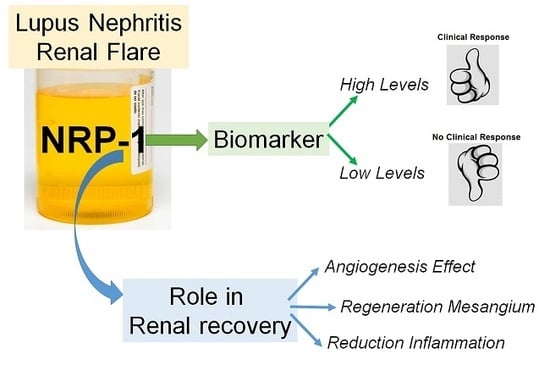
Urinary Neuropilin-1: A Predictive Biomarker for Renal Outcome in Lupus Nephritis
Abstract
:1. Introduction
2. Results
2.1. Patients
2.2. Urinary Expression of NRP-1 in Lupus Nephritis
2.3. Protein Levels of NRP-1 in Patients with Lupus Nephritis
2.4. Baseline Levels of NRP-1 Predict Clinical Response
2.5. VEGFA, VEGFR1, VEGFR2, and SEMA3A in Patients with Lupus Nephritis and Correlation with NRP-1 Levels
2.6. Immunohistochemistry Localization of NRP-1, VEGFA and SEMA3A within Renal Biopsies
2.7. Serial Monitoring of uNRP-1 Levels to Predict Response
2.8. NRP-1 Expression Levels by Primary Cells under Stimulatory Conditions
2.9. Effect of NRP-1 on Endothelial Proliferation and Migration
2.10. NRP-1 Induces Migration in Human Renal Mesangial Cells Via PDGFB
2.11. NRP-1 Induces a VEGF-Dependent T cell Cytotoxicity
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. mRNA Isolation, cDNA Synthesis and Real-Time PCR
4.3. Enzyme-Linked Immunosorbent Assay (ELISA)
4.4. Renal Histology and Immunohistochemistry
4.5. Cell Cultures
4.6. NRP-1 Expression Levels in Primary Cells
4.7. NRP-1 Inhibiton and Stimulation Conditions
4.8. Wound Healing Assay
4.9. Proliferation Assay
4.10. Migration Assay
4.11. Apoptosis Assay
4.12. Immunofluorescence Assay
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rahman, A.; Isenberg, D.A. Systemic lupus erythematosus. N. Engl. J. Med. 2008, 358, 929–939. [Google Scholar] [CrossRef]
- Parodis, I.; Zickert, A.; Sundelin, B.; Axelsson, M.; Gerhardsson, J.; Svenungsson, E.; Malmström, V.; Gunnarsson, I. Evaluation of B lymphocyte stimulator and a proliferation inducing ligand as candidate biomarkers in lupus nephritis based on clinical and histopathological outcome following induction therapy. Lupus Sci. Med. 2015, 2, e000061. [Google Scholar] [CrossRef] [PubMed]
- Cervera, R.; Khamashta, M.A.; Font, J.; Sebastiani, G.D.; Gil, A.; Lavilla, P.; Mejía, J.C.; Aydintug, A.O.; Chwalinska-Sadowsa, H.; de Ramón, E.; et al. Morbidity and mortality in systemic lupus erythematosus during a 10-year period: A comparison of early and late manifestations in a cohort of 1000 patients. Medicine 2003, 82, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Baker, K.P.; Edwards, B.M.; Main, S.H.; Choi, G.H.; Wager, R.E.; Halpern, W.G.; Lappin, P.B.; Riccobene, T.; Abramina, D.; Sekut, L.; et al. Generation and characterization of LymphoStat-B, a human monoclonal antibody that antagonizes the bioactivities of B lymphocyte stimulator. Arthritis Rheumatol. 2003, 48, 3253–3265. [Google Scholar] [CrossRef]
- Kolodkin, A.L.; Lavengood, D.V.; Rowe, E.G.; Tai, Y.T.; Giger, R.J.; Ginty, D.D. Neuropilin is a semaphorin III receptor. Cell 1997, 90, 753–762. [Google Scholar] [CrossRef]
