Heightened TLR7/9-Induced IL-10 and CXCL13 Production with Dysregulated NF-ҝB Activation in CD11chiCD11b+ Dendritic Cells in NZB/W F1 Mice
Abstract
:1. Introduction
2. Results
2.1. Expanded CD11chiCD11b+ Dendritic Cells Display Aberrant Phenotypes during SLE Development
2.2. CD11chiCD11b+ Dendritic Cells Display Heightened TLR7/9 Response during Disease State
2.3. Enhanced TLR7/9 Signaling in CD11chiCD11b+ in F1 Mice at Disease State
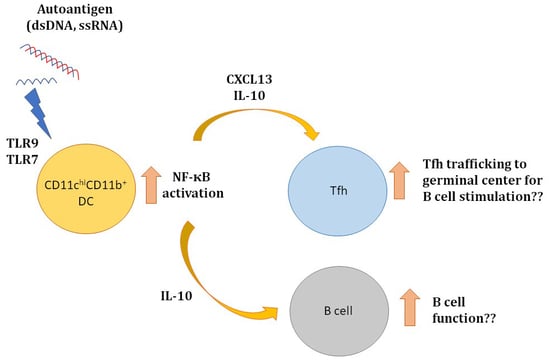
3. Discussion
4. Materials and Methods
4.1. Mouse Colonies and Disease Monitoring
4.2. Antibodies for Immunophenotyping
4.3. Purification of and Stimulation of CD11chiCD11b+ DCs
4.4. Allogeneic T Cell Proliferation Assay
4.5. Quantitative RT-PCR
4.6. ELISA
4.7. Immunoblotting
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Petri, M.; Orbai, A.M.; Alarcon, G.S.; Gordon, C.; Merrill, J.T.; Fortin, P.R.; Bruce, I.N.; Isenberg, D.; Wallace, D.J.; Nived, O.; et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012, 64, 2677–2686. [Google Scholar] [CrossRef] [PubMed]
- Swiecki, M.; Colonna, M. The multifaceted biology of plasmacytoid dendritic cells. Nat. Rev. Immunol. 2015, 15, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, Y.; Kumar, H.; Koyama, S.; Kawai, T.; Takeuchi, O.; Akira, S. Cutting Edge: TLR-Dependent Viral Recognition Along with Type I IFN Positive Feedback Signaling Masks the Requirement of Viral Replication for IFN-α Production in Plasmacytoid Dendritic Cells. J. Immunol. 2009, 182, 3960–3964. [Google Scholar] [CrossRef] [PubMed]
- Young, L.J.; Wilson, N.S.; Schnorrer, P.; Proietto, A.; ten Broeke, T.; Matsuki, Y.; Mount, A.M.; Belz, G.T.; O’Keeffe, M.; Ohmura-Hoshino, M.; et al. Differential MHC class II synthesis and ubiquitination confers distinct antigen-presenting properties on conventional and plasmacytoid dendritic cells. Nat. Immunol. 2008, 9, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Chan, V.S.F.; Nie, Y.J.; Shen, N.; Yan, S.; Mok, M.Y.; Lau, C.S. Distinct roles of myeloid and plasmacytoid dendritic cells in systemic lupus erythematosus. Autoimmun. Rev. 2012, 11, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Dall’Era, M.C.; Cardarelli, P.M.; Preston, B.T.; Witte, A.; Davis, J.C. Type I interferon correlates with serological and clinical manifestations of SLE. Ann. Rheum. Dis. 2005, 64, 1692–1697. [Google Scholar] [CrossRef] [PubMed]
- Baechler, E.C.; Batliwalla, F.M.; Karypis, G.; Gaffney, P.M.; Ortmann, W.A.; Espe, K.J.; Shark, K.B.; Grande, W.J.; Hughes, K.M.; Kapur, V.; et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc. Natl. Acad. Sci. USA 2003, 100, 2610–2615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, O.; Kavikondala, S.; Mok, M.Y.; Sun, L.; Gu, J.; Fu, R.; Chan, A.; Yeung, J.; Nie, Y.; Lau, C.S. Abnormalities in circulating plasmacytoid dendritic cells in patients with systemic lupus erythematosus. Arthritis Res. Ther. 2010, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fiore, N.; Castellano, G.; Blasi, A.; Capobianco, C.; Loverre, A.; Montinaro, V.; Netti, S.; Torres, D.; Manno, C.; Grandaliano, G.; et al. Immature myeloid and plasmacytoid dendritic cells infiltrate renal tubulointerstitium in patients with lupus nephritis. Mol. Immunol. 2008, 45, 259–265. [Google Scholar] [CrossRef]
