Roles of Extracellular HSPs as Biomarkers in Immune Surveillance and Immune Evasion
Abstract
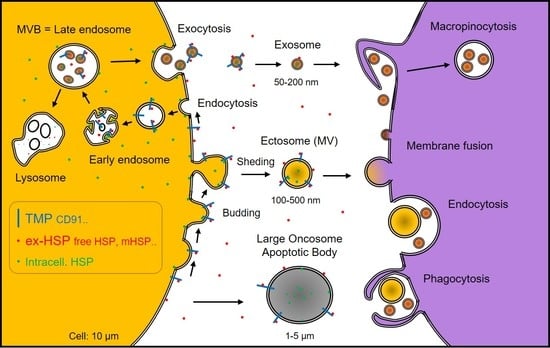
:1. Introduction
1.1. Roles of ex-HSP in Cancer Progression vs. the Host Immune System
1.2. HSP90 and Co-Chaperones
1.3. Inducibility of HSPs
1.4. Table of Contents
2. Resistance-Associated Secretory Phenotype (RASP)
2.1. HSP-Rich, Oncoprotein-Rich EVs
2.2. Ejection of Drugs and Antibodies with HSP-EVs
2.3. Release of Redundant Toxic Lipids
3. Immunomodulatory Roles of ex-HSP
3.1. Immunogenic Immunostimulatory Roles of ex-HSP
3.2. Anti-Inflammatory, Immunotolerant Roles of ex-HSP
4. Receptors for ex-HSP and HSP Peptide Complex
4.1. CD91/LRP1/A2MR
4.2. Toll-Like Receptors (TLRs)
4.3. SREC-1
4.4. CD94, Killer Cell Receptor
5. Inducible Mechanisms for HSPs
6. HSPs as Biomarkers Detectable by Liquid Biopsies
6.1. Serum HSP Biomarkers
6.2. Serum Antibodies against HSP (60, 70, and 90)
6.3. Cancer Liquid Biopsies and HSPs
7. HSP-Targeted Therapies
7.1. Clinical Trials of HSP90 Inhibitors
7.2. Potential Limitations of HSP90 Inhibitors
7.3. HSP70 Inhibitors
7.4. Anti-mHSP70 Antibody
7.5. HSF1 Inhibitors
7.6. HSP40 Inhibitors
7.7. HSP110 Inhibitor
7.8. HSP27 Inhibitors
7.9. HSP mRNA-Targeted Therapy
7.10. Nano-Vesicles as Potential DDS
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 17-AAG | 17-allylamino-17-demethoxygeldanamycin |
| A2MR | Alpha 2 macroglobulin receptor |
| AAb | Autoantibody |
| ABC | ATP-binding cassette |
| APC | Antigen-presenting cell |
| BiP | Binding immunoglobulin protein |
| CAF | Cancer-associated fibroblast |
| CBX | Chromobox protein |
| CDC37 | Cell division control 37 |
| CDK | Cyclin-dependent kinase |
| CIC | Cancer-initiating cell |
| CML | Chronic myelogenous leukemia |
| CR | Complete response |
| CRC | Colorectal cancer |
| CRPC | Castration-resistant prostate cancer |
| CSC | Cancer stem cell |
| CTC | Circulating tumor cell |
| ctDNA | Circulating tumor DNA |
| CTGF | Connective tissue growth factor |
| CTL | Cytotoxic T-lymphocyte |
| CXC | Cysteine-X-cysteine motif |
| DAMP | Damage-associated molecular pattern, danger-associated molecular pattern |
| DM | Diabetes mellitus |
| EGFR | Epidermal growth factor receptor |
| EMT | Epithelial to mesenchymal transition |
| EV | Extracellular vesicle |
| EV-Hsp | Extracellular vesicle-associated heat shock protein |
| ex-Hsp | Extracellular HSP |
| FcR | Fragment-crystallizable receptor |
| GP96 | Glycoprotein 96 |
| GRP | Glucose-regulated protein |
| HIF | Hypoxia-inducible factor |
| HP1 | Heterochromatin protein 1 |
| HSF | Heat shock factor |
| HSP | Heat shock protein |
| ILV | Intra-luminal vesicle |
| IRAK | IL-1 receptor-associated kinase |
| LPS | Lipopolysaccharide |
| LRP1 | Low-density lipoprotein receptor-related protein 1 |
| MDSC | Myeloid-derived suppressor cells |
| MHC | Major histocompatibility complex |
| MMP | Matrix metalloproteinase |
| MSC | Mesenchymal stem cell |
| mTOR | Mammalian target of rapamycin |
| MV | Microvesicle |
| MVB | Multi-vesicular body |
| Myd88 | Myeloid differentiation 88 |
| MZF1 | Myeloid zinc finger 1 |
| NK | Natural killer |
| NSCLC | Non-small-cell lung cancer |
| OncomiR | Oncogenic microRNA |
| ORP150 | Oxygen-regulated protein 150 |
| OSCC | Oral squamous cell carcinoma |
| PAMP | Pathogen-associated molecular pattern |
| PD-1 | Programmed cell death-1 |
| PD-L1 | Programmed cell death-ligand 1 |
| PI3K | Phosphatidylinositol 3-kinases |
| POC | Proof of concept |
| RA | Rheumatoid arthritis |
| RASP | Resistance-associated secretory phenotype |
| RCC | Renal cell carcinoma |
| SCAN | SREZBP-CTfin51-AW1-Number 18 cDNA |
| SCC | Squamous cell carcinoma |
| SR | Scavenger receptor |
| SREC | Scavenger receptor expressed by endothelial cells-1 |
| TAA | Tumor-associated antigen |
| TAITN | Tumor angiogenic inhibition triggered necrosis |
| TLR | Toll-like receptor |
| TRAP-1 | TNF receptor-associated protein-1 |
| Treg | Regulatory T cells |
| Tumoroid | Tumor organoid |
References
- Ono, K.; Eguchi, T.; Sogawa, C.; Calderwood, S.K.; Futagawa, J.; Kasai, T.; Seno, M.; Okamoto, K.; Sasaki, A.; Kozaki, K.I. HSP-enriched properties of extracellular vesicles involve survival of metastatic oral cancer cells. J. Cell. Biochem. 2018. [Google Scholar] [CrossRef]
- Eguchi, T.; Lang, B.J.; Murshid, A.; Prince, T.; Gong, J.; Calderwood, S.K. Regulatory roles for Hsp70 in cancer incidence and tumor progression. In Frontiers in Structural Biology; Galigniana, M.D., Ed.; Bentham Science: Sharjah, UAE, 2018; Volume 1, pp. 1–22. [Google Scholar]
- Murshid, A.; Eguchi, T.; Calderwood, S.K. Stress proteins in aging and life span. Int. J. Hyperth. 2013, 29, 442–447. [Google Scholar] [CrossRef] [Green Version]
- Neckers, L.; Blagg, B.; Haystead, T.; Trepel, J.B.; Whitesell, L.; Picard, D. Methods to validate Hsp90 inhibitor specificity, to identify off-target effects, and to rethink approaches for further clinical development. Cell Stress Chaperones 2018, 23, 467–482. [Google Scholar] [CrossRef]
- Eguchi, T.; Sogawa, C.; Okusha, Y.; Uchibe, K.; Iinuma, R.; Ono, K.; Nakano, K.; Murakami, J.; Itoh, M.; Arai, K.; et al. Organoids with Cancer Stem Cell-like Properties Secrete Exosomes and HSP90 in a 3D NanoEnvironment. PLoS ONE 2018, 13, e0191109. [Google Scholar] [CrossRef]
- Clayton, A.; Turkes, A.; Navabi, H.; Mason, M.D.; Tabi, Z. Induction of heat shock proteins in B-cell exosomes. J. Cell Sci. 2005, 118, 3631–3638. [Google Scholar] [CrossRef] [Green Version]
- Fujiwara, T.; Eguchi, T.; Sogawa, C.; Ono, K.; Murakami, J.; Ibaragi, S.; Asaumi, J.-I.; Calderwood, S.K.; Okamoto, K.; Kozaki, K.-I. Carcinogenic epithelial-mesenchymal transition initiated by oral cancer exosomes is inhibited by anti-EGFR antibody Cetuximab. Oral Oncol. 2018, 86, 251–257. [Google Scholar] [CrossRef]
- Reddy, V.S.; Madala, S.K.; Trinath, J.; Reddy, G.B. Extracellular small heat shock proteins: Exosomal biogenesis and function. Cell Stress Chaperones 2018, 23, 441–454. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Guan, S.; Fan, J.; Cheng, C.F.; Bright, A.M.; Chinn, C.; Chen, M.; Woodley, D.T. Extracellular heat shock protein-90alpha: Linking hypoxia to skin cell motility and wound healing. EMBO J. 2007, 26, 1221–1233. [Google Scholar] [CrossRef]
- Manzo, G. Natural killer cell reactivity: Activation and cytolysis mechanism models, involving heat shock protein, haemopoietic histocompatibility, major histocompatibility complex and complement molecules. Med. Hypotheses 1998, 51, 5–9. [Google Scholar] [CrossRef]
- Bottger, E.; Multhoff, G.; Kun, J.F.; Esen, M. Plasmodium falciparum-infected erythrocytes induce granzyme B by NK cells through expression of host-Hsp70. PLoS ONE 2012, 7, e33774. [Google Scholar] [CrossRef]
- Milani, V.; Noessner, E.; Ghose, S.; Kuppner, M.; Ahrens, B.; Scharner, A.; Gastpar, R.; Issels, R.D. Heat shock protein 70: Role in antigen presentation and immune stimulation. Int. J. Hyperth. 2002, 18, 563–575. [Google Scholar] [CrossRef]
- Tauro, B.J.; Greening, D.W.; Mathias, R.A.; Mathivanan, S.; Ji, H.; Simpson, R.J. Two distinct populations of exosomes are released from LIM1863 colon carcinoma cell-derived organoids. Mol. Cell. Proteom. 2013, 12, 587–598. [Google Scholar] [CrossRef]
- Smith, K.J.; Twal, W.O.; Soodavar, F.; Virella, G.; Lopes-Virella, M.F.; Hammad, S.M. Heat shock protein 70B′ (HSP70B′) expression and release in response to human oxidized low density lipoprotein immune complexes in macrophages. J. Biol. Chem. 2010, 285, 15985–15993. [Google Scholar] [CrossRef]
- Gunther, S.; Ostheimer, C.; Stangl, S.; Specht, H.M.; Mozes, P.; Jesinghaus, M.; Vordermark, D.; Combs, S.E.; Peltz, F.; Jung, M.P.; et al. Correlation of Hsp70 Serum Levels with Gross Tumor Volume and Composition of Lymphocyte Subpopulations in Patients with Squamous Cell and Adeno Non-Small Cell Lung Cancer. Front. Immunol. 2015, 6, 556. [Google Scholar] [CrossRef] [Green Version]
- Radons, J.; Multhoff, G. Immunostimulatory functions of membrane-bound and exported heat shock protein 70. Exerc. Immunol. Rev. 2005, 11, 17–33. [Google Scholar]
- Ogawa, K.; Kim, H.K.; Shimizu, T.; Abe, S.; Shiga, Y.; Calderwood, S.K. Plasma heat shock protein 72 as a biomarker of sarcopenia in elderly people. Cell Stress Chaperones 2012, 17, 349–359. [Google Scholar] [CrossRef]
- Noonan, E.J.; Fournier, G.; Hightower, L.E. Surface expression of Hsp70B′ in response to proteasome inhibition in human colon cells. Cell Stress Chaperones 2008, 13, 105–110. [Google Scholar] [CrossRef]
- Noonan, E.J.; Place, R.F.; Rasoulpour, R.J.; Giardina, C.; Hightower, L.E. Cell number-dependent regulation of Hsp70B’ expression: Evidence of an extracellular regulator. J. Cell Physiol. 2007, 210, 201–211. [Google Scholar] [CrossRef]
