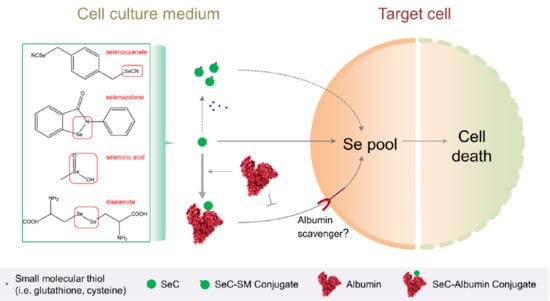
Extracellular Albumin Covalently Sequesters Selenocompounds and Determines Cytotoxicity
Abstract
:1. Introduction
2. Results
2.1. Concomitant Treatment with Albumin Decreased Selenocompound Cytotoxicity
2.2. Reduced Cellular Uptake of Selenocompounds Underlined the Diminished Cytotoxicity
2.3. Selenocompounds Covalently Bind to Extracellular Albumin
2.4. The Role of Albumin and Small Molecular Thiols in Selenocompound Transformation
2.5. Albumin was Actively Endocytosed
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Culture
4.3. Preparation of Selenocompound Stock Solutions
4.4. Assessment of Cell Viability
4.5. Measurement of Intracellular Selenium Element
4.6. Preparation of Albumin Conjugate and Serum Mixture of Selenocompounds
4.7. Selenium Speciation by X-Ray Absorbance Spectroscopy
4.8. Speciation of p-Xyleneselenocyanate by Liquid Chromatography–Mass Spectrometry
4.9. Assessment of Cellular Uptake of Albumin
4.10. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Se | Selenium |
| SeC | Selenocompound |
| GSH | Glutathione |
| Cys | Cysteine |
| BSA | Bovine serum albumin |
| FITC-BSA | Fluorescein-isothiocyanate-labeled bovine serum albumin |
| SeC–BSA | The conjugate between selenocompound and bovine serum albumin |
| SeC–FBS | The mixture of selenocompound and fetal bovine serum |
| SeC–SM | The conjugate between selenocompound and small-molecule thiol (like GSH and Cys) |
| ROS | Reactive oxygen species |
| p-XSC | p-xyleneselenocyanate |
| CysSe2 | Selenocystine |
| MeSeA | Methylseleninic acid |
| p-XSC-SM | The conjugate between p-xyleneselenocyanate and small-molecule thiol (like GSH and Cys) |
| p-XSC-BSA | The conjugate between p-xyleneselenocyanate and bovine serum albumin |
| LC-MS | Liquid chromatography-mass spectrometry |
| XAS | X-ray absorption spectroscopy |
| XANES | X-ray absorption near edge spectra |
| EXAFS | Extended X-ray absorption fine structure |
References
- Reich, H.J.; Hondal, R.J. Why Nature Chose Selenium. ACS Chem. Biol. 2016, 11, 821–841. [Google Scholar] [CrossRef]
- Gandin, V.; Khalkar, P.; Braude, J.; Fernandes, A.P. Organic selenium compounds as potential chemotherapeutic agents for improved cancer treatment. Free Radic. Biol. Med. 2018, 127, 80–97. [Google Scholar] [CrossRef]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug discov. 2009, 8, 579. [Google Scholar] [CrossRef] [PubMed]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Zhou, Y.; Zhang, H.; Demizu, Y.; Chen, Z.; Pelicano, H.; Chiao, P.J.; Achanta, G.; Arlinghaus, R.B.; Liu, J.; et al. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by β-phenylethyl isothiocyanate. Cancer Cell 2006, 10, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Wang, J.-Q.; Assaraf, Y.G.; Ren, L.; Gupta, P.; Wei, L.; Ashby, C.R., Jr.; Yang, D.-H.; Chen, Z.-S. Modulating ROS to overcome multidrug resistance in cancer. Drug Resist. Updates 2018, 41, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.-F.; Yang, Y.; Wu, L.; Ma, W.-W.; Zeng, H.-H. Ethaselen: A novel organoselenium anticancer agent targeting thioredoxin reductase 1 reverses cisplatin resistance in drug-resistant K562 cells by inducing apoptosis. J. Zhejiang Univ. Sci. B 2017, 18, 373–382. [Google Scholar] [CrossRef]
- Brodin, O.; Eksborg, S.; Wallenberg, M.; Asker-Hagelberg, C.; Larsen, E.H.; Mohlkert, D.; Lenneby-Helleday, C.; Jacobsson, H.; Linder, S.; Misra, S. Pharmacokinetics and toxicity of sodium selenite in the treatment of patients with carcinoma in a phase I clinical trial: The SECAR study. Nutrients 2015, 7, 4978–4994. [Google Scholar] [CrossRef]
