Anti-Myocardial Infarction Effects of Radix Aconiti Lateralis Preparata Extracts and Their Influence on Small Molecules in the Heart Using Matrix-Assisted Laser Desorption/Ionization–Mass Spectrometry Imaging
Abstract
:1. Introduction
2. Results
2.1. Standardization of Radix Aconiti Lateralis Preparata Extracts
2.2. RAE, FPS, and FTA Improve the Hemodynamic Status and Organ Weight Index of Rats with Myocardial Infarction
2.3. RAE, FPS, and FTA Inhibit Myocardial Injury of Rats with Myocardial Infarction
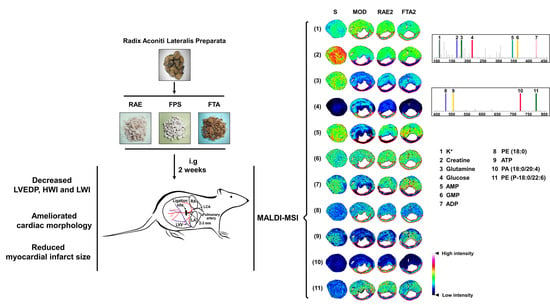
2.4. RAE and FTA Regulate Energy Metabolism-Related Molecules
2.5. RAE and FTA Decrease Phospholipids
2.6. FTA Changes Potassium Ions and Glutamine
3. Discussion
4. Materials and Methods
4.1. Reagents and Drugs
4.2. Preparation of Alkaloid Reference Substances
4.3. Quantitative Analysis of Alkaloids
4.4. Quantitative Analysis of Polysaccharides
4.5. Animals Care
4.6. Surgical Procedures
4.7. Grouping and Administration
4.8. Hemodynamics
4.9. Histopathological Examination
4.10. Matrix-Assisted Laser Desorption/Ionization–Mass Spectrometry Imaging (MALDI–MSI)
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DDAs | Diester-diterpene alkaloids |
| FPS | Fuzi polysaccharide |
| FTA | Fuzi total alkaloid |
| HE | Hematoxylin and eosin |
| HPLC | High performance liquid chromatography |
| HWI | Heart weight index |
| IS | Infarct size |
| LAD | Left anterior descending artery |
| LVEDP | Left ventricular end-diastolic pressure |
| LWI | Lung weight index |
| MALDI-MSI | Matrix assisted laser desorption/ionization–mass spectrometry imaging |
| MDAs | Monoester-diterpene alkaloids |
| METO | Metoprolol |
| PA | Phosphatidic acid |
| PE | Phosphatidylethanolamine |
| PI | Phosphatidylinositol |
| RAE | Radix Aconiti Lateralis Preparata extract |
| RALP | Radix Aconiti Lateralis Preparata |
References
- Lu, L.; Liu, M.; Sun, R.; Zheng, Y.; Zhang, P. Myocardial Infarction: Symptoms and Treatments. Cell Biochem. Biophys. 2015, 72, 865–867. [Google Scholar] [CrossRef] [PubMed]
- Boateng, S.; Sanborn, T. Acute myocardial infarction. Dis. Mon. 2013, 59, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Takagawa, J.; Zhang, Y.; Wong, M.L.; Sievers, R.E.; Kapasi, N.K.; Wang, Y.; Yeghiazarians, Y.; Lee, R.J.; Grossman, W.; Springer, M.L. Myocardial infarct size measurement in the mouse chronic infarction model: Comparison of area- and length-based approaches. J. Appl. Physiol. 2007, 102, 2104–2111. [Google Scholar] [CrossRef] [PubMed]
- Kainuma, S.; Miyagawa, S.; Fukushima, S.; Tsuchimochi, H.; Sonobe, T.; Fujii, Y.; Pearson, J.T.; Saito, A.; Harada, A.; Toda, K.; et al. Influence of coronary architecture on the variability in myocardial infarction induced by coronary ligation in rats. PLoS ONE 2017, 12, e0183323. [Google Scholar] [CrossRef] [PubMed]
