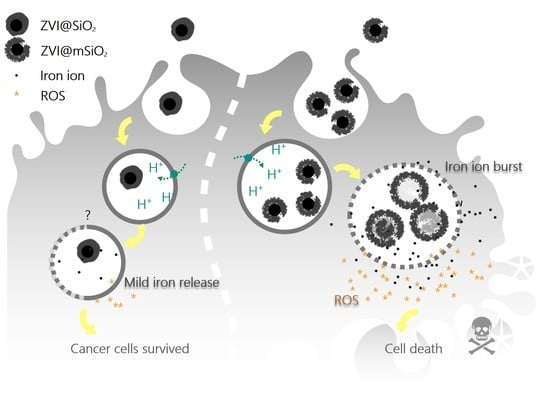
Iron Release Profile of Silica-Modified Zero-Valent Iron NPs and Their Implication in Cancer Therapy
Abstract
:1. Introduction
2. Results
2.1. Characterization of ZVI@SiO2 and ZVI@mSiO2 NPs
2.2. Different Shells of ZVI NPs Showed Distinct Cytotoxicity and ROS Induction Profile in the OEC-M1 Oral Cancer Cell Line
2.3. ZVI@mSiO2 NPs Showed Conformation Change within OEC-M1 Cells
2.4. Intracellular Iron Ion Burst Only Observed in ZVI@mSiO2 NP-treated Cells Through Lysosome Acidification
2.5. ZVI@mSiO2 Induced Necrosis and Apoptosis in Lysosome Acidification and ROS Dependent Manner
2.6. ZVI@mSiO2 NPs Inhibited Tumor Growth without Body Weight Loss
3. Discussion
4. Materials and Methods
4.1. Synthesis of ZVI@SiO2 and ZVI@mSiO2 NPs
4.2. TEM Characterization and EDS Analysis
4.3. Cell Culture
4.4. Cell Fixation and Embedding for TEM Imaging
4.5. Cell Viability Analysis
4.6. Intracellular Iron Release Assay
4.7. Intracellular ROS Analysis
4.8. Apoptosis and Necrosis Analysis
4.9. Anticancer Efficacy Evaluation In Vivo
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. C.A. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taccola, L.; Raffa, V.; Riggio, C.; Vittorio, O.; Iorio, M.C.; Vanacore, R.; Pietrabissa, A.; Cuschieri, A. Zinc oxide nanoparticles as selective killers of proliferating cells. Int. J. Nanomed. 2011, 6, 1129–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasmussen, J.W.; Martinez, E.; Louka, P.; Wingett, D.G. Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Expert Opin. Drug Deliv. 2010, 7, 1063–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.N.; Chen, D.H.; Shi, X.Y.; Lian, C.C.; Wang, T.Y.; Yeh, C.S.; Ratinac, K.R.; Thordarson, P.; Braet, F.; Shieh, D.B. Cancer-cell-specific cytotoxicity of non-oxidized iron elements in iron core-gold shell NPs. Nanomedicine 2011, 7, 420–427. [Google Scholar] [CrossRef]
- Wu, Y.N.; Yang, L.X.; Shi, X.Y.; Li, I.C.; Biazik, J.M.; Ratinac, K.R.; Chen, D.H.; Thordarson, P.; Shieh, D.B.; Braet, F. The selective growth inhibition of oral cancer by iron core-gold shell nanoparticles through mitochondria-mediated autophagy. Biomaterials 2011, 32, 4565–4573. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.J.; Wei, Y.H.; Chiu, Y.C.; Wu, S.R.; Shieh, D.B. Assessment of zero-valent iron-based nanotherapeutics for ferroptosis induction and resensitization strategy in cancer cells. Biomater. Sci. 2019, 7, 1311–1322. [Google Scholar] [CrossRef]
- Xu, C.; Yuan, Z.; Kohler, N.; Kim, J.; Chung, M.A.; Sun, S. FePt nanoparticles as an Fe reservoir for controlled Fe release and tumor inhibition. J. Am. Chem. Soc. 2009, 131, 15346–15351. [Google Scholar] [CrossRef]
- Thurber, A.; Wingett, D.G.; Rasmussen, J.W.; Layne, J.; Johnson, L.; Tenne, D.A.; Zhang, J.; Hanna, C.B.; Punnoose, A. Improving the selective cancer killing ability of ZnO nanoparticles using Fe doping. Nanotoxicology 2012, 6, 440–452. [Google Scholar] [CrossRef]
- Putz, M.V.; Ionascu, C.; Putz, A.M.; Ostafe, V. Alert-QSAR. Implications for electrophilic theory of chemical carcinogenesis. Int. J. Mol. Sci. 2011, 12, 5098–5134. [Google Scholar] [CrossRef]