- Ohtsuka, K.; Hata, M. Molecular chaperone function of mammalian Hsp70 and Hsp40—A review. Int. J. Hyperth. 2000, 16, 231–245. [Google Scholar] [CrossRef]
- Hattori, T.; Takahash, K.; Yutani, Y.; Fujisawa, T.; Nakanishi, T.; Takigawa, M. Rheumatoid arthritis-related antigen 47kDa (RA-A47) is a product of colligin-2 and acts as a human HSP47. J. Bone Miner. Metab. 2000, 18, 328–334. [Google Scholar] [CrossRef]
- Hattori, T.; von der Mark, K.; Kawaki, H.; Yutani, Y.; Kubota, S.; Nakanishi, T.; Eberspaecher, H.; de Crombrugghe, B.; Takigawa, M. Downregulation of rheumatoid arthritis-related antigen RA-A47 (HSP47/colligin-2) in chondrocytic cell lines induces apoptosis and cell-surface expression of RA-A47 in association with CD9. J. Cell. Physiol. 2005, 202, 191–204. [Google Scholar] [CrossRef]
- Caruso Bavisotto, C.; Cappello, F.; Macario, A.J.L.; Conway de Macario, E.; Logozzi, M.; Fais, S.; Campanella, C. Exosomal HSP60: A potentially useful biomarker for diagnosis, assessing prognosis, and monitoring response to treatment. Expert Rev. Mol. Diagn. 2017, 17, 815–822. [Google Scholar] [CrossRef]
- Juwono, J.; Martinus, R.D. Does Hsp60 Provide a Link between Mitochondrial Stress and Inflammation in Diabetes Mellitus? J. Diabetes Res. 2016, 2016, 8017571. [Google Scholar] [CrossRef]
- Tai, Y.L.; Chen, K.C.; Hsieh, J.T.; Shen, T.L. Exosomes in cancer development and clinical applications. Cancer Sci. 2018, 109, 2364–2374. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.; Zou, M.; Bhatia, A.; Jayaprakash, P.; Hofman, F.; Ying, Q.; Chen, M.; Woodley, D.T.; Li, W. Breast Cancer MDA-MB-231 Cells Use Secreted Heat Shock Protein-90alpha (Hsp90alpha) to Survive a Hostile Hypoxic Environment. Sci. Rep. 2016, 6, 20605. [Google Scholar] [CrossRef]
- Tsen, F.; Bhatia, A.; O’Brien, K.; Cheng, C.F.; Chen, M.; Hay, N.; Stiles, B.; Woodley, D.T.; Li, W. Extracellular heat shock protein 90 signals through subdomain II and the NPVY motif of LRP-1 receptor to Akt1 and Akt2: A circuit essential for promoting skin cell migration in vitro and wound healing in vivo. Mol. Cell. Biol. 2013, 33, 4947–4959. [Google Scholar] [CrossRef]
- Najafi, M.; Goradel, N.H.; Farhood, B.; Salehi, E.; Solhjoo, S.; Toolee, H.; Kharazinejad, E.; Mortezaee, K. Tumor microenvironment: Interactions and therapy. J. Cell. Physiol. 2019, 234, 5700–5721. [Google Scholar] [CrossRef]
- Hance, M.W.; Dole, K.; Gopal, U.; Bohonowych, J.E.; Jezierska-Drutel, A.; Neumann, C.A.; Liu, H.; Garraway, I.P.; Isaacs, J.S. Secreted Hsp90 is a novel regulator of the epithelial to mesenchymal transition (EMT) in prostate cancer. J. Biol. Chem. 2012, 287, 37732–37744. [Google Scholar] [CrossRef]
- Nolan, K.D.; Franco, O.E.; Hance, M.W.; Hayward, S.W.; Isaacs, J.S. Tumor-secreted Hsp90 subverts polycomb function to drive prostate tumor growth and invasion. J. Biol. Chem. 2015, 290, 8271–8282. [Google Scholar] [CrossRef]
- Nagaraju, G.P.; Long, T.E.; Park, W.; Landry, J.C.; Taliaferro-Smith, L.; Farris, A.B.; Diaz, R.; El-Rayes, B.F. Heat shock protein 90 promotes epithelial to mesenchymal transition, invasion, and migration in colorectal cancer. Mol. Carcinog. 2015, 54, 1147–1158. [Google Scholar] [CrossRef]
- Nolan, K.D.; Kaur, J.; Isaacs, J.S. Secreted heat shock protein 90 promotes prostate cancer stem cell heterogeneity. Oncotarget 2017, 8, 19323–19341. [Google Scholar] [CrossRef]
- Wiersma, V.R.; Michalak, M.; Abdullah, T.M.; Bremer, E.; Eggleton, P. Mechanisms of Translocation of ER Chaperones to the Cell Surface and Immunomodulatory Roles in Cancer and Autoimmunity. Front. Oncol. 2015, 5, 7. [Google Scholar] [CrossRef]
- Hoos, A.; Levey, D.L. Vaccination with heat shock protein-peptide complexes: From basic science to clinical applications. Expert Rev. Vaccines 2003, 2, 369–379. [Google Scholar] [CrossRef]
- Blachere, N.E.; Udono, H.; Janetzki, S.; Li, Z.; Heike, M.; Srivastava, P.K. Heat shock protein vaccines against cancer. J. Immunother. Emphas. Tumor Immunol. 1993, 14, 352–356. [Google Scholar] [CrossRef]
- Srivastava, P.K.; Udono, H. Heat shock protein-peptide complexes in cancer immunotherapy. Curr. Opin. Immunol. 1994, 6, 728–732. [Google Scholar] [CrossRef]
- Blachere, N.E.; Li, Z.; Chandawarkar, R.Y.; Suto, R.; Jaikaria, N.S.; Basu, S.; Udono, H.; Srivastava, P.K. Heat shock protein-peptide complexes, reconstituted in vitro, elicit peptide-specific cytotoxic T lymphocyte response and tumor immunity. J. Exp. Med. 1997, 186, 1315–1322. [Google Scholar] [CrossRef]
- Neckers, L.; Workman, P. Hsp90 molecular chaperone inhibitors: Are we there yet? Clin. Cancer Res. 2012, 18, 64–76. [Google Scholar] [CrossRef]
- Trepel, J.; Mollapour, M.; Giaccone, G.; Neckers, L. Targeting the dynamic HSP90 complex in cancer. Nat. Rev. Cancer 2010, 10, 537–549. [Google Scholar] [CrossRef] [Green Version]
- Saini, J.; Sharma, P.K. Clinical, Prognostic and Therapeutic Significance of Heat Shock Proteins in Cancer. Curr. Drug Targets 2018, 19, 1478–1490. [Google Scholar] [CrossRef]
- Dimas, D.T.; Perlepe, C.D.; Sergentanis, T.N.; Misitzis, I.; Kontzoglou, K.; Patsouris, E.; Kouraklis, G.; Psaltopoulou, T.; Nonni, A. The Prognostic Significance of Hsp70/Hsp90 Expression in Breast Cancer: A Systematic Review and Meta-analysis. Anticancer Res. 2018, 38, 1551–1562. [Google Scholar] [CrossRef]
- Haque, A.; Alam, Q.; Alam, M.Z.; Azhar, E.I.; Sait, K.H.; Anfinan, N.; Mushtaq, G.; Kamal, M.A.; Rasool, M. Current Understanding of HSP90 as a Novel Therapeutic Target: An Emerging Approach for the Treatment of Cancer. Curr. Pharm. Des. 2016, 22, 2947–2959. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Jin, F.; Wang, Y.; Zheng, D.; Liu, J.; Zhang, Z.; Wang, R.; Dong, D.; Zheng, K.; Wang, Y. Hsp90 inhibitor AT-533 blocks HSV-1 nuclear egress and assembly. J. Biochem. 2018, 164, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jin, F.; Wang, R.; Li, F.; Wu, Y.; Kitazato, K.; Wang, Y. HSP90: A promising broad-spectrum antiviral drug target. Arch. Virol. 2017, 162, 3269–3282. [Google Scholar] [CrossRef]
- Kinnaird, J.H.; Singh, M.; Gillan, V.; Weir, W.; Calder, E.D.; Hostettler, I.; Tatu, U.; Devaney, E.; Shiels, B.R. Characterization of HSP90 isoforms in transformed bovine leukocytes infected with Theileria annulata. Cell. Microbiol. 2017, 19. [Google Scholar] [CrossRef] [PubMed]
- Bukong, T.N.; Momen-Heravi, F.; Kodys, K.; Bala, S.; Szabo, G. Exosomes from hepatitis C infected patients transmit HCV infection and contain replication competent viral RNA in complex with Ago2-miR122-HSP90. PLoS Pathog. 2014, 10, e1004424. [Google Scholar] [CrossRef] [PubMed]
- Ocana, G.J.; Sims, E.K.; Watkins, R.A.; Ragg, S.; Mather, K.J.; Oram, R.A.; Mirmira, R.G.; DiMeglio, L.A.; Blum, J.S.; Evans-Molina, C. Analysis of serum Hsp90 as a potential biomarker of beta cell autoimmunity in type 1 diabetes. PLoS ONE 2019, 14, e0208456. [Google Scholar] [CrossRef] [PubMed]
- Tukaj, S.; Wegrzyn, G. Anti-Hsp90 therapy in autoimmune and inflammatory diseases: A review of preclinical studies. Cell Stress Chaperones 2016, 21, 213–218. [Google Scholar] [CrossRef]
- Tukaj, S.; Zillikens, D.; Kasperkiewicz, M. Heat shock protein 90: A pathophysiological factor and novel treatment target in autoimmune bullous skin diseases. Exp. Dermatol. 2015, 24, 567–571. [Google Scholar] [CrossRef]
- Preuss, K.D.; Pfreundschuh, M.; Weigert, M.; Fadle, N.; Regitz, E.; Kubuschok, B. Sumoylated HSP90 is a dominantly inherited plasma cell dyscrasias risk factor. J. Clin. Investig. 2015, 125, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Shukla, H.D.; Pitha, P.M. Role of hsp90 in systemic lupus erythematosus and its clinical relevance. Autoimmune Dis. 2012, 2012, 728605. [Google Scholar] [CrossRef]
- He, D.; Song, X.; Li, L. Geranylgeranylacetone protects against cerebral ischemia and reperfusion injury: HSP90 and eNOS phosphorylation involved. Brain Res. 2015, 1599, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Businaro, R.; Profumo, E.; Tagliani, A.; Buttari, B.; Leone, S.; D’Amati, G.; Ippoliti, F.; Leopizzi, M.; D’Arcangelo, D.; Capoano, R.; et al. Heat-shock protein 90: A novel autoantigen in human carotid atherosclerosis. Atherosclerosis 2009, 207, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Kramer, J.; Harcos, P.; Prohaszka, Z.; Horvath, L.; Karadi, I.; Singh, M.; Csaszar, A.; Romics, L.; Fust, G. Frequencies of certain complement protein alleles and serum levels of anti-heat-shock protein antibodies in cerebrovascular diseases. Stroke 2000, 31, 2648–2652. [Google Scholar] [CrossRef]
- Workman, P.; Burrows, F.; Neckers, L.; Rosen, N. Drugging the cancer chaperone HSP90: Combinatorial therapeutic exploitation of oncogene addiction and tumor stress. Ann. N. Y. Acad. Sci. 2007, 1113, 202–216. [Google Scholar] [CrossRef] [PubMed]