- Schramek, H.; Sarközi, R.; Lauterberg, C.; Kronbichler, A.; Pirklbauer, M.; Albrecht, R.; Noppert, S.J.; Perco, P.; Rudnicki, M.; Strutz, F.M.; et al. Neuropilin-1 and neuropilin-2 are differentially expressed in human proteinuric nephropathies and cytokine-stimulated proximal tubular cells. Lab. Investig. 2009, 89, 1304–1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, C.; Jiang, X. The effect of anti-angiogenic drugs on regulatory T cells in the tumor microenvironment. Biomed. Pharmacother. 2017, 88, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Tordjman, R.; Lepelletier, Y.; Lemarchandel, V.; Cambot, M.; Gaulard, P.; Hermine, O.; Roméo, P.H. A neuronal receptor, neuropilin-1, is essential for the initiation of the primary immune response. Nat. Immunol. 2002, 3, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Perälä, N.; Sariola, H.; Immonen, T. More than nervous: The emerging roles of plexins. Differentiation 2012, 83, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Glinka, Y.; Prud’homme, G.J. Neuropilin-1 is a receptor for transforming growth factor-1, activates its latent form, and promotes regulatory T cell activity. J. Leukoc. Biol. 2008, 84, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Bruder, D.; Probst-Kepper, M.; Westendorf, A.M.; Geffers, R.; Beissert, S.; Loser, K.; von Boehmer, H.; Buer, J.; Hansen, W. Neuropilin-1: A surface marker of regulatory T cells. Eur. J. Immunol. 2004, 34, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Sarris, M.; Andersen, K.G.; Randow, F.; Mayr, L.; Betz, A.G. Neuropilin-1 expression on regulatory T cells enhances their interactions with dendritic cells during antigen recognition. Immunity 2008, 28, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Mizui, M.; Kikutani, H. Neuropilin-1: The glue between regulatory T cells and dendritic cells? Immunity 2008, 28, 302–303. [Google Scholar] [CrossRef] [PubMed]
- Staton, C.A.; Kumar, I.; Reed, N.W.; Brown, N.J. Neuropilins in physiological and pathological angiogenesis. J. Pathol. 2007, 212, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Fuh, G.; Garcia, K.C.; de Vos, A.M. The interaction of neuropilin-1 with vascular endothelial growth factor and its receptor Flt-1. J. Biol. Chem. 2000, 275, 26690–26695. [Google Scholar] [CrossRef]
- Gagnon, M.L.; Bielenberg, D.R.; Grechtman, Z.; Miao, H.Q.; Takashima, S.; Soker, S.; Kagsbrun, M. Identification of a natural soluble neuropilin-1 that binds vascular endothelial growth factor: In vivo expression and antitumor activity. Proc. Natl. Acad. Sci. USA 2000, 97, 2573–2578. [Google Scholar] [CrossRef]
- Karihaloo, A.; Karumanchi, S.A.; Cantley, W.L.; Venkatesha, S.; Cantley, L.G.; Kale, S. Vascular endothelial growth factor induces branching morphogenesis/tubulogenesis in renal epithelial cells in a neuropilin-dependent fashion. Mol. Cell. Biol. 2005, 25, 7441–7448. [Google Scholar] [CrossRef]
- Villegas, G.; Tufro, A. Ontogeny of semaphorins 3A and 3F and their receptors neuropilins 1 and 2 in the kidney. Mech. Dev. 2002, 119, S149–S153. [Google Scholar] [CrossRef]