- Putz, M.V.; Duda-Seiman, C.; Duda-Seiman, D.; Putz, A.M.; Alexandrescu, I.; Mernea, M.; Avram, S. Chemical Structure-Biological Activity Models for Pharmacophores’ 3D-Interactions. Int. J. Mol. Sci. 2016, 17, 1087. [Google Scholar] [CrossRef]
- Ahamed, M.; Alhadlaq, H.A.; Khan, M.A.M.; Akhtar, M.J. Selective killing of cancer cells by iron oxide nanoparticles mediated through reactive oxygen species via p53 pathway. J. Nanopart Res. 2013, 15, 1225–1234. [Google Scholar] [CrossRef]
- Festjens, N.; Vanden Berghe, T.; Vandenabeele, P. Necrosis, a well-orchestrated form of cell demise: signalling cascades, important mediators and concomitant immune response. Biochim. Biophys Acta. 2006, 1757, 1371–1387. [Google Scholar] [CrossRef] [PubMed]
- Zong, W.X.; Thompson, C.B. Necrotic death as a cell fate. Gene Dev. 2006, 20, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fawcett, H.; Mader, J.S.; Robichaud, M.; Giacomantonio, C.; Hoskin, D.W. Contribution of reactive oxygen species and caspase-3 to apoptosis and attenuated ICAM-1 expression by paclitaxel-treated MDA-MB-435 breast carcinoma cells. Int. J. Oncol. 2005, 27, 1717–1726. [Google Scholar] [CrossRef] [PubMed]
- Simizu, S.; Takada, M.; Umezawa, K.; Imoto, M. Requirement of caspase-3(-like) protease-mediated hydrogen peroxide production for apoptosis induced by various anticancer drugs. J. Biol. Chem. 1998, 273, 26900–26907. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, X.; Sun, Y.; Liang, L.; Pan, B.; Zhang, W.; Guan, X. Advances in Sulfidation of Zerovalent Iron for Water Decontamination. Env. Sci. Technol. 2017, 51, 13533–13544. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C.; Care, S.; Crane, R. Nanoscale Metallic Iron for Environmental Remediation: Prospects and Limitations. Water Air Soil Pollut. 2012, 223, 1363–1382. [Google Scholar] [CrossRef] [PubMed]
- Gheju, M. Hexavalent Chromium Reduction with Zero-Valent Iron (ZVI) in Aquatic Systems. Water Air Soil Poll. 2011, 222, 103–148. [Google Scholar] [CrossRef]
- Ludwig, R.D.; Smyth, D.J.A.; Blowes, D.W.; Spink, L.E.; Wilkin, R.T.; Jewett, D.G.; Weisener, C.J. Treatment of Arsenic, Heavy Metals, and Acidity Using a Mixed ZVI-Compost PRB. Env. Sci. Technol. 2009, 43, 1970–1976. [Google Scholar] [CrossRef] [PubMed]
- Enami, S.; Sakamoto, Y.; Colussi, A.J. Fenton chemistry at aqueous interfaces. Proc. Natl. Acad. Sci. USA 2014, 111, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.G.; Dong, S.; Catalano, C.; Moore, R.; Liang, X.; Mekalanos, J.J. Generation of reactive oxygen species by lethal attacks from competing microbes. Proc. Natl. Acad. Sci. USA 2015, 112, 2181–2186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsueh, Y.H.; Tsai, P.H.; Lin, K.S.; Ke, W.J.; Chiang, C.L. Antimicrobial effects of zero-valent iron nanoparticles on gram-positive Bacillus strains and gram-negative Escherichia coli strains. J. Nanobiotechnology 2017, 15, 77. [Google Scholar] [CrossRef] [PubMed]
- Lefevre, E.; Bossa, N.; Wiesner, M.R.; Gunsch, C.K. A review of the environmental implications of in situ remediation by nanoscale zero valent iron (nZVI): Behavior, transport and impacts on microbial communities. Sci. Total Env. 2016, 565, 889–901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, F.; Li, L.; Chen, D. Mesoporous silica nanoparticles: synthesis, biocompatibility and drug delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef] [PubMed]