- Vermi, W.; Lonardi, S.; Morassi, M.; Rossini, C.; Tardanico, R.; Venturini, M.; Sala, R.; Tincani, A.; Poliani, P.L.; Calzavara-Pinton, P.G.; et al. Cutaneous distribution of plasmacytoid dendritic cells in lupus erythematosus. Selective tropism at the site of epithelial apoptotic damage. Immunobiology 2009, 214, 877–886. [Google Scholar] [CrossRef]
- Jin, O.; Kavikondala, S.; Sun, L.; Fu, R.; Mok, M.Y.; Chan, A.; Yeung, J.; Lau, C.S. Systemic lupus erythematosus patients have increased number of circulating plasmacytoid dendritic cells, but decreased myeloid dendritic cells with deficient CD83 expression. Lupus 2008, 17, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Nowatzky, J.; Jiménez-Branda, S.; Greenberg, J.D.; Clancy, R.; Buyon, J.; Bhardwaj, N. Active systemic lupus erythematosus is associated with decreased blood conventional dendritic cells. Exp. Mol. Pathol. 2013, 95, 121–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, D.; Mehta, H.; McCune, W.J.; Kaplan, M.J. Aberrant Phenotype and Function of Myeloid Dendritic Cells in Systemic Lupus Erythematosus. J. Immunol. 2006, 177, 5878–5889. [Google Scholar] [CrossRef] [PubMed]
- Boulé, M.W.; Broughton, C.; Mackay, F.; Akira, S.; Marshak-Rothstein, A.; Rifkin, I.R. Toll-like Receptor 9–Dependent and –Independent Dendritic Cell Activation by Chromatin–Immunoglobulin G Complexes. J. Exp. Med. 2004, 199, 1631–1640. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, K.; Richez, C.; Maciaszek, J.W.; Agrawal, N.; Akira, S.; Marshak-Rothstein, A.; Rifkin, I.R. Murine Dendritic Cell Type I IFN Production Induced by Human IgG-RNA Immune Complexes Is IFN Regulatory Factor (IRF)5 and IRF7 Dependent and Is Required for IL-6 Production. J. Immunol. 2007, 178, 6876–6885. [Google Scholar] [CrossRef] [PubMed]
- Richez, C.; Blanco, P.; Rifkin, I.; Moreau, J.-F.; Schaeverbeke, T. Role for toll-like receptors in autoimmune disease: The example of systemic lupus erythematosus. Jt. Bone Spine 2011, 78, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, A.; Herrmann, M.; Kleber, S.; Beckmann-Welle, M.; Fehsel, K.; Martin-Villalba, A.; Lehmann, P.; Ruzicka, T.; Krammer, P.H.; Kolb-Bachofen, V. Accumulation of apoptotic cells in the epidermis of patients with cutaneous lupus erythematosus after ultraviolet irradiation. Arthritis Rheum. 2006, 54, 939–950. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, M.; Voll, R.E.; Zoller, O.M.; Hagenhofer, M.; Ponner, B.B.; Kalden, J.R. Impaired phagocytosis of apoptotic cell material by monocyte-derived macrophages from patients with systemic lupus erythematosus. Arthritis Rheum. 1998, 41, 1241–1250. [Google Scholar] [CrossRef]