- Calderwood, S.K.; Khaleque, M.A.; Sawyer, D.B.; Ciocca, D.R. Heat shock proteins in cancer: Chaperones of tumorigenesis. Trends Biochem. Sci. 2006, 31, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Yano, M.; Naito, Z.; Tanaka, S.; Asano, G. Expression and roles of heat shock proteins in human breast cancer. Jpn. J. Cancer Res. 1996, 87, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Marchenko, N.D.; Schulz, R.; Fischer, V.; Velasco-Hernandez, T.; Talos, F.; Moll, U.M. Functional inactivation of endogenous MDM2 and CHIP by HSP90 causes aberrant stabilization of mutant p53 in human cancer cells. Mol. Cancer Res. 2011, 9, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Shintani, S.; Zhang, T.; Aslam, A.; Sebastian, K.; Yoshimura, T.; Hamakawa, H. P53-dependent radiosensitizing effects of Hsp90 inhibitor 17-allylamino-17-demethoxygeldanamycin on human oral squamous cell carcinoma cell lines. Int. J. Oncol. 2006, 29, 1111–1117. [Google Scholar] [CrossRef]
- Peng, X.; Guo, X.; Borkan, S.C.; Bharti, A.; Kuramochi, Y.; Calderwood, S.; Sawyer, D.B. Heat shock protein 90 stabilization of ErbB2 expression is disrupted by ATP depletion in myocytes. J. Biol. Chem. 2005, 280, 13148–13152. [Google Scholar] [CrossRef] [PubMed]
- Lauwers, E.; Wang, Y.C.; Gallardo, R.; Van der Kant, R.; Michiels, E.; Swerts, J.; Baatsen, P.; Zaiter, S.S.; McAlpine, S.R.; Gounko, N.V.; et al. Hsp90 Mediates Membrane Deformation and Exosome Release. Mol. Cell 2018, 71, 689–702. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, T.; Prince, T.L.; Tran, M.T.; Sogawa, C.; Lang, B.J.; Calderwood, S.K. MZF1 and SCAND1 Reciprocally Regulate CDC37 Gene Expression in Prostate Cancer. Cancers 2019, 11, 792. [Google Scholar] [CrossRef] [PubMed]
- Calderwood, S.K. Cdc37 as a co-chaperone to Hsp90. Subcell. Biochem. 2015, 78, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Gray, P.J.; Prince, T.; Cheng, J.; Stevenson, M.A.; Calderwood, S.K. Targeting the oncogene and kinome chaperone CDC37. Nat. Rev. Cancer 2008, 8, 491–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calderwood, S.K.; Repasky, E.A.; Neckers, L.; Hightower, L.E. The IXth CSSI international symposium on heat shock proteins in biology and medicine: Stress responses in health and disease: Alexandria Old Town, Alexandria, Virginia, November 10–13, 2018. Cell Stress Chaperones 2019. [Google Scholar] [CrossRef] [PubMed]
- Ciocca, D.R.; Arrigo, A.P.; Calderwood, S.K. Heat shock proteins and heat shock factor 1 in carcinogenesis and tumor development: An update. Arch. Toxicol. 2013, 87, 19–48. [Google Scholar] [CrossRef] [PubMed]
- Ciocca, D.; Clark, G.; Tandon, A.; Fuqua, S.; Welch, W.; McGuire, W. Heat shock protein hsp70 in patients with axillary lymph node-negative breast cancer: Prognostic implications. J. Natl. Cancer Inst. 1993, 85, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, T.; Calderwood, S.K.; Takigawa, M.; Kubota, S.; Kozaki, K.I. Intracellular MMP3 Promotes HSP Gene Expression in Collaboration with Chromobox Proteins. J. Cell. Biochem. 2017, 118, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Saunderson, S.C.; McLellan, A.D. Role of Lymphocyte Subsets in the Immune Response to Primary B Cell-Derived Exosomes. J. Immunol. 2017, 199, 2225–2235. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, T.; Prince, T.; Wegiel, B.; Calderwood, S.K. Role and Regulation of Myeloid Zinc Finger Protein 1 in Cancer. J. Cell. Biochem. 2015, 116, 2146–2154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rak, J.; Guha, A. Extracellular vesicles—Vehicles that spread cancer genes. Bioessays 2012, 34, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Eguchi, T.; Sogawa, C.; Ono, K.; Murakami, J.; Ibaragi, S.; Asaumi, J.; Okamoto, K.; Calderwood, S.; Kozaki, K. Anti-EGFR antibody Cetuximab is secreted by oral squamous cell carcinoma and alters EGF-driven mesenchymal transition. Biochem. Biophys. Res. Commun. 2018, 503, 1267–1272. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.H.; Wan, Y.L.; Lin, Y.; Zhang, W.; Yang, M.; Li, G.L.; Lin, H.M.; Shang, C.Z.; Chen, Y.J.; Min, J. Anticancer drugs cause release of exosomes with heat shock proteins from human hepatocellular carcinoma cells that elicit effective natural killer cell antitumor responses in vitro. J. Biol. Chem. 2012, 287, 15874–15885. [Google Scholar] [CrossRef] [PubMed]
- Namba, Y.; Sogawa, C.; Okusha, Y.; Kawai, H.; Itagaki, M.; Ono, K.; Murakami, J.; Aoyama, E.; Ohyama, K.; Asaumi, J.I.; et al. Depletion of Lipid Efflux Pump ABCG1 Triggers the Intracellular Accumulation of Extracellular Vesicles and Reduces Aggregation and Tumorigenesis of Metastatic Cancer Cells. Front. Oncol. 2018, 8, 376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Udono, H.; Srivastava, P.K. Heat shock protein 70-associated peptides elicit specific cancer immunity. J. Exp. Med. 1993, 178, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- Osterloh, A.; Breloer, M. Heat shock proteins: Linking danger and pathogen recognition. Med. Microbiol. Immunol. 2008, 197, 1–8. [Google Scholar] [CrossRef]
- van Eden, W.; Jansen, M.A.A.; Ludwig, I.; van Kooten, P.; van der Zee, R.; Broere, F. The Enigma of Heat Shock Proteins in Immune Tolerance. Front. Immunol. 2017, 8, 1599. [Google Scholar] [CrossRef] [PubMed]
- Alard, J.E.; Dueymes, M.; Youinou, P.; Jamin, C. Modulation of endothelial cell damages by anti-Hsp60 autoantibodies in systemic autoimmune diseases. Autoimmun. Rev. 2007, 6, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Torigoe, T.; Tamura, Y.; Sato, N. Heat shock proteins and immunity: Application of hyperthermia for immunomodulation. Int. J. Hyperth. 2009, 25, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Ueda, G.; Tamura, Y.; Hirai, I.; Kamiguchi, K.; Ichimiya, S.; Torigoe, T.; Hiratsuka, H.; Sunakawa, H.; Sato, N. Tumor-derived heat shock protein 70-pulsed dendritic cells elicit tumor-specific cytotoxic T lymphocytes (CTLs) and tumor immunity. Cancer Sci. 2004, 95, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.F.; Ren, W.; Rollins, L.; Pittman, P.; Shah, M.; Shen, L.; Gu, Q.; Strube, R.; Hu, F.; Chen, S.Y. A broadly applicable, personalized heat shock protein-mediated oncolytic tumor vaccine. Cancer Res. 2003, 63, 7321–7329. [Google Scholar]
- Srivastava, P.K.; Udono, H.; Blachere, N.E.; Li, Z. Heat shock proteins transfer peptides during antigen processing and CTL priming. Immunogenetics 1994, 39, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, V.; Coppa, J.; Carrabba, M.G.; Rivoltini, L.; Schiavo, M.; Regalia, E.; Mariani, L.; Camerini, T.; Marchiano, A.; Andreola, S.; et al. Vaccination with autologous tumor-derived heat-shock protein gp96 after liver resection for metastatic colorectal cancer. Clin. Cancer Res. 2003, 9, 3235–3245. [Google Scholar] [PubMed]
- Kurotaki, T.; Tamura, Y.; Ueda, G.; Oura, J.; Kutomi, G.; Hirohashi, Y.; Sahara, H.; Torigoe, T.; Hiratsuka, H.; Sunakawa, H.; et al. Efficient cross-presentation by heat shock protein 90-peptide complex-loaded dendritic cells via an endosomal pathway. J. Immunol. 2007, 179, 1803–1813. [Google Scholar] [CrossRef] [PubMed]
- Udono, H.; Srivastava, P.K. Comparison of tumor-specific immunogenicities of stress-induced proteins gp96, hsp90, and hsp70. J. Immunol. 1994, 152, 5398–5403. [Google Scholar]
- Kutomi, G.; Tamura, Y.; Okuya, K.; Yamamoto, T.; Hirohashi, Y.; Kamiguchi, K.; Oura, J.; Saito, K.; Torigoe, T.; Ogawa, S.; et al. Targeting to static endosome is required for efficient cross-presentation of endoplasmic reticulum-resident oxygen-regulated protein 150-peptide complexes. J. Immunol. 2009, 183, 5861–5869. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zheng, L. Tumor immunotherapy based on tumor-derived heat shock proteins (Review). Oncol. Lett. 2013, 6, 1543–1549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tosti, G.; Cocorocchio, E.; Pennacchioli, E.; Ferrucci, P.F.; Testori, A.; Martinoli, C. Heat-shock proteins-based immunotherapy for advanced melanoma in the era of target therapies and immunomodulating agents. Expert Opin. Biol. Ther. 2014, 14, 955–967. [Google Scholar] [CrossRef] [PubMed]
- Parmiani, G.; De Filippo, A.; Pilla, L.; Castelli, C.; Rivoltini, L. Heat shock proteins gp96 as immunogens in cancer patients. Int. J. Hyperth. 2006, 22, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Belli, F.; Testori, A.; Rivoltini, L.; Maio, M.; Andreola, G.; Sertoli, M.R.; Gallino, G.; Piris, A.; Cattelan, A.; Lazzari, I.; et al. Vaccination of metastatic melanoma patients with autologous tumor-derived heat shock protein gp96-peptide complexes: Clinical and immunologic findings. J. Clin. Oncol. 2002, 20, 4169–4180. [Google Scholar] [CrossRef] [PubMed]
- Caudill, M.M.; Li, Z. HSPPC-96: A personalised cancer vaccine. Expert Opin. Biol. Ther. 2001, 1, 539–547. [Google Scholar] [CrossRef]