- Asefa, T.; Tao, Z. Biocompatibility of mesoporous silica nanoparticles. Chem. Res. Toxicol. 2012, 25, 2265–2284. [Google Scholar] [CrossRef] [PubMed]
- Uskokovic, V.; Pernal, S.; Wu, V.M. Earthicle: The Design of a Conceptually New Type of Particle. ACS Appl. Mater. Inter. 2017, 9, 1305–1321. [Google Scholar] [CrossRef]
- Anbouhi, T.S.; Esfidvajani, E.M.; Nemati, F.; Haghighat, S.; Sari, S.; Attar, F.; Pakaghideh, A.; Sohrabi, M.J.; Mousavi, S.E.; Falahati, M. Albumin binding, anticancer and antibacterial properties of synthesized zero valent iron nanoparticles. Int. J. Nanomed. 2019, 14, 243–256. [Google Scholar] [CrossRef]
- Wu, Y.N.; Wu, P.C.; Yang, L.X.; Ratinac, K.R.; Thordarson, P.; Jahn, K.A.; Chen, D.H.; Shieh, D.B.; Braet, F. The anticancer properties of iron core-gold shell nanoparticles in colorectal cancer cells. Int. J. Nanomed. 2013, 8, 3321–3331. [Google Scholar] [CrossRef]
- Wu, Y.N.; Shieh, D.B.; Yang, L.X.; Sheu, H.S.; Zheng, R.Z.; Thordarson, P.; Chen, D.H.; Braet, F. Characterization of Iron Core(-)Gold Shell Nanoparticles for Anti-Cancer Treatments: Chemical and Structural Transformations During Storage and Use. Materials 2018, 11, 2572. [Google Scholar] [CrossRef]
- Sabella, S.; Carney, R.P.; Brunetti, V.; Malvindi, M.A.; Al-Juffali, N.; Vecchio, G.; Janes, S.M.; Bakr, O.M.; Cingolani, R.; Stellacci, F.; et al. A general mechanism for intracellular toxicity of metal-containing nanoparticles. Nanoscale 2014, 6, 7052–7061. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.I.; Brown, R.N.; Kempel, L.C.; Kofinas, P. Controlled synthesis of core-shell iron-silica nanoparticles and their magneto-dielectric properties in polymer composites. Nanotechnology 2011, 22, 105601. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S.; Haynes, C.L. Impacts of mesoporous silica nanoparticle size, pore ordering, and pore integrity on hemolytic activity. J. Am. Chem. Soc. 2010, 132, 4834–4842. [Google Scholar] [CrossRef] [PubMed]
- Keenan, C.R.; Goth-Goldstein, R.; Lucas, D.; Sedlak, D.L. Oxidative stress induced by zero-valent iron nanoparticles and Fe(II) in human bronchial epithelial cells. Env. Sci. Technol. 2009, 43, 4555–4560. [Google Scholar] [CrossRef]
- Katsoyiannis, I.A.; Ruettimann, T.; Hug, S.J. pH dependence of Fenton reagent generation and As(III) oxidation and removal by corrosion of zero valent iron in aerated water. Env. Sci. Technol. 2008, 42, 7424–7430. [Google Scholar] [CrossRef] [PubMed]
- Misinzo, G.; Delputte, P.L.; Nauwynck, H.J. Inhibition of endosome-lysosome system acidification enhances porcine circovirus 2 infection of porcine epithelial cells. J. Virol. 2008, 82, 1128–1135. [Google Scholar] [CrossRef]
- Sun, Z.; Yang, L.; Chen, K.F.; Chen, G.W.; Peng, Y.P.; Chen, J.K.; Suo, G.; Yu, J.; Wang, W.C.; Lin, C.H. Nano zerovalent iron particles induce pulmonary and cardiovascular toxicity in an in vitro human co-culture model. Nanotoxicology 2016, 10, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Yu, M.; Meka, A.; Liu, Y.; Zhang, J.; Yang, Y.; Yu, C. Understanding the contribution of surface roughness and hydrophobic modification of silica nanoparticles to enhanced therapeutic protein delivery. J. Mater. Chem. B 2016, 4, 212–219. [Google Scholar] [CrossRef]