- Thomas, S.; Vanuystel, J.; Gruden, G.; Rodríguez, V.; Burt, D.; Gnudi, L.; Hartley, B.; Viberti, G. Vascular endothelial growth factor receptors in human mesangium in vitro and in glomerular diseases. J. Am. Soc. Nephrol. 2000, 11, 1236–1243. [Google Scholar]
- Vadasz, Z.; Haj, T.; Halasz, K.; Rosner, I.; Slobodin, G.; Attias, D.; Kessel, A.; Kessler, O.; Neufeld, G.; Toubi, E. Semaphorin 3A is a marker for disease activity and a potential immunoregulator in systemic lupus erythematosus. Arthritis Res. Ther. 2012, 14, R146. [Google Scholar] [CrossRef]
- Vadasz, Z.; Haj, T.; Balber, A.; Peri, R.; Rosner, I.; Slobodin, G.; Kessel, A.; Toubi, E. A regulatory role for CD72 expression on B cells in systemic lupus erythematosus. Semin. Arthritis Rheum. 2014, 43, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Vadasz, Z.; Ben-Izhak, O.; Bejar, J.; Sabo, E.; Kessel, A.; Storch, S.; Toubi, E. The involvement of immune semaphorins and neuropilin-1 in lupus nephritis. Lupus 2011, 20, 1466–1473. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.E.; Korbet, S.M.; Katz, R.S.; Schwartz, M.M.; Lewis, E.J.; Collaboratie Study Group. Value of a Complete or Partial Remission in Severe Lupus Nephritis. Clin. J. Am. Soc. Nephrol. 2008, 3, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Austin, H.A., 3rd; Muenz, L.R.; Joyce, K.M.; Antonovych, T.A.; Kullick, M.E.; Klippel, J.H.; Decker, J.L.; Balow, J.E. Prognostic factors in lupus nephritis. Contribution of renal histologic data. Am. J. Med. 1983, 75, 382–391. [Google Scholar] [CrossRef]
- Ayodele, O.E.; Okpechi, I.G.; Swanepoel, C.R. Predictors of poor renal outcome in patients with biopsy-proven lupus nephritis. Nephrology 2010, 15, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Avihingsanon, Y.; Benjachat, T.; Tassanarong, A.; Sodsai, P.; Kittikovit, V.; Hirankarn, N. Decreased renal expression of vascular endothelial growth factor in lupus nephritis is associated with worse prognosis. Kidney Int. 2009, 75, 1340–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hankenson, K.D.; Dishowitz, M.; Gray, C.; Schenker, M. Angiogenesis in bone regeneration. Injury 2011, 42, 556–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniel, C.; Lüdke, A.; Wagner, A.; Todorov, V.T.; Hohenstein, B.; Hugo, C. Transgelin is a marker of repopulating mesangial cells after injury and promotes their proliferation and migration. Lab. Invest. 2012, 92, 812–826. [Google Scholar] [CrossRef] [Green Version]
- Bartlett, C.S.; Scott, R.P.; Carota, I.A.; Wnuk, M.L.; Kanwar, Y.S.; Miner, J.H.; Quaggin, S.E. Glomerular mesangial cell recruitment and function require the co-receptor neuropilin-1. Am. J. Physiol. Ren. Physiol. 2017, 313, F1232–F1242. [Google Scholar] [CrossRef]
- Gavalas, N.G.; Tsjatas, M.; Tsitsilonis, O.; Politi, E.; Ioannou, K.; Ziogas, A.C.; Rodolakis, A.; Vlahos, G.; Thomakos, N.; Haidopoulos, D.; et al. VEGF directly suppresses activation of T cells from ascites secondary to ovarian cancer via VEGF receptor type 2. Br. J. Cancer 2012, 107, 1869–1875. [Google Scholar] [CrossRef] [Green Version]