- Srivastava, P.K. Therapeutic cancer vaccines. Curr. Opin. Immunol. 2006, 18, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Wood, C.G.; Mulders, P. Vitespen: A preclinical and clinical review. Future Oncol. 2009, 5, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Maki, R.G.; Livingston, P.O.; Lewis, J.J.; Janetzki, S.; Klimstra, D.; Desantis, D.; Srivastava, P.K.; Brennan, M.F. A phase I pilot study of autologous heat shock protein vaccine HSPPC-96 in patients with resected pancreatic adenocarcinoma. Dig. Dis. Sci. 2007, 52, 1964–1972. [Google Scholar] [CrossRef] [PubMed]
- Pilla, L.; Patuzzo, R.; Rivoltini, L.; Maio, M.; Pennacchioli, E.; Lamaj, E.; Maurichi, A.; Massarut, S.; Marchiano, A.; Santantonio, C.; et al. A phase II trial of vaccination with autologous, tumor-derived heat-shock protein peptide complexes Gp96, in combination with GM-CSF and interferon-alpha in metastatic melanoma patients. Cancer Immunol. Immunother. 2006, 55, 958–968. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Liu, H.L.; Zhang, X.R.; Xu, J.P.; Hu, W.K.; Liang, M.; Chen, S.Y.; Hu, F.; Chu, D.T. A phase I trial of intratumoral administration of recombinant oncolytic adenovirus overexpressing HSP70 in advanced solid tumor patients. Gene Ther. 2009, 16, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Gross, C.; Hansch, D.; Gastpar, R.; Multhoff, G. Interaction of heat shock protein 70 peptide with NK cells involves the NK receptor CD94. Biol. Chem. 2003, 384, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Yoshimura, K.; Matsui, H.; Shindo, Y.; Tamesa, T.; Tokumitsu, Y.; Hashimoto, N.; Tokuhisa, Y.; Sakamoto, K.; Sakai, K.; et al. Dendritic cells transfected with heat-shock protein 70 messenger RNA for patients with hepatitis C virus-related hepatocellular carcinoma: A phase 1 dose escalation clinical trial. Cancer Immunol. Immunother. 2015, 64, 1047–1056. [Google Scholar] [CrossRef]
- Sedlacek, A.L.; Kinner-Bibeau, L.B.; Binder, R.J. Phenotypically distinct helper NK cells are required for gp96-mediated anti-tumor immunity. Sci. Rep. 2016, 6, 29889. [Google Scholar] [CrossRef] [Green Version]
- van Eden, W.; Wendling, U.; Paul, L.; Prakken, B.; van Kooten, P.; van der Zee, R. Arthritis protective regulatory potential of self-heat shock protein cross-reactive T cells. Cell Stress Chaperones 2000, 5, 452–457. [Google Scholar] [CrossRef]
- Van Eden, W.; Wick, G.; Albani, S.; Cohen, I. Stress, heat shock proteins, and autoimmunity: How immune responses to heat shock proteins are to be used for the control of chronic inflammatory diseases. Ann. N. Y. Acad. Sci. 2007, 1113, 217–237. [Google Scholar] [CrossRef]
- Borges, T.J.; Wieten, L.; van Herwijnen, M.J.; Broere, F.; van der Zee, R.; Bonorino, C.; van Eden, W. The anti-inflammatory mechanisms of Hsp70. Front. Immunol. 2012, 3, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Eden, W.; van Herwijnen, M.; Wagenaar, J.; van Kooten, P.; Broere, F.; van der Zee, R. Stress proteins are used by the immune system for cognate interactions with anti-inflammatory regulatory T cells. FEBS Lett. 2013, 587, 1951–1958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Herwijnen, M.J.; Van Der Zee, R.; Van Eden, W.; Broere, F. Heat shock proteins can be targets of regulatory T cells for therapeutic intervention in rheumatoid arthritis. Int. J. Hyperth. 2013, 29, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Habich, C.; Baumgart, K.; Kolb, H.; Burkart, V. The receptor for heat shock protein 60 on macrophages is saturable, specific, and distinct from receptors for other heat shock proteins. J. Immunol. 2002, 168, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.F.; Fan, J.; Fedesco, M.; Guan, S.; Li, Y.; Bandyopadhyay, B.; Bright, A.M.; Yerushalmi, D.; Liang, M.; Chen, M.; et al. Transforming growth factor alpha (TGFalpha)-stimulated secretion of HSP90alpha: Using the receptor LRP-1/CD91 to promote human skin cell migration against a TGFbeta-rich environment during wound healing. Mol. Cell. Biol. 2008, 28, 3344–3358. [Google Scholar] [CrossRef] [PubMed]
- Kawata, K.; Kubota, S.; Eguchi, T.; Aoyama, E.; Moritani, N.H.; Kondo, S.; Nishida, T.; Takigawa, M. Role of LRP1 in transport of CCN2 protein in chondrocytes. J. Cell Sci. 2012, 125, 2965–2972. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.J.; Messmer, M.N.; Binder, R.J. Establishment of tumor-associated immunity requires interaction of heat shock proteins with CD91. Cancer Immunol. Res. 2014, 2, 217–228. [Google Scholar] [CrossRef]
- Ohashi, K.; Burkart, V.; Flohe, S.; Kolb, H. Cutting edge: Heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J. Immunol. 2000, 164, 558–561. [Google Scholar] [CrossRef]
- Vabulas, R.M.; Ahmad-Nejad, P.; Ghose, S.; Kirschning, C.J.; Issels, R.D.; Wagner, H. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J. Biol. Chem. 2002, 277, 15107–15112. [Google Scholar] [CrossRef]
- Asea, A.; Rehli, M.; Kabingu, E.; Boch, J.A.; Bare, O.; Auron, P.E.; Stevenson, M.A.; Calderwood, S.K. Novel signal transduction pathway utilized by extracellular HSP70: Role of toll-like receptor (TLR) 2 and TLR4. J. Biol. Chem. 2002, 277, 15028–15034. [Google Scholar] [CrossRef]
- Tamura, Y.; Torigoe, T.; Kutomi, G.; Hirata, K.; Sato, N. New paradigm for intrinsic function of heat shock proteins as endogenous ligands in inflammation and innate immunity. Curr. Mol. Med. 2012, 12, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Zhu, B.; Murshid, A.; Adachi, H.; Song, B.; Lee, A.; Liu, C.; Calderwood, S.K. T cell activation by heat shock protein 70 vaccine requires TLR signaling and scavenger receptor expressed by endothelial cells-1. J. Immunol. 2009, 183, 3092–3098. [Google Scholar] [CrossRef] [PubMed]
- Murshid, A.; Gong, J.; Calderwood, S.K. The role of heat shock proteins in antigen cross presentation. Front. Immunol. 2012, 3, 63. [Google Scholar] [CrossRef] [PubMed]
- Murshid, A.; Gong, J.; Stevenson, M.A.; Calderwood, S.K. Heat shock proteins and cancer vaccines: Developments in the past decade and chaperoning in the decade to come. Expert Rev. Vaccines 2011, 10, 1553–1568. [Google Scholar] [CrossRef] [PubMed]
- Murshid, A.; Gong, J.; Calderwood, S.K. Heat shock protein 90 mediates efficient antigen cross presentation through the scavenger receptor expressed by endothelial cells-I. J. Immunol. 2010, 185, 2903–2917. [Google Scholar] [CrossRef] [PubMed]
- Murshid, A.; Gong, J.; Calderwood, S.K. Hsp90-peptide complexes stimulate antigen presentation through the class II pathway after binding scavenger receptor SREC-I. Immunobiology 2014, 219, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Murshid, A.; Gong, J.; Ahmad, R.; Borges, T.J.; Calderwood, S.K. Scavenger receptor SREC-I promotes double stranded RNA-mediated TLR3 activation in human monocytes. Immunobiology 2015, 220, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Murshid, A.; Gong, J.; Prince, T.; Borges, T.J.; Calderwood, S.K. Scavenger receptor SREC-I mediated entry of TLR4 into lipid microdomains and triggered inflammatory cytokine release in RAW 264.7 cells upon LPS activation. PLoS ONE 2015, 10, e0122529. [Google Scholar] [CrossRef]
- Murshid, A.; Borges, T.J.; Lang, B.J.; Calderwood, S.K. The Scavenger Receptor SREC-I Cooperates with Toll-Like Receptors to Trigger Inflammatory Innate Immune Responses. Front. Immunol. 2016, 7, 226. [Google Scholar] [CrossRef]
- Murshid, A.; Theriault, J.; Gong, J.; Calderwood, S.K. Investigating receptors for extracellular heat shock proteins. Methods Mol. Biol. 2011, 787, 289–302. [Google Scholar] [CrossRef]
- Hromadnikova, I.; Li, S.; Kotlabova, K.; Dickinson, A.M. Influence of In Vitro IL-2 or IL-15 Alone or in Combination with Hsp 70 Derived 14-Mer Peptide (TKD) on the Expression of NK Cell Activatory and Inhibitory Receptors on Peripheral Blood T Cells, B Cells and NKT Cells. PLoS ONE 2016, 11, e0151535. [Google Scholar] [CrossRef] [PubMed]
- Hromadnikova, I.; Pirkova, P.; Sedlackova, L. Influence of in vitro IL-2 or IL-15 alone or in combination with Hsp-70-derived 14-mer peptide (TKD) on the expression of NK cell activatory and inhibitory receptors. Mediat. Inflamm. 2013, 2013, 405295. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.H.; Lee, U.Y.; Park, E.M.; Han, M.Y.; Lee, Y.S.; Park, Y.M. Role of protein kinase Cdelta in transmitting hypoxia signal to HSF and HIF-1. J. Cell. Physiol. 2001, 188, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.; Khaliq, A.; Jarvis-Evans, J.; Boulton, M.; Arrol, S.; Mackness, M.; McLeod, D. Hypoxia induces HSP 70 gene expression in human hepatoma (HEP G2) cells. Biochem. Mol. Biol. Int. 1995, 36, 907–912. [Google Scholar] [PubMed]
- Ikeda, J.; Kaneda, S.; Kuwabara, K.; Ogawa, S.; Kobayashi, T.; Matsumoto, M.; Yura, T.; Yanagi, H. Cloning and expression of cDNA encoding the human 150 kDa oxygen-regulated protein, ORP150. Biochem. Biophys. Res. Commun. 1997, 230, 94–99. [Google Scholar] [CrossRef]