- Baumann, I.; Kolowos, W.; Voll, R.E.; Manger, B.; Gaipl, U.; Neuhuber, W.L.; Kirchner, T.; Kalden, J.R.; Herrmann, M. Impaired uptake of apoptotic cells into tingible body macrophages in germinal centers of patients with systemic lupus erythematosus. Arthritis Rheum. 2002, 46, 191–201. [Google Scholar] [CrossRef]
- Dieker, J.; Tel, J.; Pieterse, E.; Thielen, A.; Rother, N.; Bakker, M.; Fransen, J.; Dijkman, H.B.P.M.; Berden, J.H.; de Vries, J.M.; et al. Circulating Apoptotic Microparticles in Systemic Lupus Erythematosus Patients Drive the Activation of Dendritic Cell Subsets and Prime Neutrophils for NETosis. Arthritis Rheumatol. 2016, 68, 462–472. [Google Scholar] [CrossRef]
- Karrich, J.J.; Jachimowski, L.C.M.; Uittenbogaart, C.H.; Blom, B. The Plasmacytoid Dendritic Cell as the Swiss Army Knife of the Immune System: Molecular Regulation of Its Multifaceted Functions. J. Immunol. 2014, 193, 5772. [Google Scholar] [CrossRef] [PubMed]
- Bynote, K.K.; Hackenberg, J.M.; Korach, K.S.; Lubahn, D.B.; Lane, P.H.; Gould, K.A. Estrogen receptor-α deficiency attenuates autoimmune disease in (NZB/NZW)F1 mice. Genes Immun. 2008, 9, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Mathian, A.; Weinberg, A.; Gallegos, M.; Banchereau, J.; Koutouzov, S. IFN-alpha induces early lethal lupus in preautoimmune (New Zealand Black x New Zealand White) F1 but not in BALB/c mice. J. Immunol. 2005, 174, 2499–2506. [Google Scholar] [CrossRef] [PubMed]
- Gleisner, M.A.; Reyes, P.; Alfaro, J.; Solanes, P.; Simon, V.; Crisostomo, N.; Sauma, D.; Rosemblatt, M.; Bono, M.R. Dendritic and stromal cells from the spleen of lupic mice present phenotypic and functional abnormalities. Mol. Immunol. 2013, 54, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Blasius, A.L.; Giurisato, E.; Cella, M.; Schreiber, R.D.; Shaw, A.S.; Colonna, M. Bone Marrow Stromal Cell Antigen 2 Is a Specific Marker of Type I IFN-Producing Cells in the Naive Mouse, but a Promiscuous Cell Surface Antigen following IFN Stimulation. J. Immunol. 2006, 177, 3260–3265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merad, M.; Sathe, P.; Helft, J.; Miller, J.; Mortha, A. The Dendritic Cell Lineage: Ontogeny and Function of Dendritic Cells and Their Subsets in the Steady State and the Inflamed Setting. Annu. Rev. Immunol. 2013, 31. [Google Scholar] [CrossRef]
- Yan, S.; Yim, L.Y.; Tam, R.C.Y.; Chan, A.; Lu, L.; Lau, C.S.; Chan, V.S.F. MicroRNA-155 Mediates Augmented CD40 Expression in Bone Marrow Derived Plasmacytoid Dendritic Cells in Symptomatic Lupus-Prone NZB/W F1 Mice. Int. J. Mol. Sci. 2016, 17, 1282. [Google Scholar] [CrossRef]
- Ise, W.; Fujii, K.; Shiroguchi, K.; Ito, A.; Kometani, K.; Takeda, K.; Kawakami, E.; Yamashita, K.; Suzuki, K.; Okada, T.; et al. T Follicular Helper Cell-Germinal Center B Cell Interaction Strength Regulates Entry into Plasma Cell or Recycling Germinal Center Cell Fate. Immunity 2018, 48, 702–715. [Google Scholar] [CrossRef]
