AMP pathway. Neuroscience 2000, 95, 859–868. [Google Scholar] [CrossRef]
- Lehnert, B.E.; Goodwin, E.H. Extracellular factor(s) following exposure to alpha particles can cause sister chromatid exchanges in normal human cells. Cancer Res. 1997, 57, 2164–2171. [Google Scholar] [PubMed]
- Mothersill, C.; Seymour, C.B. Radiation-induced bystander effects-implications for cancer. Nat. Rev. Cancer 2001, 4, 158–164. [Google Scholar] [CrossRef]
- Merrifield, M.; Kovalchuk, O. Issue in low dose radiation biology: A new research frontier. Front. Genet. 2013, 4, 1–5. [Google Scholar] [CrossRef]
- Maeda, J.; Yurkon, C.R.; Fujii, Y.; Fujisawa, H.; Kato, S.; Brents, C.A.; Uesaka, M.; Fujimori, A.; Kitamura, H.; Kato, T. Solution radioactivated by hadron radiation can increase chromatid exchanges. PLoS ONE 2015, 10, e0144619. [Google Scholar] [CrossRef]
- Burtt, J.J.; Thompson, P.A.; Lafrenie, R.M. Non-targeted effects and radiation-induced carcinogenesis: A review. J. Radiol. Prot. 2016, 36, R23–R35. [Google Scholar] [CrossRef]
- Hall, E.J. The bystander effect. Health Phys. 2003, 85, 31–35. [Google Scholar] [CrossRef]
- Morgan, W.F. Non-targeted and delayed effects of exposure to ionizing radiation: I. Radiation-induced genomic instability and bystander effects in vitro. Radiat. Res. 2003, 159, 567–580. [Google Scholar] [CrossRef]
- Cucinotta, F.A.; Cacao, E. Non-targeted effects model predict significantly higher Mars mission cancer risk than targeted effects models. Sci. Rep. 2017, 7, 1832. [Google Scholar] [CrossRef] [PubMed]
- Matsuya, Y.; Sasaki, K.; Yoshii, Y.; Okuyama, G.; Date, H. Integrated modeling of cell responses after irradiation for DNA-targeted effects and non-targeted effects. Sci. Rep. 2018, 8, 4849. [Google Scholar] [CrossRef] [PubMed]
- George, K.; Willingham, V.; Cucinotta, F.A. Stability of chromosome aberrations in the blood lymphocytes of astronauts measured after space flight by FISH chromosome painting. Radiat. Res. 2005, 156, 731–738. [Google Scholar] [CrossRef]
- George, K.; Rhone, J.; Beitman, A.; Cucinotta, F.A. Cytogenetic damage in the blood lymphocytes of astronauts: Effect of repeat long-duration space missions. Mutat. Res. 2013, 756, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Ballarini, F.; Altieri, S.; Bortolussi, S.; Carante, M.; Giroletti, E.; Protti, N. The role of DNA cluster damage and chromosome aberrations in radiation-induced cell killing: A theoretical approach. Radiat. Prot. Dosim. 2015, 166, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Yannone, S.M.; Roy, S.; Chan, D.W.; Murphy, M.B.; Huang, S.; Campisi, J. Warner syndrome protein is regulated and phosphorylated by DNA dependent protein kinase. J. Biol. Chem. 2001, 276, 38242–38248. [Google Scholar] [CrossRef]
- La Tessa, C.; Sivertz, M.; Chiang, I.H.; Lowenstein, D.; Rusek, A. Overview of NASA space radiation laboratory. Life Sci. Space Res. 2016, 11, 18–23. [Google Scholar] [CrossRef]
- George, K.A.; Hada, M.; Elliott, T.; Kawata, T.; Pluth, J.M.; Cucinotta, F.A. Dose response of γ-rays and iron nuclei induction of chromosomal aberrations in normal and repair deficient cell lines. Radiat. Res. 2009, 171, 752–763. [Google Scholar] [CrossRef]
- Hada, M.; Huff, J.L.; Patel, Z.S.; Kawata, T.; Pluth, J.M.; George, K.A.; Cucinotta, F.A. AT cells are not radiosensitive for chromosomal exchanges at low dose. Mutat. Res. 2011, 716, 76–83. [Google Scholar] [CrossRef]
- Hada, M.; Meador, J.A.; Cucinotta, F.A.; Gonda, S.R.; Wu, H. Chromosome aberrations induced by dual exposure of protons and iron ions. Radiat. Environ. Biophys. 2007, 46, 125–129. [Google Scholar] [CrossRef]