- Son, T.W.; Yun, S.P.; Yong, M.S.; Seo, B.N.; Ryu, J.M.; Youn, H.Y.; Oh, Y.M.; Han, H.J. Netrin-1 protects hypoxia-induced mitochondrial apoptosis through HSP27 expression via DCC- and integrin alpha6beta4-dependent Akt, GSK-3beta, and HSF-1 in mesenchymal stem cells. Cell Death Dis. 2013, 4, e563. [Google Scholar] [CrossRef]
- Liu, X.; Chen, S.; Tu, J.; Cai, W.; Xu, Q. HSP90 inhibits apoptosis and promotes growth by regulating HIF-1alpha abundance in hepatocellular carcinoma. Int. J. Mol. Med. 2016, 37, 825–835. [Google Scholar] [CrossRef]
- Fujii, T.; Otsuki, T.; Moriya, T.; Sakaguchi, H.; Kurebayashi, J.; Yata, K.; Uno, M.; Kobayashi, T.; Kimura, T.; Jo, Y.; et al. Effect of hypoxia on human seminoma cells. Int. J. Oncol. 2002, 20, 955–962. [Google Scholar] [CrossRef]
- Patel, A.; van de Poll, M.C.; Greve, J.W.; Buurman, W.A.; Fearon, K.C.; McNally, S.J.; Harrison, E.M.; Ross, J.A.; Garden, O.J.; Dejong, C.H.; et al. Early stress protein gene expression in a human model of ischemic preconditioning. Transplantation 2004, 78, 1479–1487. [Google Scholar] [CrossRef]
- Semenza, G.L. The hypoxic tumor microenvironment: A driving force for breast cancer progression. Biochim. Biophys. Acta 2016, 1863, 382–391. [Google Scholar] [CrossRef]
- Keith, B.; Simon, M.C. Hypoxia-inducible factors, stem cells, and cancer. Cell 2007, 129, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Koong, A.C.; Denko, N.C.; Hudson, K.M.; Schindler, C.; Swiersz, L.; Koch, C.; Evans, S.; Ibrahim, H.; Le, Q.T.; Terris, D.J.; et al. Candidate genes for the hypoxic tumor phenotype. Cancer Res. 2000, 60, 883–887. [Google Scholar] [CrossRef]
- Yoshida, S.; Kawai, H.; Eguchi, T.; Sukegawa, S.; Oo, M.W.; Anqi, C.; Takabatake, K.; Nakano, K.; Okamoto, K.; Nagatsuka, H. Tumor Angiogenic Inhibition Triggered Necrosis (TAITN) in Oral Cancer. Cells 2019, 8, 761. [Google Scholar] [CrossRef] [PubMed]
- Sahu, D.; Zhao, Z.; Tsen, F.; Cheng, C.F.; Park, R.; Situ, A.J.; Dai, J.; Eginli, A.; Shams, S.; Chen, M.; et al. A potentially common peptide target in secreted heat shock protein-90alpha for hypoxia-inducible factor-1alpha-positive tumors. Mol. Biol. Cell 2012, 23, 602–613. [Google Scholar] [CrossRef] [PubMed]
- Sogawa, C.; Eguchi, T.; Okusha, Y.; Ono, K.; Ohyama, K.; Iizuka, M.; Kawasaki, R.; Hamada, Y.; Takigawa, M.; Sogawa, N.; et al. A reporter system evaluates tumorigenesis, metastasis, beta-catenin/MMP regulation, and druggability. Tissue Eng. Part A 2019. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S.; Kubota, S.; Shimo, T.; Nishida, T.; Yosimichi, G.; Eguchi, T.; Takigawa, M. Connective tissue growth factor increased by hypoxia may initiate angiogenesis in collaboration with matrix metalloproteinases. Carcinogenesis 2002, 23, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Arai, K.; Eguchi, T.; Rahman, M.M.; Sakamoto, R.; Masuda, N.; Nakatsura, T.; Calderwood, S.K.; Kozaki, K.; Itoh, M. A Novel High-Throughput 3D Screening System for EMT Inhibitors: A Pilot Screening Discovered the EMT Inhibitory Activity of CDK2 Inhibitor SU9516. PLoS ONE 2016, 11, e0162394. [Google Scholar] [CrossRef]
- Jayaprakash, P.; Dong, H.; Zou, M.; Bhatia, A.; O’Brien, K.; Chen, M.; Woodley, D.T.; Li, W. Hsp90alpha and Hsp90beta together operate a hypoxia and nutrient paucity stress-response mechanism during wound healing. J. Cell Sci. 2015, 128, 1475–1480. [Google Scholar] [CrossRef]
- Cheng, C.F.; Sahu, D.; Tsen, F.; Zhao, Z.; Fan, J.; Kim, R.; Wang, X.; O’Brien, K.; Li, Y.; Kuang, Y.; et al. A fragment of secreted Hsp90alpha carries properties that enable it to accelerate effectively both acute and diabetic wound healing in mice. J. Clin. Investig. 2011, 121, 4348–4361. [Google Scholar] [CrossRef]
- Zusman, I.; Ben-Hur, H. Serological markers for detection of cancer (Review). Int. J. Mol. Med. 2001, 7, 547–556. [Google Scholar] [CrossRef]
- Salvermoser, L.; Dressel, S.; Schleissheimer, S.; Stangl, S.; Diederichs, C.; Wergin, M.; Bley, C.R.; Haller, B.; Multhoff, G. Hsp70 serum levels in pet dogs-a potential diagnostic biomarker for spontaneous round cell tumors. Cell Stress Chaperones 2019, 24, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Peake, J.; Nosaka, K.; Okutsu, M.; Abbiss, C.R.; Surriano, R.; Bishop, D.; Quod, M.J.; Lee, H.; Martin, D.T.; et al. Changes in markers of muscle damage, inflammation and HSP70 after an Ironman Triathlon race. Eur. J. Appl. Physiol. 2006, 98, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Lin, C.F.; Skinner, K.A.; Schiffhauer, L.M.; Peacock, J.; Hicks, D.G.; Redmond, E.M.; Morrow, D.; Huston, A.; Shayne, M.; et al. Heat shock protein 27 differentiates tolerogenic macrophages that may support human breast cancer progression. Cancer Res. 2011, 71, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Stope, M.B.; Klinkmann, G.; Diesing, K.; Koensgen, D.; Burchardt, M.; Mustea, A. Heat Shock Protein HSP27 Secretion by Ovarian Cancer Cells Is Linked to Intracellular Expression Levels, Occurs Independently of the Endoplasmic Reticulum Pathway and HSP27’s Phosphorylation Status, and Is Mediated by Exosome Liberation. Dis. Markers 2017, 2017, 1575374. [Google Scholar] [CrossRef] [PubMed]
- Thuringer, D.; Berthenet, K.; Cronier, L.; Solary, E.; Garrido, C. Primary tumor- and metastasis-derived colon cancer cells differently modulate connexin expression and function in human capillary endothelial cells. Oncotarget 2015, 6, 28800–28815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, J.T.; Liu, Y.K.; Song, H.Y.; Dai, Z.; Qin, L.X.; Almofti, M.R.; Fang, C.Y.; Lu, H.J.; Yang, P.Y.; Tang, Z.Y. Heat-shock protein 27: A potential biomarker for hepatocellular carcinoma identified by serum proteome analysis. Proteomics 2005, 5, 4581–4588. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Ye, J.; Huang, Q.; Chen, W.; Wang, L.; Lin, W.; Lin, J.; Lin, X. Heat shock protein 27 is over-expressed in tumor tissues and increased in sera of patients with gastric adenocarcinoma. Clin. Chem. Lab. Med. 2010, 48, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.C.; Wu, M.S.; Wang, H.P.; Tien, Y.W.; Lin, J.T. Serum heat shock protein 27 is increased in chronic pancreatitis and pancreatic carcinoma. Pancreas 2009, 38, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Gruden, G.; Bruno, G.; Chaturvedi, N.; Burt, D.; Schalkwijk, C.; Pinach, S.; Stehouwer, C.D.; Witte, D.R.; Fuller, J.H.; Perin, P.C. Serum heat shock protein 27 and diabetes complications in the EURODIAB prospective complications study: A novel circulating marker for diabetic neuropathy. Diabetes 2008, 57, 1966–1970. [Google Scholar] [CrossRef]
- Burut, D.F.; Borai, A.; Livingstone, C.; Ferns, G. Serum heat shock protein 27 antigen and antibody levels appear to be related to the macrovascular complications associated with insulin resistance: A pilot study. Cell Stress Chaperones 2010, 15, 379–386. [Google Scholar] [CrossRef]
- Martin-Ventura, J.L.; Duran, M.C.; Blanco-Colio, L.M.; Meilhac, O.; Leclercq, A.; Michel, J.B.; Jensen, O.N.; Hernandez-Merida, S.; Tunon, J.; Vivanco, F.; et al. Identification by a differential proteomic approach of heat shock protein 27 as a potential marker of atherosclerosis. Circulation 2004, 110, 2216–2219. [Google Scholar] [CrossRef] [PubMed]
- Seibert, T.A.; Hibbert, B.; Chen, Y.X.; Rayner, K.; Simard, T.; Hu, T.; Cuerrier, C.M.; Zhao, X.; de Belleroche, J.; Chow, B.J.; et al. Serum heat shock protein 27 levels represent a potential therapeutic target for atherosclerosis: Observations from a human cohort and treatment of female mice. J. Am. Coll. Cardiol. 2013, 62, 1446–1454. [Google Scholar] [CrossRef] [PubMed]
- Park, H.K.; Park, E.C.; Bae, S.W.; Park, M.Y.; Kim, S.W.; Yoo, H.S.; Tudev, M.; Ko, Y.H.; Choi, Y.H.; Kim, S.; et al. Expression of heat shock protein 27 in human atherosclerotic plaques and increased plasma level of heat shock protein 27 in patients with acute coronary syndrome. Circulation 2006, 114, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Hecker, J.G.; McGarvey, M. Heat shock proteins as biomarkers for the rapid detection of brain and spinal cord ischemia: A review and comparison to other methods of detection in thoracic aneurysm repair. Cell Stress Chaperones 2011, 16, 119–131. [Google Scholar] [CrossRef]
- Shi, L.; Chevolot, Y.; Souteyrand, E.; Laurenceau, E. Autoantibodies against heat shock proteins as biomarkers for the diagnosis and prognosis of cancer. Cancer Biomark. 2017, 18, 105–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mellby, L.D.; Nyberg, A.P.; Johansen, J.S.; Wingren, C.; Nordestgaard, B.G.; Bojesen, S.E.; Mitchell, B.L.; Sheppard, B.C.; Sears, R.C.; Borrebaeck, C.A.K. Serum Biomarker Signature-Based Liquid Biopsy for Diagnosis of Early-Stage Pancreatic Cancer. J. Clin. Oncol. 2018, 36, 2887–2894. [Google Scholar] [CrossRef] [PubMed]