- Hacker, H.; Redecke, V.; Blagoev, B.; Kratchmarova, I.; Hsu, L.-C.; Wang, G.G.; Kamps, M.P.; Raz, E.; Wagner, H.; Hacker, G.; et al. Specificity in Toll-like receptor signalling through distinct effector functions of TRAF3 and TRAF6. Nature 2006, 439, 204–207. [Google Scholar] [CrossRef]
- Pimenta, E.M.; De, S.; Weiss, R.; Feng, D.; Hall, K.; Kilic, S.; Bhanot, G.; Ganesan, S.; Ran, S.; Barnes, B.J. IRF5 is a novel regulator of CXCL13 expression in breast cancer that regulates CXCR5+ B- and T-cell trafficking to tumor-conditioned media. Immunol. Cell Biol. 2015, 93, 486–499. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, S.; Nagai, S.; Sato, T.; Akadegawa, K.; Yoneyama, H.; Zhang, Y.; Onai, N.; Matsushima, K. Increased circulating CD11b+CD11c+ dendritic cells (DC) in aged BWF1 mice which can be matured by TNF-alpha into BLC/CXCL13-producing DC. Eur. J. Immunol. 2002, 32, 1881–1887. [Google Scholar] [CrossRef]
- Talaei, N.; Cheung, Y.-H.; Landolt-Marticorena, C.; Noamani, B.; Li, T.; Wither, J.E. T Cell and Dendritic Cell Abnormalities Synergize to Expand Pro-Inflammatory T Cell Subsets Leading to Fatal Autoimmunity in B6.NZBc1 Lupus-Prone Mice. PLoS ONE 2013, 8, e75166. [Google Scholar] [CrossRef] [PubMed]
- Teichmann, L.L.; Ols, M.L.; Kashgarian, M.; Reizis, B.; Kaplan, D.H.; Shlomchik, M.J. Dendritic Cells in Lupus are not Required for Activation of T and B Cells but Promote their Expansion Resulting in Tissue Damage. Immunity 2010, 33, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Sauma, D.; Crisóstomo, N.; Fuentes, C.; Gleisner, M.A.; Hidalgo, Y.; Fuenzalida, M.J.; Rosemblatt, M.; Bono, M.R. Adoptive transfer of autoimmune splenic dendritic cells to lupus-prone mice triggers a B lymphocyte humoral response. Immunol. Res. 2017, 65, 957–968. [Google Scholar] [CrossRef] [Green Version]
- Wan, S.; Xia, C.; Morel, L. IL-6 Produced by Dendritic Cells from Lupus-Prone Mice Inhibits CD4+CD25+ T Cell Regulatory Functions. J. Immunol. 2007, 178, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Bethunaickan, R.; Berthier, C.C.; Ramanujam, M.; Sahu, R.; Zhang, W.; Sun, Y.; Bottinger, E.P.; Ivashkiv, L.; Kretzler, M.; Davidson, A. A Unique Hybrid Renal Mononuclear Phagocyte Activation Phenotype in Murine Systemic Lupus Erythematosus Nephritis. J. Immunol. 2011, 186, 4994–5003. [Google Scholar] [CrossRef] [Green Version]
- Furuta, K.; Ishido, S.; Roche, P.A. Encounter with antigen-specific primed CD4 T cells promotes MHC class II degradation in dendritic cells. Proc. Natl. Acad. Sci. USA 2012, 109, 19380–19385. [Google Scholar] [CrossRef] [Green Version]
- Ishida, H.; Muchamuel, T.; Sakaguchi, S.; Andrade, S.; Menon, S.; Howard, M. Continuous administration of anti-interleukin 10 antibodies delays onset of autoimmunity in NZB/W F1 mice. J. Exp. Med. 1994, 179, 305–310. [Google Scholar] [CrossRef]