- Ziogas, A.C.; Gavalas, N.G.; Tsiatas, M.; Tsitsilonis, O.; Politi, E.; Terpos, E.; Rodolakis, A.; Vlahos, G.; Thomakos, N.; Haidopoulos, D.; et al. VEGF directly suppresses activation of T cells from ovarian cancer patients and healthy individuals via VEGF receptor Type 2. Int. J. Cancer 2012, 130, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Couzi, L.; Merville, P.; Deminière, C.; Moreau, J.F.; Combe, C.; Pellegrin, J.L.; Viallard, J.F.; Blanco, P. Predominance of CD8+ T lymphocytes among periglomerular infiltrating cells and link to the prognosis of class III and class IV lupus nephritis. Arthritis Rheum. 2007, 56, 2362–2370. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Bag, A.K.; Singh, R.K.; Talmadge, J.E.; Batra, S.K.; Datta, K. Multifaceted Role of Neuropilins in the Immune System: Potential Targets for Immunotherapy. Front. Immunol. 2017, 10, 1228. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, M.C. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997, 40, 1725. [Google Scholar] [CrossRef]
- Gladman, D.D.; Ibanez, D.; Urowitz, M.B. Systemic lupus erythematosus disease activity index 2000. J. Rheumatol. 2002, 29, 288–291. [Google Scholar] [PubMed]
- Renal Disease Subcommittee of the American College of Rheumatology Ad Hoc Committee on Systemic Lupus Erythematosus Response Criteria. The American College of Rheumatology response criteria for proliferative and membranous renal disease in systemic lupus erythematosus clinical trials. Arthritis Rheum. 2006, 54, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Betsias, G.K.; Tektonidou, M.; Amoura, Z.; Aringer, M.; Bajema, I.; Berden, J.H.; Boletis, J.; Cervera, R.; Dörner, T.; Doria, A.; et al. Joint European League Against Rheumatism and Europena Renal association-European Dialysis and transplant Association (EULAR/ERA-EDTA) recommendations for the management of adult and paediatric lupus nephritis. Ann. Rheum. Dis. 2012, 71, 1771–1782. [Google Scholar] [CrossRef] [PubMed]
- Weening, J.J.; D’Agati, V.D.; Schwartz, M.M.; Seshan, S.V.; Alpers, C.E.; Appel, G.B.; Balow, J.E.; Brujin, J.A.; Cook, T.; Ferrario, F.; et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J. Am. Soc. Nephrol. 2004, 15, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Hernández, J.; Torres-Salido, M.T.; Medrano, A.S.; Tarrés, M.V.; Ordi-Ros, J. Long-term outcomes--mycophenolate mofetil treatment for lupus nephritis with addition of tacrolimus for resistant cases. Nephrol. Dial. Transplant. 2010, 25, 3939–3948. [Google Scholar] [CrossRef] [Green Version]






| Characteristics | Active Lupus Nephritis (n = 70) | Active Non-Renal SLE (n = 25) | Other Glomerular Diseases (n = 25) | Healthy Controls (n = 25) | p Value a |
|---|---|---|---|---|---|
| Demographic | |||||
| Age, yrs | 41 ± 10 | 34 ± 6 | 35 ± 5 | 30 ± 4 | 0.4857 (0.787) [0.885] |