- Marin-Garcia, J. Cell death in the pathogenesis and progression of heart failure. Heart Fail. Rev. 2016, 21, 117–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahoo, S.; Losordo, D.W. Exosomes and cardiac repair after myocardial infarction. Circ. Res. 2014, 114, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Heusch, G.; Gersh, B.J. The pathophysiology of acute myocardial infarction and strategies of protection beyond reperfusion: A continual challenge. Eur. Heart J. 2017, 38, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Roolvink, V.; Ibanez, B.; Ottervanger, J.P.; Pizarro, G.; van Royen, N.; Mateos, A.; Dambrink, J.E.; Escalera, N.; Lipsic, E.; Albarran, A.; et al. Early Intravenous Beta-Blockers in Patients With ST-Segment Elevation Myocardial Infarction Before Primary Percutaneous Coronary Intervention. J. Am. Coll. Cardiol. 2016, 67, 2705–2715. [Google Scholar] [CrossRef]
- Johns, T.N.; Olson, B.J. Experimental myocardial infarction. I. A method of coronary occlusion in small animals. Ann. Surg. 1954, 140, 675–682. [Google Scholar] [CrossRef]
- Smith, S.A.; Mammen, P.P.; Mitchell, J.H.; Garry, M.G. Role of the exercise pressor reflex in rats with dilated cardiomyopathy. Circulation 2003, 108, 1126–1132. [Google Scholar] [CrossRef]
- Smith, S.A.; Williams, M.A.; Mitchell, J.H.; Mammen, P.P.; Garry, M.G. The capsaicin-sensitive afferent neuron in skeletal muscle is abnormal in heart failure. Circulation 2005, 111, 2056–2065. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, Y.; Deng, Y.; Li, H. MicroRNA-223 Regulates Cardiac Fibrosis After Myocardial Infarction by Targeting RASA1. Cell Physiol. Biochem. 2018, 46, 1439–1454. [Google Scholar] [CrossRef]
- Feng, G.; Yan, Z.; Li, C.; Hou, Y. microRNA-208a in an early stage myocardial infarction rat model and the effect on cAMP-PKA signaling pathway. Mol. Med. Rep. 2016, 14, 1631–1635. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Miyamoto, K.; Yokoyama, N.; Sugi, M.; Kagioka, A.; Kitao, Y.; Adachi, T.; Ohsawa, M.; Mizukami, H.; Makino, T. Processed aconite root and its active ingredient neoline may alleviate oxaliplatin-induced peripheral neuropathic pain. J. Ethnopharmacol. 2016, 186, 44–52. [Google Scholar] [CrossRef]
- Liu, X.X.; Jian, X.X.; Cai, X.F.; Chao, R.B.; Chen, Q.H.; Chen, D.L.; Wang, X.L.; Wang, F.P. Cardioactive C(1)(9)-diterpenoid alkaloids from the lateral roots of Aconitum carmichaeli “Fu Zi”. Chem. Pharm. Bull. 2012, 60, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.Y.; Wang, S.J.; Teng, J.L.; Ji, X.M.; Wu, Z.C.; Ma, Q.C.; Fu, X.J. Effects of Radix aconiti lateralis preparata and Rhizoma zingiberis on energy metabolism and expression of the genes related to metabolism in rats. Chin. J. Integr. Med. 2012, 18, 23–29. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, J.; Cui, Y.; Wu, X. Pharmacological effects of Chinese herb aconite (fuzi) on cardiovascular system. J. Tradit. Chin. Med. 2012, 32, 308–313. [Google Scholar] [CrossRef]
- Zheng, S.Y.; Sun, J.; Zhao, X.; Xu, J.G. Protective effect of shen-fu on myocardial ischemia-reperfusion injury in rats. Am. J. Chin. Med. 2004, 32, 209–220. [Google Scholar] [CrossRef]