- Yin, Z.; Bahtiyar, G.; Zhang, N.; Liu, L.; Zhu, P.; Robert, M.E.; McNiff, J.; Madaio, M.P.; Craft, J. IL-10 Regulates Murine Lupus. J. Immunol. 2002, 169, 2148–2155. [Google Scholar] [CrossRef] [Green Version]
- Blenman, K.R.M.; Duan, B.; Xu, Z.; Wan, S.; Atkinson, M.A.; Flotte, T.R.; Croker, B.P.; Morel, L. IL-10 regulation of lupus in the NZM2410 murine model. Lab. Investig. 2006, 86, 1136–1148. [Google Scholar] [CrossRef] [PubMed]
- Godsell, J.; Rudloff, I.; Kandane-Rathnayake, R.; Hoi, A.; Nold, M.F.; Morand, E.F.; Harris, J. Clinical associations of IL-10 and IL-37 in systemic lupus erythematosus. Sci. Rep. 2016, 6, 34604. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cho, B.; Suzuki, K.; Xu, Y.; Green, J.A.; An, J.; Cyster, J.G. Follicular dendritic cells help establish follicle identity and promote B cell retention in germinal centers. J. Exp. Med. 2011, 208, 2497–2510. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.K.; Wong, P.T.Y.; Tam, L.S.; Li, E.K.; Chen, D.P.; Lam, C.W.K. Elevated Production of B Cell Chemokine CXCL13 is Correlated with Systemic Lupus Erythematosus Disease Activity. J. Clin. Immunol. 2010, 30, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.T.; Shiao, Y.M.; Wu, T.H.; Chen, W.S.; Hsu, Y.H.; Tsai, S.F.; Tsai, C.Y. Serum BLC/CXCL13 Concentrations and Renal Expression of CXCL13/CXCR5 in Patients with Systemic Lupus Erythematosus and Lupus Nephritis. J. Rheumatol. 2009, 37, 45. [Google Scholar] [CrossRef] [PubMed]
- Vinuesa, C.G.; Cook, M.C.; Angelucci, C.; Athanasopoulos, V.; Rui, L.; Hill, K.M.; Yu, D.; Domaschenz, H.; Whittle, B.; Lambe, T.; et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature 2005, 435, 452–458. [Google Scholar] [CrossRef]
- Simpson, N.; Gatenby, P.A.; Wilson, A.; Malik, S.; Fulcher, D.A.; Tangye, S.G.; Manku, H.; Vyse, T.J.; Roncador, G.; Huttley, G.A.; et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum. 2010, 62, 234–244. [Google Scholar] [CrossRef]
- Ishikawa, S.; Sato, T.; Abe, M.; Nagai, S.; Onai, N.; Yoneyama, H.; Zhang, Y.; Suzuki, T.; Hashimoto, S.; Shirai, T. Aberrant high expression of B lymphocyte chemokine (BLC/CXCL13) by C11b+CD11c+ dendritic cells in murine lupus and preferential chemotaxis of B1 cells towards BLC. J. Exp. Med. 2001, 193, 1393–1402. [Google Scholar] [CrossRef]
- Sun, S. The non-canonical NF-κB pathway in immunity and inflammation. Nat. Rev. Immunol. 2017, 17, 545–558. [Google Scholar] [CrossRef]
- Teichmann Lino, L.; Schenten, D.; Medzhitov, R.; Kashgarian, M.; Shlomchik Mark, J. Signals via the Adaptor MyD88 in B Cells and DCs Make Distinct and Synergistic Contributions to Immune Activation and Tissue Damage in Lupus. Immunity 2013, 38, 528–540. [Google Scholar] [CrossRef] [Green Version]