- Berghuis, A.M.S.; Koffijberg, H.; Prakash, J.; Terstappen, L.W.; IJzerman, M. Detecting Blood-Based Biomarkers in Metastatic Breast Cancer: A Systematic Review of Their Current Status and Clinical Utility. Int. J. Mol. Sci. 2017, 18, 363. [Google Scholar] [CrossRef]
- Crowley, E.; Di Nicolantonio, F.; Loupakis, F.; Bardelli, A. Liquid biopsy: Monitoring cancer-genetics in the blood. Nat. Rev. Clin. Oncol. 2013, 10, 472–484. [Google Scholar] [CrossRef]
- Schmidt, H.; Kulasinghe, A.; Perry, C.; Nelson, C.; Punyadeera, C. A liquid biopsy for head and neck cancers. Expert Rev. Mol. Diagn. 2016, 16, 165–172. [Google Scholar] [CrossRef]
- Rapado-Gonzalez, O.; Majem, B.; Muinelo-Romay, L.; Lopez-Lopez, R.; Suarez-Cunqueiro, M.M. Cancer Salivary Biomarkers for Tumours Distant to the Oral Cavity. Int. J. Mol. Sci. 2016, 17, 1531. [Google Scholar] [CrossRef]
- Hu, Z.; Dong, J.; Wang, L.E.; Ma, H.; Liu, J.; Zhao, Y.; Tang, J.; Chen, X.; Dai, J.; Wei, Q.; et al. Serum microRNA profiling and breast cancer risk: The use of miR-484/191 as endogenous controls. Carcinogenesis 2012, 33, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Nagata, M.; Muto, S.; Horie, S. Molecular Biomarkers in Bladder Cancer: Novel Potential Indicators of Prognosis and Treatment Outcomes. Dis. Markers 2016, 2016, 8205836. [Google Scholar] [CrossRef] [PubMed]
- Birkenkamp-Demtroder, K.; Nordentoft, I.; Christensen, E.; Hoyer, S.; Reinert, T.; Vang, S.; Borre, M.; Agerbaek, M.; Jensen, J.B.; Orntoft, T.F.; et al. Genomic Alterations in Liquid Biopsies from Patients with Bladder Cancer. Eur. Urol. 2016, 70, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Alberice, J.V.; Amaral, A.F.; Armitage, E.G.; Lorente, J.A.; Algaba, F.; Carrilho, E.; Marquez, M.; Garcia, A.; Malats, N.; Barbas, C. Searching for urine biomarkers of bladder cancer recurrence using a liquid chromatography-mass spectrometry and capillary electrophoresis-mass spectrometry metabolomics approach. J. Chromatogr. A 2013, 1318, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Souverijn, J.H. Multitarget stool DNA testing for colorectal-cancer screening. N. Engl. J. Med. 2014, 371, 187. [Google Scholar] [CrossRef] [PubMed]
- Huth, L.; Jakel, J.; Dahl, E. Molecular Diagnostic Applications in Colorectal Cancer. Microarrays 2014, 3, 168–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Touat, M.; Duran-Pena, A.; Alentorn, A.; Lacroix, L.; Massard, C.; Idbaih, A. Emerging circulating biomarkers in glioblastoma: Promises and challenges. Expert Rev. Mol. Diagn. 2015, 15, 1311–1323. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Gu, W.; Nagpal, S.; Gephart, M.H.; Quake, S.R. Brain tumor mutations detected in cerebral spinal fluid. Clin. Chem. 2015, 61, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Baraniskin, A.; Kuhnhenn, J.; Schlegel, U.; Chan, A.; Deckert, M.; Gold, R.; Maghnouj, A.; Zollner, H.; Reinacher-Schick, A.; Schmiegel, W.; et al. Identification of microRNAs in the cerebrospinal fluid as marker for primary diffuse large B-cell lymphoma of the central nervous system. Blood 2011, 117, 3140–3146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Pol, E.; Boing, A.N.; Harrison, P.; Sturk, A.; Nieuwland, R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol. Rev. 2012, 64, 676–705. [Google Scholar] [CrossRef]
- Heitzer, E.; Haque, I.S.; Roberts, C.E.S.; Speicher, M.R. Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat. Rev. Genet. 2019, 20, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Siravegna, G.; Marsoni, S.; Siena, S.; Bardelli, A. Integrating liquid biopsies into the management of cancer. Nat. Rev. Clin. Oncol. 2017, 14, 531–548. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.C.M.; Massie, C.; Garcia-Corbacho, J.; Mouliere, F.; Brenton, J.D.; Caldas, C.; Pacey, S.; Baird, R.; Rosenfeld, N. Liquid biopsies come of age: Towards implementation of circulating tumour DNA. Nat. Rev. Cancer 2017, 17, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Alix-Panabieres, C.; Pantel, K. Challenges in circulating tumour cell research. Nat. Rev. Cancer 2014, 14, 623–631. [Google Scholar] [CrossRef]
- Alix-Panabieres, C.; Pantel, K. Clinical Applications of Circulating Tumor Cells and Circulating Tumor DNA as Liquid Biopsy. Cancer Discov. 2016, 6, 479–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bardelli, A.; Pantel, K. Liquid Biopsies, What We Do Not Know (Yet). Cancer Cell 2017, 31, 172–179. [Google Scholar] [CrossRef] [Green Version]
- Giannopoulou, L.; Zavridou, M.; Kasimir-Bauer, S.; Lianidou, E.S. Liquid biopsy in ovarian cancer: The potential of circulating miRNAs and exosomes. Transl. Res. 2019, 205, 77–91. [Google Scholar] [CrossRef]
- Ochel, H.J.; Eichhorn, K.; Gademann, G. Geldanamycin: The prototype of a class of antitumor drugs targeting the heat shock protein 90 family of molecular chaperones. Cell Stress Chaperones 2001, 6, 105–112. [Google Scholar] [CrossRef]
- Whitesell, L.; Mimnaugh, E.G.; De Costa, B.; Myers, C.E.; Neckers, L.M. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: Essential role for stress proteins in oncogenic transformation. Proc. Natl. Acad. Sci. USA 1994, 91, 8324–8328. [Google Scholar] [CrossRef]
- Egorin, M.J.; Zuhowski, E.G.; Rosen, D.M.; Sentz, D.L.; Covey, J.M.; Eiseman, J.L. Plasma pharmacokinetics and tissue distribution of 17-(allylamino)-17-demethoxygeldanamycin (NSC 330507) in CD2F1 mice1. Cancer Chemother. Pharmacol. 2001, 47, 291–302. [Google Scholar] [CrossRef]
- Schulte, T.W.; Neckers, L.M. The benzoquinone ansamycin 17-allylamino-17-demethoxygeldanamycin binds to HSP90 and shares important biologic activities with geldanamycin. Cancer Chemother. Pharmacol. 1998, 42, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Tatokoro, M.; Koga, F.; Yoshida, S.; Kihara, K. Heat shock protein 90 targeting therapy: State of the art and future perspective. EXCLI J. 2015, 14, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Goetz, M.P.; Toft, D.; Reid, J.; Ames, M.; Stensgard, B.; Safgren, S.; Adjei, A.A.; Sloan, J.; Atherton, P.; Vasile, V.; et al. Phase I trial of 17-allylamino-17-demethoxygeldanamycin in patients with advanced cancer. J. Clin. Oncol. 2005, 23, 1078–1087. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, G.S.; McCollum, A.K.; Ames, M.M.; Mandrekar, S.J.; Reid, J.M.; Adjei, A.A.; Toft, D.O.; Safgren, S.L.; Erlichman, C. A phase I trial of twice-weekly 17-allylamino-demethoxy-geldanamycin in patients with advanced cancer. Clin. Cancer Res. 2006, 12, 6087–6093. [Google Scholar] [CrossRef] [PubMed]
- Felip, E.; Barlesi, F.; Besse, B.; Chu, Q.; Gandhi, L.; Kim, S.W.; Carcereny, E.; Sequist, L.V.; Brunsvig, P.; Chouaid, C.; et al. Phase 2 Study of the HSP-90 Inhibitor AUY922 in Previously Treated and Molecularly Defined Patients with Advanced Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2018, 13, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.L.; Yu, H.A.; Hart, E.M.; Weitner, B.B.; Rademaker, A.W.; Patel, J.D.; Kris, M.G.; Riely, G.J. Phase I/II Study of HSP90 Inhibitor AUY922 and Erlotinib for EGFR-Mutant Lung Cancer With Acquired Resistance to Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors. J. Clin. Oncol. 2015, 33, 1666–1673. [Google Scholar] [CrossRef] [PubMed]
- Ischia, J.; Saad, F.; Gleave, M. The promise of heat shock protein inhibitors in the treatment of castration resistant prostate cancer. Curr. Opin. Urol. 2013, 23, 194–200. [Google Scholar] [CrossRef]
- Reddy, N.; Voorhees, P.M.; Houk, B.E.; Brega, N.; Hinson, J.M., Jr.; Jillela, A. Phase I trial of the HSP90 inhibitor PF-04929113 (SNX5422) in adult patients with recurrent, refractory hematologic malignancies. Clin. Lymphoma Myeloma Leuk. 2013, 13, 385–391. [Google Scholar] [CrossRef]
- Isambert, N.; Delord, J.P.; Soria, J.C.; Hollebecque, A.; Gomez-Roca, C.; Purcea, D.; Rouits, E.; Belli, R.; Fumoleau, P. Debio0932, a second-generation oral heat shock protein (HSP) inhibitor, in patients with advanced cancer-results of a first-in-man dose-escalation study with a fixed-dose extension phase. Ann. Oncol. 2015, 26, 1005–1011. [Google Scholar] [CrossRef]
- Spigel, D.R.; Shipley, D.L.; Waterhouse, D.M.; Jones, S.F.; Ward, P.J.; Shih, K.C.; Hemphill, B.; McCleod, M.; Whorf, R.C.; Page, R.D.; et al. A Randomized, Double-Blinded, Phase II Trial of Carboplatin and Pemetrexed with or without Apatorsen (OGX-427) in Patients with Previously Untreated Stage IV Non-Squamous-Non-Small-Cell Lung Cancer: The SPRUCE Trial. Oncologist 2019. [Google Scholar] [CrossRef]