- Karelia, D.N.; Kim, S.; Ramisetti, S.; Jiang, C.; Amin, S.; Sharma, A.K.; Lu, J. Novel selenium-containing aspirin molecule AS-10 suppresses androgen receptor signaling and induces apoptosis of LNCap prostate cancer cells. In Proceedings of the AACR Annual Meeting 2017, Washington, DC, USA, 1–5 April 2017. [Google Scholar]
- Wagner, M.S.; Schultze, E.; Oliveira, T.L.; de Leon, P.M.M.; Thurow, H.S.; Campos, V.F.; Oliveira, I.; de Souza, D.; Rodrigues, O.E.D.; Collares, T. Revitalizing the AZT through of the selenium: An approach in human triple negative breast cancer cell line. Front. Oncol. 2018, 8, 8. [Google Scholar] [CrossRef]
- Khandelwal, S.; Gollahon, L.; Spallholz, J.; Boylan, M.; Garcia-Hernandez, M.D.M. Cytotoxicity of selenium trastuzumab and bevacizumab immunoconjugates against triple negative breast cancer cells. In Proceedings of the AACR Annual Meeting 2017, Washington, DC, USA, 1–5 April 2017. [Google Scholar]
- Fernandes, A.P.; Gandin, V. Selenium compounds as therapeutic agents in cancer. Biochim. Biophys. Acta 2015, 1850, 1642–1660. [Google Scholar] [CrossRef]
- Nogueira, C.W.; Rocha, J.B. Toxicology and pharmacology of selenium: Emphasis on synthetic organoselenium compounds. Arch. Toxicol. 2011, 85, 1313–1359. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.T.; Kurasaki, K.; Suzuki, N. Selenocysteine β-lyase and methylselenol demethylase in the metabolism of Se-methylated selenocompounds into selenide. Biochim. Biophys. Acta Gen. Subj. 2007, 1770, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Spallholz, J.E.; Palace, V.P.; Reid, T.W. Methioninase and selenomethionine but not Se-methylselenocysteine generate methylselenol and superoxide in an In Vitro chemiluminescent assay: Implications for the nutritional carcinostatic activity of selenoamino acids. Biochem. Pharmacol. 2004, 67, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Weekley, C.; Aitken, J.; Finney, L.; Vogt, S.; Witting, P.; Harris, H. Selenium Metabolism in Cancer Cells: The Combined Application of XAS and XFM Techniques to the Problem of Selenium Speciation in Biological Systems. Nutrients 2013, 5, 1734. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Endo, M.; Shinohara, F.; Echigo, S.; Rikiishi, H. Differential apoptotic response of human cancer cells to organoselenium compounds. Cancer Chemother. Pharmacol. 2010, 66, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-F.; Shen, H.-M.; Ong, C.-N. Ebselen Induces Apoptosis in HepG2 Cells through Rapid Depletion of Intracellular Thiols. Arch. Biochem. Biophys. 2000, 374, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Azad, G.K.; Tomar, R.S. Ebselen, a promising antioxidant drug: Mechanisms of action and targets of biological pathways. Mol. Biol. Rep. 2014, 41, 4865–4879. [Google Scholar] [CrossRef]
- Wu, W.; Karelia, D.; Pramanik, K.; Amin, S.G.; Sharma, A.K.; Jiang, C.; Lu, J. Phenylbutyl isoselenocyanate induces reactive oxygen species to inhibit androgen receptor and to initiate p53-mediated apoptosis in LNCaP prostate cancer cells. Mol. Carcinog. 2018, 57, 1055–1066. [Google Scholar] [CrossRef]
- Sanmartín, C.; Plano, D.; Sharma, A.K.; Palop, J.A. Selenium Compounds, Apoptosis and Other Types of Cell Death: An Overview for Cancer Therapy. Int. J. Mol. Sci. 2012, 13, 9649–9672. [Google Scholar] [CrossRef]
- Turell, L.; Radi, R.; Alvarez, B. The thiol pool in human plasma: The central contribution of albumin to redox processes. Free Radic.Biol. Med. 2013, 65, 244–253. [Google Scholar] [CrossRef] [Green Version]
- Sohn, O.S.; Desai, D.H.; Das, A.; Rodriguez, J.G.; Amin, S.G.; El-Bayoumy, K. Comparative excretion and tissue distribution of selenium in mice and rats following treatment with the chemopreventive agent 1,4-phenylenebis(methylene)selenocyanate. Chem.-Biol. Interact. 2005, 151, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Benkessou, F.; Twelkmeyer, B.; Wang, S.; Ginman, T.; Ottosson, H.; Abedi-Valugerdi, M.; Subirana, M.A.; Zhao, Y.; Hassan, M. Rapid and Robust Quantification of p-Xyleneselenocyanate in Plasma via Derivatization. Anal. Chem. 2017, 89, 7586–7592. [Google Scholar] [CrossRef] [PubMed]