- Sun, F.; Teng, J.; Yu, P.; Li, W.; Chang, J.; Xu, H. Involvement of TWEAK and the NF-κB signaling pathway in lupus nephritis. Exp. Ther. Med. 2018, 15, 2611–2619. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, H.; Huang, Y.; Wang, H.; Wang, S.; Zhao, C.; Liang, Y.; Yang, N. Bay11-7082 attenuates murine lupus nephritis via inhibiting NLRP3 inflammasome and NF-κB activation. Int. Immunopharmacol. 2013, 17, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Brightbill, H.D.; Suto, E.; Blaquiere, N.; Ramamoorthi, N.; Sujatha-Bhaskar, S.; Gogol, E.B.; Castanedo, G.M.; Jackson, B.T.; Kwon, Y.C.; Haller, S.; et al. NF-κB inducing kinase is a therapeutic target for systemic lupus erythematosus. Nat. Commun. 2018, 9, 179. [Google Scholar] [CrossRef] [PubMed]
- Saxena, V.; Ondr, J.K.; Magnusen, A.F.; Munn, D.H.; Katz, J.D. The countervailing actions of myeloid and plasmacytoid dendritic cells control autoimmune diabetes in the nonobese diabetic mouse. J. Immunol. 2007, 179, 5041–5053. [Google Scholar] [CrossRef] [PubMed]
- Guilliams, M.; Dutertre, C.A.; Scott, C.L.; McGovern, N.; Sichien, D.; Chakarov, S.; Van Gassen, S.; Chen, J.; Poidinger, M.; De Prijck, S.; et al. Unsupervised High-Dimensional Analysis Aligns Dendritic Cells across Tissues and Species. Immunity 2016, 45, 669–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Genes | Primer Sequence (Forward) | Primer Sequence (Reverse) | Product Size (bp) |
|---|---|---|---|
| Il-6 | GAAGTTCCTCTCTGCAAGAGAC | CCAGAGAACATGTGTAATTAAGC | 178 |
| Il-10 | GCCGGGAAGACAATAACTGC | TTCAGCTTCTCACCCAGGGA | 280 |
| Tnfa | AGCACAGAAAGCATGATCCGCG | AGAAGATGATCTGAGTGTGA | 249 |
| Baff | TCCAGCAGTTTCACAGCGAT | CCGGTGTCAGGAGTTTGACT | 157 |
| Cxcl13 | CACGGTATTCTGGAAGCCCA | AGACAGACTTTTGCTTTGGACA | 231 |
| Myd88 | TGTTCTTGAACCCTCGGACG | TTCTGGCAGTCCTCCTCGAT | 241 |
| Traf3 | CAGCGCACGGCACAGAATTT | CTTGTAGCCTCCTTGCTCCG | 149 |
| Irf5 | CTAGCCTGGATGTGGCATGT | CTCTTTAGCCCAGGCCTTGAA | 314 |
| Nfkb1 | CCACAAGGGGACATGAAGCA | GATGGTACCCCCAGAGACCT | 198 |
| β-actin | AGATCAAGATCATTGCTCCTCCT | ACGCAGCTCAGTAACAGTCC | 174 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yim, L.Y.; Lau, C.S.; Chan, V.S.-F. Heightened TLR7/9-Induced IL-10 and CXCL13 Production with Dysregulated NF-ҝB Activation in CD11chiCD11b+ Dendritic Cells in NZB/W F1 Mice. Int. J. Mol. Sci. 2019, 20, 4639. https://doi.org/10.3390/ijms20184639
Yim LY, Lau CS, Chan VS-F. Heightened TLR7/9-Induced IL-10 and CXCL13 Production with Dysregulated NF-ҝB Activation in CD11chiCD11b+ Dendritic Cells in NZB/W F1 Mice. International Journal of Molecular Sciences. 2019; 20(18):4639. https://doi.org/10.3390/ijms20184639
Chicago/Turabian StyleYim, Lok Yan, Chak Sing Lau, and Vera Sau-Fong Chan. 2019. "Heightened TLR7/9-Induced IL-10 and CXCL13 Production with Dysregulated NF-ҝB Activation in CD11chiCD11b+ Dendritic Cells in NZB/W F1 Mice" International Journal of Molecular Sciences 20, no. 18: 4639. https://doi.org/10.3390/ijms20184639
APA StyleYim, L. Y., Lau, C. S., & Chan, V. S. -F. (2019). Heightened TLR7/9-Induced IL-10 and CXCL13 Production with Dysregulated NF-ҝB Activation in CD11chiCD11b+ Dendritic Cells in NZB/W F1 Mice. International Journal of Molecular Sciences, 20(18), 4639. https://doi.org/10.3390/ijms20184639