- Hendriks, L.E.L.; Dingemans, A.C. Heat shock protein antagonists in early stage clinical trials for NSCLC. Expert Opin. Investig. Drugs 2017, 26, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Thao, L.; Sensintaffar, J.; Zhang, L.; Boehm, M.F.; Fritz, L.C.; Burrows, F.J. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature 2003, 425, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Massey, A.J.; Williamson, D.S.; Browne, H.; Murray, J.B.; Dokurno, P.; Shaw, T.; Macias, A.T.; Daniels, Z.; Geoffroy, S.; Dopson, M.; et al. A novel, small molecule inhibitor of Hsc70/Hsp70 potentiates Hsp90 inhibitor induced apoptosis in HCT116 colon carcinoma cells. Cancer Chemother. Pharmacol. 2010, 66, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Miyajima, N.; Tsutsumi, S.; Sourbier, C.; Beebe, K.; Mollapour, M.; Rivas, C.; Yoshida, S.; Trepel, J.B.; Huang, Y.; Tatokoro, M.; et al. The HSP90 inhibitor ganetespib synergizes with the MET kinase inhibitor crizotinib in both crizotinib-sensitive and -resistant MET-driven tumor models. Cancer Res. 2013, 73, 7022–7033. [Google Scholar] [CrossRef] [PubMed]
- Ernst, A.; Anders, H.; Kapfhammer, H.; Orth, M.; Hennel, R.; Seidl, K.; Winssinger, N.; Belka, C.; Unkel, S.; Lauber, K. HSP90 inhibition as a means of radiosensitizing resistant, aggressive soft tissue sarcomas. Cancer Lett. 2015, 365, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Spiegelberg, D.; Dascalu, A.; Mortensen, A.C.; Abramenkovs, A.; Kuku, G.; Nestor, M.; Stenerlow, B. The novel HSP90 inhibitor AT13387 potentiates radiation effects in squamous cell carcinoma and adenocarcinoma cells. Oncotarget 2015, 6, 35652–35666. [Google Scholar] [CrossRef]
- Eroglu, Z.; Chen, Y.A.; Gibney, G.T.; Weber, J.S.; Kudchadkar, R.R.; Khushalani, N.I.; Markowitz, J.; Brohl, A.S.; Tetteh, L.F.; Ramadan, H.; et al. Combined BRAF and HSP90 Inhibition in Patients with Unresectable BRAF (V600E)-Mutant Melanoma. Clin. Cancer Res. 2018, 24, 5516–5524. [Google Scholar] [CrossRef]
- Kang, J.; Young Lee, J.; Tas, I.; More, K.N.; Kim, H.; Park, J.H.; Chang, D.J. Radiosynthesis, biological evaluation and preliminary microPET study of (18)F-labeled 5-resorcinolic triazolone derivative based on ganetespib targeting HSP90. Bioorg. Med. Chem. Lett. 2018, 28, 3658–3664. [Google Scholar] [CrossRef]
- Chou, S.D.; Murshid, A.; Eguchi, T.; Gong, J.; Calderwood, S.K. HSF1 regulation of beta-catenin in mammary cancer cells through control of HuR/elavL1 expression. Oncogene 2015, 34, 2178–2188. [Google Scholar] [CrossRef]
- Calderwood, S.K.; Xie, Y.; Wang, X.; Khaleque, M.A.; Chou, S.D.; Murshid, A.; Prince, T.; Zhang, Y. Signal Transduction Pathways Leading to Heat Shock Transcription. Sign. Transduct. Insights 2010, 2, 13–24. [Google Scholar] [CrossRef]
- Wang, Y.; Theriault, J.R.; He, H.; Gong, J.; Calderwood, S.K. Expression of a dominant negative heat shock factor-1 construct inhibits aneuploidy in prostate carcinoma cells. J. Biol. Chem. 2004, 279, 32651–32659. [Google Scholar] [CrossRef]
- Wang, X.; Grammatikakis, N.; Siganou, A.; Stevenson, M.A.; Calderwood, S.K. Interactions between extracellular signal-regulated protein kinase 1, 14-3-3epsilon, and heat shock factor 1 during stress. J. Biol. Chem. 2004, 279, 49460–49469. [Google Scholar] [CrossRef]
- Xie, Y.; Zhong, R.; Chen, C.; Calderwood, S.K. Heat shock factor 1 contains two functional domains that mediate transcriptional repression of the c-fos and c-fms genes. J. Biol. Chem. 2003, 278, 4687–4698. [Google Scholar] [CrossRef]
- Murshid, A.; Chou, S.D.; Prince, T.; Zhang, Y.; Bharti, A.; Calderwood, S.K. Protein kinase A binds and activates heat shock factor 1. PLoS ONE 2010, 5, e13830. [Google Scholar] [CrossRef]
- Tang, D.; Khaleque, M.A.; Jones, E.L.; Theriault, J.R.; Li, C.; Wong, W.H.; Stevenson, M.A.; Calderwood, S.K. Expression of heat shock proteins and heat shock protein messenger ribonucleic acid in human prostate carcinoma in vitro and in tumors in vivo. Cell Stress Chaperones 2005, 10, 46–58. [Google Scholar] [CrossRef] [Green Version]
- Meriin, A.B.; Narayanan, A.; Meng, L.; Alexandrov, I.; Varelas, X.; Cisse, II.; Sherman, M.Y. Hsp70-Bag3 complex is a hub for proteotoxicity-induced signaling that controls protein aggregation. Proc. Natl. Acad. Sci. USA 2018, 115, E7043–E7052. [Google Scholar] [CrossRef]
- Ruan, L.; Zhou, C.; Jin, E.; Kucharavy, A.; Zhang, Y.; Wen, Z.; Florens, L.; Li, R. Cytosolic proteostasis through importing of misfolded proteins into mitochondria. Nature 2017, 543, 443–446. [Google Scholar] [CrossRef]
- Melentijevic, I.; Toth, M.L.; Arnold, M.L.; Guasp, R.J.; Harinath, G.; Nguyen, K.C.; Taub, D.; Parker, J.A.; Neri, C.; Gabel, C.V.; et al. elegans neurons jettison protein aggregates and mitochondria under neurotoxic stress. Nature 2017, 542, 367–371. [Google Scholar] [CrossRef]
- Leak, R.K. Heat shock proteins in neurodegenerative disorders and aging. J. Cell Commun. Signal. 2014, 8, 293–310. [Google Scholar] [CrossRef]
- Neef, D.W.; Jaeger, A.M.; Thiele, D.J. Heat shock transcription factor 1 as a therapeutic target in neurodegenerative diseases. Nat. Rev. Drug Discov. 2011, 10, 930–944. [Google Scholar] [CrossRef] [Green Version]
- Anckar, J.; Sistonen, L. Regulation of HSF1 function in the heat stress response: Implications in aging and disease. Annu. Rev. Biochem. 2011, 80, 1089–1115. [Google Scholar] [CrossRef]
- Chou, S.D.; Prince, T.; Gong, J.; Calderwood, S.K. mTOR is essential for the proteotoxic stress response, HSF1 activation and heat shock protein synthesis. PLoS ONE 2012, 7, e39679. [Google Scholar] [CrossRef]
- Kijima, T.; Prince, T.L.; Tigue, M.L.; Yim, K.H.; Schwartz, H.; Beebe, K.; Lee, S.; Budzynski, M.A.; Williams, H.; Trepel, J.B.; et al. HSP90 inhibitors disrupt a transient HSP90-HSF1 interaction and identify a noncanonical model of HSP90-mediated HSF1 regulation. Sci. Rep. 2018, 8, 6976. [Google Scholar] [CrossRef]
- Prince, T.L.; Kijima, T.; Tatokoro, M.; Lee, S.; Tsutsumi, S.; Yim, K.; Rivas, C.; Alarcon, S.; Schwartz, H.; Khamit-Kush, K.; et al. Client Proteins and Small Molecule Inhibitors Display Distinct Binding Preferences for Constitutive and Stress-Induced HSP90 Isoforms and Their Conformationally Restricted Mutants. PLoS ONE 2015, 10, e0141786. [Google Scholar] [CrossRef]
- Zou, J.; Guo, Y.; Guettouche, T.; Smith, D.F.; Voellmy, R. Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell 1998, 94, 471–480. [Google Scholar] [CrossRef]
- Nadler, S.G.; Eversole, A.C.; Tepper, M.A.; Cleaveland, J.S. Elucidating the mechanism of action of the immunosuppressant 15-deoxyspergualin. Ther. Drug Monit. 1995, 17, 700–703. [Google Scholar] [CrossRef]
- Nadler, S.G.; Tepper, M.A.; Schacter, B.; Mazzucco, C.E. Interaction of the immunosuppressant deoxyspergualin with a member of the Hsp70 family of heat shock proteins. Science 1992, 258, 484–486. [Google Scholar] [CrossRef]
- Dhingra, K.; Valero, V.; Gutierrez, L.; Theriault, R.; Booser, D.; Holmes, F.; Buzdar, A.; Fraschini, G.; Hortobagyi, G. Phase II study of deoxyspergualin in metastatic breast cancer. Investig. New Drugs 1994, 12, 235–241. [Google Scholar] [CrossRef]
- Multhoff, G.; Radons, J. Radiation, inflammation, and immune responses in cancer. Front. Oncol. 2012, 2, 58. [Google Scholar] [CrossRef]
- Stangl, S.; Gehrmann, M.; Riegger, J.; Kuhs, K.; Riederer, I.; Sievert, W.; Hube, K.; Mocikat, R.; Dressel, R.; Kremmer, E.; et al. Targeting membrane heat-shock protein 70 (Hsp70) on tumors by cmHsp70.1 antibody. Proc. Natl. Acad. Sci. USA 2011, 108, 733–738. [Google Scholar] [CrossRef]
- Krause, S.W.; Gastpar, R.; Andreesen, R.; Gross, C.; Ullrich, H.; Thonigs, G.; Pfister, K.; Multhoff, G. Treatment of colon and lung cancer patients with ex vivo heat shock protein 70-peptide-activated, autologous natural killer cells: A clinical phase i trial. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2004, 10, 3699–3707. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, N.; Hirayoshi, K.; Kudo, H.; Takechi, H.; Aoike, A.; Kawai, K.; Nagata, K. Inhibition of the activation of heat shock factor in vivo and in vitro by flavonoids. Mol. Cell. Biol. 1992, 12, 3490–3498. [Google Scholar] [CrossRef] [PubMed]
- Yokota, S.; Kitahara, M.; Nagata, K. Benzylidene lactam compound, KNK437, a novel inhibitor of acquisition of thermotolerance and heat shock protein induction in human colon carcinoma cells. Cancer Res. 2000, 60, 2942–2948. [Google Scholar] [PubMed]
- Xia, Y.; Liu, Y.; Rocchi, P.; Wang, M.; Fan, Y.; Qu, F.; Iovanna, J.L.; Peng, L. Targeting heat shock factor 1 with a triazole nucleoside analog to elicit potent anticancer activity on drug-resistant pancreatic cancer. Cancer Lett. 2012, 318, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Goloudina, A.R.; Demidov, O.N.; Garrido, C. Inhibition of HSP70: A challenging anti-cancer strategy. Cancer Lett. 2012, 325, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Cassel, J.A.; Ilyin, S.; McDonnell, M.E.; Reitz, A.B. Novel inhibitors of heat shock protein Hsp70-mediated luciferase refolding that bind to DnaJ. Bioorg. Med. Chem. 2012, 20, 3609–3614. [Google Scholar] [CrossRef]