- Nojiri, S.; Joh, T. Albumin suppresses human hepatocellular carcinoma proliferation and the cell cycle. Int. J. Mol. Sci. 2014, 15, 5163–5174. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; Yamaguchi, M. Role of albumin in osteoblastic cells: Enhancement of cell proliferation and suppression of alkaline phosphatase activity. Int. J. Mol. Med. 2004, 14, 1077–1081. [Google Scholar] [CrossRef] [PubMed]
- Olm, E.; Fernandes, A.P.; Hebert, C.; Rundlöf, A.-K.; Larsen, E.H.; Danielsson, O.; Björnstedt, M. Extracellular thiol-assisted selenium uptake dependent on the x-cystine transporter explains the cancer-specific cytotoxicity of selenite. Proc. Natl. Acad. Sci. USA 2009, 106, 11400–11405. [Google Scholar] [CrossRef] [PubMed]
- Pietrella, D. Antimicrobial Activity of Organoselenium Compounds. In Organoselenium Chemistry: Between Synthesis and Biochemistry; Bentham Science Publishers: Sharjah, UAE, 2014; pp. 328–344. [Google Scholar] [Green Version]
- Bern, M.; Sand, K.M.K.; Nilsen, J.; Sandlie, I.; Andersen, J.T. The role of albumin receptors in regulation of albumin homeostasis: Implications for drug delivery. J. Control. Release 2015, 211, 144–162. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Chuang, V.T.G.; Maruyama, T.; Otagiri, M. Albumin–drug interaction and its clinical implication. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 5435–5443. [Google Scholar] [CrossRef] [PubMed]
- Cavalieri, R.R.; Scott, K.G. Sodium selenite se 75: A more specific agent for scanning tumors. JAMA 1968, 206, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Simonelli, L.; Marini, C.; Olszewski, W.; Avila Perez, M.; Ramanan, N.; Guilera, G.; Cuartero, V.; Klementiev, K. CLÆSS: The hard X-ray absorption beamline of the ALBA CELLS synchrotron. Cogent Phys. 2016, 3, 1231987. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef]





| SeC | Atom | Number | E0 (eV) | R (Å) | σ2 (Å2) | R-Factor |
|---|---|---|---|---|---|---|
| CysSe2 | C | 1 | 4.8 | 2.003 | 0.006 | 0.022 |
| S | 0.4 | 2.139 | 0.004 | |||
| Se | 0.6 | 2.322 | 0.001 | |||
| MeSeA | O | 1 | 4.0 | 1.682 | 0.003 | 0.008 |
| C | 1 | 2.359 | 0.004 | |||
| S | 1 | 2.203 | 0.008 | |||
| Ebselen | C | 1 | 5.0 | 1.920 | 0.004 | 0.016 |
| S | 0.4 | 2.191 | 0.002 | |||
| Se | 0.6 | 2.355 | 0.004 | |||
| p-XSC | C | 1 | 6.5 | 2.009 | 0.003 | 0.017 |
| S | 1 | 2.185 | 0.002 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, W.; Boada, R.; He, R.; Xiao, T.; Ye, F.; Simonelli, L.; Valiente, M.; Zhao, Y.; Hassan, M. Extracellular Albumin Covalently Sequesters Selenocompounds and Determines Cytotoxicity. Int. J. Mol. Sci. 2019, 20, 4734. https://doi.org/10.3390/ijms20194734
Zheng W, Boada R, He R, Xiao T, Ye F, Simonelli L, Valiente M, Zhao Y, Hassan M. Extracellular Albumin Covalently Sequesters Selenocompounds and Determines Cytotoxicity. International Journal of Molecular Sciences. 2019; 20(19):4734. https://doi.org/10.3390/ijms20194734
Chicago/Turabian StyleZheng, Wenyi, Roberto Boada, Rui He, Tingting Xiao, Fei Ye, Laura Simonelli, Manuel Valiente, Ying Zhao, and Moustapha Hassan. 2019. "Extracellular Albumin Covalently Sequesters Selenocompounds and Determines Cytotoxicity" International Journal of Molecular Sciences 20, no. 19: 4734. https://doi.org/10.3390/ijms20194734
APA StyleZheng, W., Boada, R., He, R., Xiao, T., Ye, F., Simonelli, L., Valiente, M., Zhao, Y., & Hassan, M. (2019). Extracellular Albumin Covalently Sequesters Selenocompounds and Determines Cytotoxicity. International Journal of Molecular Sciences, 20(19), 4734. https://doi.org/10.3390/ijms20194734