- Luomahaara, S.; Lindholm, C.; Mustonen, R.; Salomaa, S. Distribution of radiation-induced exchange aberrations in human chromosomes 1, 2 and 4. Int. J. Radiat. Biol. 1999, 75, 1551–1556. [Google Scholar] [CrossRef] [PubMed]
- Lucas, J.N.; Poggensee, M.; Straume, T. The persistence of chromosome translocations in a radiation worker accidentally exposed to tritium. Cytogenet. Cell Genet. 1992, 60, 255–256. [Google Scholar] [CrossRef] [PubMed]




| Dose (Gy) | Treatment | Cells Scored (#) | Frequency of Chromosome Aberrations (%) | ||
|---|---|---|---|---|---|
| Simple | Complex | Total | |||
| (a) | |||||
| 0 | control | 3701 | 0.40 ± 0.16 | 0 | 0.40 ± 0.16 |
| 0 | cPTIO | 2546 | 0.29 ± 0.19 | 0 | 0.29 ± 0.19 |
| 0.2 | control | 549 | 10.83 ± 2.21 | 0.45 ± 0.45 | 11.28 ± 2.26 |
| 0.2 | cPTIO | 416 | 4.17 ± 1.58 | 0 | 4.17 ± 1.58 |
| (b) | |||||
| 0 | control | 3701 | 0.40 ± 0.16 | 0 | 0.40 ± 0.16 |
| 0 | Media transferred 1 | 657 | 3.77 ± 1.19 | 0.75 ± 0.53 | 4.53 ± 1.31 |
| 0 | Media transferred + Cptio 2 | 1038 | 0.72 ± 0.41 | 0.24 ± 0.24 | 0.95 ± 0.48 |
| 0 | Media transferred 3 | 1003 | 0.25 ± 0.25 | 0 | 0.25 ± 0.25 |
| Dose (Gy) | Hit Per Cell | Treatment | Cell Score’D | Frequency of Chromosome Aberrations | ||
|---|---|---|---|---|---|---|
| Simple | Complex | Total | ||||
| 0 | 0 | control | 3701 | 0.40 ± 0.16 | 0 | 0.40 ± 0.16 |
| 0 | 0 | cPTIO | 2546 | 0.29 ± 0.17 | 0 | 0.29 ± 0.17 |
| 0.02 | 0.12 | control | 2236 | 1.33 ± 0.38 | 0 | 1.33 ± 0.38 |
| 0.02 | 0.12 | cPTIO | 1075 | 2.31 ± 0.73 | 0 | 2.31 ± 0.73 |
| 0.04 | 0.23 | control | 1030 | 1.92 ± 0.68 | 0.24 ± 0.24 | 2.17 ± 0.72 |
| 0.04 | 0.23 | cPTIO | 4630 | 2.62 ± 0.37 | 0.54 ± 0.17 | 3.16 ± 0.41 |
| 0.06 | 0.35 | control | 1157 | 4.28 ± 0.96 | 0 | 5.78 ± 1.11 |
| 0.06 | 0.35 | cPTIO | 1035 | 1.44 ± 0.59 | 0.48 ± 0.34 | 1.92 ± 0.68 |
| 0.08 | 0.47 | control | 1341 | 4.07 ± 0.87 | 0.18 ± 0.18 | 4.25 ± 0.89 |
| 0.08 | 0.47 | cPTIO | 1130 | 2.63 ± 0.76 | 0.88 ± 0.44 | 3.51 ± 0.88 |
| 0.10 | 0.59 | control | 2212 | 5.71 ± 0.80 | 0.44 ± 0.22 | 6.16 ± 0.83 |
| 0.10 | 0.59 | cPTIO | 630 | 2.75 ± 1.04 | 1.18 ± 0.68 | 3.93 ± 1.24 |
| 0.20 | 1.17 | control | 2112 | 5.75 ± 0.82 | 1.06 ± 0.35 | 6.81 ± 0.89 |
| 0.20 | 1.17 | cPTIO | 2127 | 3.15 ± 0.61 | 1.28 ± 0.39 | 4.43 ± 0.72 |
| 0.40 | 2.34 | control | 497 | 16.95 ± 2.91 | 4.99 ± 1.58 | 21.94 ± 3.31 |
| 0.40 | 2.34 | cPTIO | 875 | 11.04 ± 1.77 | 0.85 ± 0.49 | 11.89 ± 1.84 |
| 0.80 | 4.69 | control | 197 | 49.06 ± 7.86 | 7.55 ± 3.08 | 56.60 ± 8.44 |
| 0.80 | 4.69 | cPTIO | 325 | 26.69 ± 4.51 | 9.91 ± 2.75 | 36.60 ± 5.28 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hada, M.; Saganti, P.B.; Cucinotta, F.A. Nitric Oxide Is Involved in Heavy Ion-Induced Non-Targeted Effects in Human Fibroblasts. Int. J. Mol. Sci. 2019, 20, 4327. https://doi.org/10.3390/ijms20184327
Hada M, Saganti PB, Cucinotta FA. Nitric Oxide Is Involved in Heavy Ion-Induced Non-Targeted Effects in Human Fibroblasts. International Journal of Molecular Sciences. 2019; 20(18):4327. https://doi.org/10.3390/ijms20184327
Chicago/Turabian StyleHada, Megumi, Premkumar B. Saganti, and Francis A. Cucinotta. 2019. "Nitric Oxide Is Involved in Heavy Ion-Induced Non-Targeted Effects in Human Fibroblasts" International Journal of Molecular Sciences 20, no. 18: 4327. https://doi.org/10.3390/ijms20184327
APA StyleHada, M., Saganti, P. B., & Cucinotta, F. A. (2019). Nitric Oxide Is Involved in Heavy Ion-Induced Non-Targeted Effects in Human Fibroblasts. International Journal of Molecular Sciences, 20(18), 4327. https://doi.org/10.3390/ijms20184327