- Lin, Y.Z.; Epstein, D.L.; Liton, P.B. Intralysosomal Iron Induces Lysosomal Membrane Permeabilization and Cathepsin D-Mediated Cell Death in Trabecular Meshwork Cells Exposed to Oxidative Stress. Invest. Ophth. Vis. Sci. 2010, 51, 6483–6495. [Google Scholar] [CrossRef]
- Panzarini, E.; Mariano, S.; Carata, E.; Mura, F.; Rossi, M.; Dini, L. Intracellular Transport of Silver and Gold Nanoparticles and Biological Responses: An Update. Int. J. Mol. Sci. 2018, 19, 1305. [Google Scholar] [CrossRef]
- Feng, Q.Y.; Liu, Y.P.; Huang, J.; Chen, K.; Huang, J.X.; Xiao, K. Uptake, distribution, clearance, and toxicity of iron oxide nanoparticles with different sizes and coatings. Sci. Rep. 2018, 8, 2082. [Google Scholar] [CrossRef]
- Gao, F.; Ma, N.; Zhou, H.; Wang, Q.; Zhang, H.; Wang, P.; Hou, H.; Wen, H.; Li, L. Zinc oxide nanoparticles-induced epigenetic change and G2/M arrest are associated with apoptosis in human epidermal keratinocytes. Int. J. Nanomed. 2016, 11, 3859–3874. [Google Scholar] [CrossRef]
- Belade, E.; Armand, L.; Martinon, L.; Kheuang, L.; Fleury-Feith, J.; Baeza-Squiban, A.; Lanone, S.; Billon-Galland, M.A.; Pairon, J.C.; Boczkowski, J. A comparative transmission electron microscopy study of titanium dioxide and carbon black nanoparticles uptake in human lung epithelial and fibroblast cell lines. Toxicol. In Vitro 2012, 26, 57–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heneweer, C.; Holland, J.P.; Divilov, V.; Carlin, S.; Lewis, J.S. Magnitude of Enhanced Permeability and Retention Effect in Tumors with Different Phenotypes: Zr-89-Albumin as a Model System. J. Nucl. Med. 2011, 52, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Yang, C.Y.; Meng, C.L. Regulation of PG synthase by EGF and PDGF in human oral, breast, stomach, and fibrosarcoma cancer cell lines. J. Dent. Res. 1994, 73, 1407–1415. [Google Scholar] [CrossRef] [PubMed]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.-X.; Wu, Y.-N.; Wang, P.-W.; Su, W.-C.; Shieh, D.-B. Iron Release Profile of Silica-Modified Zero-Valent Iron NPs and Their Implication in Cancer Therapy. Int. J. Mol. Sci. 2019, 20, 4336. https://doi.org/10.3390/ijms20184336
Yang L-X, Wu Y-N, Wang P-W, Su W-C, Shieh D-B. Iron Release Profile of Silica-Modified Zero-Valent Iron NPs and Their Implication in Cancer Therapy. International Journal of Molecular Sciences. 2019; 20(18):4336. https://doi.org/10.3390/ijms20184336
Chicago/Turabian StyleYang, Li-Xing, Ya-Na Wu, Pei-Wen Wang, Wu-Chou Su, and Dar-Bin Shieh. 2019. "Iron Release Profile of Silica-Modified Zero-Valent Iron NPs and Their Implication in Cancer Therapy" International Journal of Molecular Sciences 20, no. 18: 4336. https://doi.org/10.3390/ijms20184336
APA StyleYang, L. -X., Wu, Y. -N., Wang, P. -W., Su, W. -C., & Shieh, D. -B. (2019). Iron Release Profile of Silica-Modified Zero-Valent Iron NPs and Their Implication in Cancer Therapy. International Journal of Molecular Sciences, 20(18), 4336. https://doi.org/10.3390/ijms20184336