| Gender (Female/male) | 56/14 | 21/4 | 16/9 | 16/9 | 0.112 (0.723) [0.112] |
| Race/ethnicity, n (%) | |||||
| White Hispanic | 65 (93) 4 (6) | 22 (88) 2 (8) | 23 (92) 1 (4) | 22 (88) 2 (8) | 0.966 (0.966) [0.500] |
| Other | 1 (1) | 1 (4) | 1 (4) | 1 (4) | |
| Laboratory parameters | |||||
| Serum creatinine, mg/dL | 1.1 ± 0.5 | 0.8 ± 0.1 | 1.9 ± 0.8 | 0.8 ± 0.3 | 0.035 (0.006) [0.001] |
| eGFR (mL/min) | 82 ± 30 | 104 ± 16 | 48 ± 36 | 95 ± 22 | 0.091 (0.001) [0.006] |
| Urea (mg/dL) | 54 ± 29 | 34 ± 14 | 81 ± 48 | 28 ± 11 | 0.076 (0.152) [0.012] |
| Anti-dsDNA Abs, IU/mL | 287 ± 68 | 39 ± 29 | n.a. | n.a. | (0.087) |
| Serum C3, mg/dL | 78 ± 31 | 94 ± 20 | n.a. | n.a. | (0.101) |
| Serum C4, mg/dL | 12.8 ± 9.2 | 13.4 ± 10 | n.a. | n.a. | (0.090) |
| Proteinuria, g/24 h | 3.3 ± 3.0 | 0.2± 0.1 | 9,1 ± 4,8 | n.a. | (0.010) [0.001] |
| Leukocytes (cel/µL) | 95 ± 114 | n.a. | n.a. | n.a. | |
| Erythrocytes (cel/µL) | 133 ± 131 | n.a. | n.a. | n.a. | |
| Disease index (SLEDAI-2K) | |||||
| Total score | 16 ± 2 | 8 ± 2 | n.a. | n.a. | (0.006) |
| Renal score | 12 ± 1 | 0 | n.a. | n.a. | |
| Extra-Renal score | 4 ± 2 | 8 ± 2 | n.a. | n.a. | (0.001) |
| Renal Flare, n (%) | |||||
| Proteinuric | 59 (84) | n.a. | n.a. | n.a. | |
| Nephritic | 11 (16) | n.a. | n.a. | n.a. | |
| First flare | 41 (59) | n.a. | n.a. | n.a. | |
| Relapsing flare | 29 (41) | n.a. | n.a. | n.a. | |
| Renal Biopsy, n (%) | |||||
| Class III | 9 (13) | n.a. | n.a. | n.a. | |
| Class IV | 53 (76) | n.a. | n.a. | n.a. | |
| Class V | 8 (11) | n.a. | n.a. | n.a. | |
| Activity Index | 7.5 ± 3.6 | n.a. | n.a. | n.a. | |
| Chronicity Index | 2.0 ± 1.9 | n.a. | n.a. | n.a. |
| Characteristics | Responders | Non-Responders | |
|---|---|---|---|
| Active LN Cohort (n = 38) | Active LN Cohort (n = 32) | p Value | |
| Demographic | |||
| Age (years) | 42 ± 12 | 39 ± 8 | 0.517 |
| Gender (Female/male) | 33/5 | 27/5 | |
| Race/ethnicity, n (%) | |||
| White | 35 (92) | 30 (94) | 0.811 |
| Hispanic Other | 2 (5) 1 (3) | 2 (6) 0 | |
| Laboratory parameters | |||
| Serum creatinine, mg/dL | 1.0 ± 0.4 | 1.3 ± 0.6 | 0.025 |
| eGFR (mL/min) | 88 ± 26 | 75 ± 33 | 0.167 |
| Urea (mg/dL) | 48 ± 27 | 61 ± 30 | 0.054 |
| Anti-dsDNA Abs, IU/mL | 344 ± 84 | 220 ± 68 | 0.781 |
| Serum C3, mg/dL | 77 ± 32 | 79 ± 29 | 0.878 |
| Serum C4, mg/dL | 12.0 ± 9.6 | 13.7 ± 8.8 | 0.248 |
| Proteinuria, g/24 h | 3.0 ± 3.2 | 3.4 ± 2.7 | 0.563 |
| Leukocytes (cel/µL) | 95 ± 114 | 101 ± 190 | 0.495 |
| Erythrocytes (cel/µL) | 133 ± 131 | 187 ± 397 | 0.158 |
| Disease index (SLEDAI-2K) | |||
| Total score | 14 ± 3 | 15 ± 2 | 0.145 |
| Renal score | 10 ± 2 | 9 ± 3 | 0.112 |
| Extra-Renal score | 5 ± 3 | 4 ± 2 | 0.184 |
| Renal Flare, n (%) | |||
| Proteinuric | 38 (100) | 14 (44) | <0.0001 |
| Nephritic | 0 | 18 (56) | <0.0001 |
| First flare | 27 (71) | 14 (44) | 0.021 |
| Relapsing flare | 11 (29) | 18 (56) | 0.021 |
| Renal Biopsy, n (%) | |||
| Class III | 4 (11%) | 3 (9%) | 0.714 |