- Moses, M.A.; Kim, Y.S.; Rivera-Marquez, G.M.; Oshima, N.; Watson, M.J.; Beebe, K.E.; Wells, C.; Lee, S.; Zuehlke, A.D.; Shao, H.; et al. Targeting the Hsp40/Hsp70 Chaperone Axis as a Novel Strategy to Treat Castration-Resistant Prostate Cancer. Cancer Res. 2018, 78, 4022–4035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorard, C.; de Thonel, A.; Collura, A.; Marisa, L.; Svrcek, M.; Lagrange, A.; Jego, G.; Wanherdrick, K.; Joly, A.L.; Buhard, O.; et al. Expression of a mutant HSP110 sensitizes colorectal cancer cells to chemotherapy and improves disease prognosis. Nat. Med. 2011, 17, 1283–1289. [Google Scholar] [CrossRef]
- Chatterjee, S.; Burns, T.F. Targeting Heat Shock Proteins in Cancer: A Promising Therapeutic Approach. Int. J. Mol. Sci. 2017, 18, 1978. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, J.-C.; Tuukkanen, A.; Schroeder, M.; Fahrig, T.; Fahrig, R. RP101 (brivudine) binds to heat shock protein HSP27 (HSPB1) and enhances survival in animals and pancreatic cancer patients. J. Cancer Res. Clin. Oncol. 2011, 137, 1349–1361. [Google Scholar] [CrossRef]
- Choi, S.-H.; Lee, Y.-J.; Seo, W.D.; Lee, H.-J.; Nam, J.-W.; Lee, Y.J.; Kim, J.; Seo, E.-K.; Lee, Y.-S. Altered cross-linking of HSP27 by zerumbone as a novel strategy for overcoming HSP27-mediated radioresistance. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 1196–1205. [Google Scholar] [CrossRef] [PubMed]
- Seigneuric, R.; Gobbo, J.; Colas, P.; Garrido, C. Targeting cancer with peptide aptamers. Oncotarget 2011, 2, 557–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamada, M.; So, A.; Muramaki, M.; Rocchi, P.; Beraldi, E.; Gleave, M. Hsp27 knockdown using nucleotide-based therapies inhibit tumor growth and enhance chemotherapy in human bladder cancer cells. Mol. Cancer Ther. 2007, 6, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, M.; Zhou, J.; Zhang, X. HSP27, 70 and 90, anti-apoptotic proteins, in clinical cancer therapy (Review). Int. J. Oncol. 2014, 45, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Meng, X.; Wang, D.; Zhang, K.; Dai, W.; Dong, H.; Zhang, X. Intelligent MnO2/Cu2-xS for Multimode Imaging Diagnostic and Advanced Single-Laser Irradiated Photothermal/Photodynamic Therapy. ACS Appl. Mater. Interfaces 2018, 10, 17732–17741. [Google Scholar] [CrossRef] [PubMed]



| Subfamily Name | Prototypical Members | Exosome HSP | Free HSP | Membrane HSP |
|---|---|---|---|---|
| HSP90 | Hsp90α HSP90AA1 Hsp90β HSP90AB1 Gp96 Grp94/Hsp90B TRAP1 Hsp75/Hsp90L | Cancer [1] B cell [6] | 3D Tumoroid [5] HDF [9] | Tumor cells APCs Infected erythrocyte [10,11,12] |
| HSP70 HspA(1-12) | Hsp72 HspA1A/HspA1B Hsc70, BiP HspA5 Hsp70B’ HspA6 See [2] in detail | Cancer [1,13] B cell [6] | Mφ [14], Cancer [5,15] Exercise [16] Sarcopenia [17] | Cancer [18,19] |
| Small HSP HspB(1–10) | Hsp27 HspB1 αB-crystallin HspB5 | B cell [6], Sera, body fluids [8] | Sera, body fluids [8] | ? |
| Large HSP | Hsp105, Hsp110 Grp170 Orp150 | Cancer [1] | ? | ? |
| HSP40DnaJ | Tid1DnaJA3 [20] ERdj4 DnaJB9 RME8 DnaJC13 | ? | ? | ? |
| HSP47 | Colligin-2 RA-A47 | ? | RA sera [21] | Chondrocyte [22] |
| Chaperonin | TRiC CCT Hsp60, Hsp10 | Cancer [1,23] | DM [24] | ? |
| Immunostimulatory HSP | Immunotolerant HSP | |
|---|---|---|
| Target diseases | Cancers Infectious diseases | Rheumatoid arthritis Type 1 diabetes Atherosclerosis Multiple sclerosis |
| APC | DCs Mφ | Tolerogenic DCs |
| Immune cells | Antigen-specific CD8+ CTL NK cells, NKT cells | MDSC Treg |
| HSP antigens | Gp96 Grp94/TRA, BiP HspA5 HSP90, HSP70 Grp170 Orp150 Small HSPs | Microbial HSP70/HSP60 |
| Effects | Antigen cross-presentation T cell cross-priming Tumor cytolysis | Immune tolerance Anti-inflammatory Immunosuppressive |
| Concept, Material | Disease | Phase | Note, Outcome |
|---|---|---|---|
| Autologous tumor-derived HSP peptide complexes (HSPPCs) | RCC Melanoma | III | Had clinical activities. In Phase III trials for advanced melanoma and RCC patients, efficacy, safety, and feasibility were demonstrated [34,87,88]. However, the limitations were apparent, and specific alternatives have been developed. |
| CML, CRC LymphomaPancreatic cancer Gastric cancer | I/II | ||
| Autologous tumor-derived HSP Gp96-peptide complexes HSPPC-96 Vitespen® Oncophage | RCC Metastatic melanoma | III | Feasible, devoid of significant toxicity, induced clinical and tumor-specific T-cell responses in vaccinated patients [89,90]. Promising in enhancing survival of patients [91,92]. |
| CRC, RCC Glioblastoma Lung cancer Melanoma | I/II | Almost devoid of side effects aside from minor injection-site reactions [93]. | |
| Preparation of HSPPC-96 | Pancreatic adenocarcinoma | I | No correlation between immune response and prognosis. Feasible prep of HSPPC-96 [94]. |
| HSPPC-96 + GM-CSF + IFN-α | Metastatic melanoma | II | Gained tumor-specific T cell-mediated and NK responses, but immune, clinical responses were not gained compared with monotherapy [95]. |
| Recombinant oncolytic adenovirus overexpressing HSP70 (H103) | Advanced solid tumors | I | CR + partial response was 11.1% (3/27), and the clinical benefit rate (CR + partial response + minor response + stable disease) was 48.1%. CD4+ and CD8+ T cells and NK cells were elevated [96]. |
| Dendritic cells transfected with HSP70 mRNA (HSP70-DC) | HCV-related HCC | I | Safe and feasible. Almost no adverse effects in grade III/IV. CR without any recurrence (2), stable disease (5), a progression of the disease (5). Infiltrating CD8+T cells and granzyme B in tumors. |
| Receptor | Key events | Expression | Notes |
|---|---|---|---|
| CD91/LRP1/A2MR | Hypoxia response EMT Antitumor immunity | Cancer cell APCDermal fibroblast Exosome |
|
| TLR2 TLR3 TLR4 TLR9 | Immune response DAMP/PAMP signal | APC Epithelial cell |
|
| SREC-1 | Immune response Antigen cross-priming | APC |
|
| CD94/KLRD1 | Cytotoxicity targeting tumor and infected cells | NK cell CD8+ CTL NKT cell |
|
| Concept | Disease | Phase | Note, Outcome |
|---|---|---|---|
| HSP90 inhibitor 17-AAG | Metastatic breast cancer Melanoma | I/II | Side effects occurred such as tiredness, nausea, diarrhea, and liver damage. HSP70 was induced in PBMC [184,185]. |
| HSP90 inhibitor Ganetespib® | NSCLC | III | Not positive in unselected NSCLC. Therefore, drug development was halted. More promising in ALK-rearranged NSCLC patients. |
| HSP90 inhibitor Retaspimycin® | NSCLC | III | |
| HSP90 inhibitor AUY922 | NSCLC | III | |
| Stage IV NSCLC | II | Active particularly among patients with ALK rearrangements and EGFR mutations [186]. | |
| HSP90 inhibitor AUY922 + Erlotinib | EGFR-mutant lung cancer | I/II | Evaluated in acquired resistance to EGFR-TKI. Partial responses, but the duration of treatment was limited by toxicities, especially night blindness. Did not meet its primary endpoint [187]. |
| HSP90 inhibitors | CRPC | I/II | Negligible anticancer activity and dose-limiting toxicity profiles [188]. |
| Oral HSP90 inhibitor PF-04929113 (SNX5422) | Recurrent, refractory hematologic malignancies | I | Alternate-day oral dosing at 74 mg/m (2) for 21/28 days was tolerated with reversible toxicity. Myeloma and lymphoma patients were responsive [189]. |
| Oral HSP inhibitor Debio0932 | NSCLC Breast cancer | I | Has limited clinical activity with manageable toxicity [190]. |
| HSP27-targeted antisense oligonucleotide OGX-427 Apatorsen® | Squamous NSCLC | I | Tested, as overexpression of Hsp27 in squamous NSCLC is a mechanism of chemoresistance. |
| Metastatic non-squamous NSCLC | II | A combination of carboplatin and pemetrexed was evaluated. Well tolerated but did not improve outcomes in the first-line setting [191]. | |
| Advanced bladder cancer | II | A combination of cisplatin and apatorsen was tested. | |
| CRPC | II/III | Has shown good biological activity [188]. | |
| HSP70 inhibitor 15-deoxyspergualin | Metastatic breast cancer | II | Neuromuscular side effects with no benefit for disease. |
| Anti-HSP70 antibody recognizing TKD | NSCLC | I/II | Safe in phase I. Evaluated in combination with radio-, chemotherapy. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taha, E.A.; Ono, K.; Eguchi, T. Roles of Extracellular HSPs as Biomarkers in Immune Surveillance and Immune Evasion. Int. J. Mol. Sci. 2019, 20, 4588. https://doi.org/10.3390/ijms20184588
Taha EA, Ono K, Eguchi T. Roles of Extracellular HSPs as Biomarkers in Immune Surveillance and Immune Evasion. International Journal of Molecular Sciences. 2019; 20(18):4588. https://doi.org/10.3390/ijms20184588
Chicago/Turabian StyleTaha, Eman A., Kisho Ono, and Takanori Eguchi. 2019. "Roles of Extracellular HSPs as Biomarkers in Immune Surveillance and Immune Evasion" International Journal of Molecular Sciences 20, no. 18: 4588. https://doi.org/10.3390/ijms20184588
APA StyleTaha, E. A., Ono, K., & Eguchi, T. (2019). Roles of Extracellular HSPs as Biomarkers in Immune Surveillance and Immune Evasion. International Journal of Molecular Sciences, 20(18), 4588. https://doi.org/10.3390/ijms20184588