| Class IV | 29 (76%) | 26 (82%) | 0.618 |
| Class V | 5 (13%) | 3 (9%) | 0.441 |
| Activity Index | 7.4± 3.2 | 7.6 ± 4.0 | 0.864 |
| Chronicity Index | 1.7 ± 2.1 | 2.2 ± 1.8 | 0.203 |
| Characteristics | Responders (n = 22) | Non-Responders (n = 17) | p Value |
|---|---|---|---|
| Demographic | |||
| Age (years) | 42 ± 13 | 38 ± 9 | 0.198 |
| Gender (Female/male) | 17/5 | 8/9 | |
| Race/ethnicity, n (%) | |||
| White | 21 (95) | 14 (82) | 0.193 |
| Hispanics | 1 (5) | 3 (18) | |
| Laboratory parameters | |||
| Serum creatinine, mg/dL | 1.0 ± 0.7 | 1.3 ± 0.6 | 0.026 |
| eGFR (mL/min) | 88 ± 26 | 79 ± 33 | 0.172 |
| Urea (mg/dL) | 51 ± 27 | 61 ± 30 | 0.074 |
| Anti-dsDNA Abs, IU/mL | 324 ± 89 | 280 ± 85 | 0.819 |
| Serum C3, mg/dL | 75 ± 28 | 78 ± 30 | 0.941 |
| Serum C4, mg/dL | 12.4 ± 9.0 | 13.2 ± 8.7 | 0.637 |
| Proteinuria, g/24 h | 3.4 ± 3.1 | 3.3 ± 2.9 | 0.266 |
| Leukocytes (cel/µL) | 105 ± 114 | 101 ± 150 | 0.214 |
| Erythrocytes (cel/µL) | 183 ± 121 | 159 ± 197 | 0.431 |
| Disease index (SLEDAI-2K) | |||
| Total score | 14 ± 3 | 15 ± 2 | 0.092 |
| Renal score | 10 ± 2 | 9 ± 3 | 0.249 |
| Extra-Renal score | 5 ± 3 | 4 ± 2 | 0.142 |
| Renal Flare, n (%) | |||
| Nephritic | 0 | 11 (65) | 0.082 |
| Proteinuric | 22 (100) | 6 (35) | 0.082 |
| First episode | 13 (59) | 7 (41) | 0.107 |
| Relapsing | 9 (41) | 10 (59) | 0.107 |
| Renal Biopsy, n (%) | |||
| Class III | 3 (14) | 2 (12) | 0.212 |
| Class IV | 15 (68) | 12 (71) | 0.966 |
| Class V | 4 (18) | 3 (18) | 0.791 |
| Activity Index | 7.0± 3.4 | 7.4 ± 3.8 | 0.936 |
| Chronicity Index | 1.5 ± 2.0 | 2.0 ± 1.5 | 0.377 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Salido, M.T.; Sanchis, M.; Solé, C.; Moliné, T.; Vidal, M.; Vidal, X.; Solà, A.; Hotter, G.; Ordi-Ros, J.; Cortés-Hernández, J. Urinary Neuropilin-1: A Predictive Biomarker for Renal Outcome in Lupus Nephritis. Int. J. Mol. Sci. 2019, 20, 4601. https://doi.org/10.3390/ijms20184601
Torres-Salido MT, Sanchis M, Solé C, Moliné T, Vidal M, Vidal X, Solà A, Hotter G, Ordi-Ros J, Cortés-Hernández J. Urinary Neuropilin-1: A Predictive Biomarker for Renal Outcome in Lupus Nephritis. International Journal of Molecular Sciences. 2019; 20(18):4601. https://doi.org/10.3390/ijms20184601
Chicago/Turabian StyleTorres-Salido, Maria Teresa, Mireia Sanchis, Cristina Solé, Teresa Moliné, Marta Vidal, Xavier Vidal, Anna Solà, Georgina Hotter, Josep Ordi-Ros, and Josefina Cortés-Hernández. 2019. "Urinary Neuropilin-1: A Predictive Biomarker for Renal Outcome in Lupus Nephritis" International Journal of Molecular Sciences 20, no. 18: 4601. https://doi.org/10.3390/ijms20184601
APA StyleTorres-Salido, M. T., Sanchis, M., Solé, C., Moliné, T., Vidal, M., Vidal, X., Solà, A., Hotter, G., Ordi-Ros, J., & Cortés-Hernández, J. (2019). Urinary Neuropilin-1: A Predictive Biomarker for Renal Outcome in Lupus Nephritis. International Journal of Molecular Sciences, 20(18), 4601. https://doi.org/10.3390/ijms20184601