- Yamada, K.; Suzuki, E.; Nakaki, T.; Watanabe, S.; Kanba, S. Aconiti tuber increases plasma nitrite and nitrate levels in humans. J. Ethnopharmacol. 2005, 96, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; L’Imperio, V.; Denti, V.; Mazza, M.; Ivanova, M.; Stella, M.; Piga, I.; Chinello, C.; Ajello, E.; Pieruzzi, F.; et al. High Spatial Resolution MALDI-MS Imaging in the Study of Membranous Nephropathy. Proteom. Clin. Appl. 2019, 13, e1800016. [Google Scholar] [CrossRef]
- Liu, H.; Li, W.; He, Q.; Xue, J.; Wang, J.; Xiong, C.; Pu, X.; Nie, Z. Mass Spectrometry Imaging of Kidney Tissue Sections of Rat Subjected to Unilateral Ureteral Obstruction. Sci. Rep. 2017, 7, 41954. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lu, X.; Wang, J.; Li, P.; Li, H.; Wei, S.; Zhou, X.; Li, K.; Wang, L.; Wang, R.; et al. Zingiberis rhizoma mediated enhancement of the pharmacological effect of aconiti lateralis radix praeparata against acute heart failure and the underlying biological mechanisms. Biomed. Pharmacother. 2017, 96, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Ammar, M.R.; Kassas, N.; Bader, M.F.; Vitale, N. Phosphatidic acid in neuronal development: A node for membrane and cytoskeleton rearrangements. Biochimie 2014, 107 Pt A, 51–57. [Google Scholar] [CrossRef]
- Mejia, E.M.; Hatch, G.M. Mitochondrial phospholipids: Role in mitochondrial function. J. Bioenerg. Biomembr. 2016, 48, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Wischmeyer, P.E.; Jayakar, D.; Williams, U.; Singleton, K.D.; Riehm, J.; Bacha, E.A.; Jeevanandam, V.; Christians, U.; Serkova, N. Single dose of glutamine enhances myocardial tissue metabolism, glutathione content, and improves myocardial function after ischemia-reperfusion injury. JPEN J. Parenter. Enteral. Nutr. 2003, 27, 396–403. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, Y.; Lv, S.J.; Wang, L.; Liang, G.P.; Wan, Q.X.; Peng, X. Effects of glutamine treatment on myocardial damage and cardiac function in rats after severe burn injury. Int. J. Clin. Exp. Pathol. 2012, 5, 651–659. [Google Scholar] [PubMed]
- Palmer, B.F.; Clegg, D.J. Physiology and pathophysiology of potassium homeostasis. Adv. Physiol. Educ. 2016, 40, 480–490. [Google Scholar] [CrossRef] [Green Version]
- Yu, B.; Cao, Y.; Xiong, Y.K. Pharmacokinetics of aconitine-type alkaloids after oral administration of Fuzi (Aconiti Lateralis Radix Praeparata) in rats with chronic heart failure by microdialysis and ultra-high performance liquid chromatography-tandem mass spectrometry. J. Ethnopharmacol. 2015, 165, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J.; Guo, Z.Z.; Zhu, Y.F.; Huang, Z.J.; Gong, X.; Li, Y.H.; Son, W.J.; Li, X.Y.; Lou, Y.M.; Zhu, L.J.; et al. A systematic review of pharmacokinetic studies on herbal drug Fuzi: Implications for Fuzi as personalized medicine. Phytomedicine 2018, 44, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, F.; Li, Y.; Li, W.; Xu, J.; Du, H. A review of traditional and current methods used to potentially reduce toxicity of Aconitum roots in Traditional Chinese Medicine. J. Ethnopharmacol. 2017, 207, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Tang, L.; Zhou, X.; Wang, T.; Kou, Z.; Wang, Z. A review on phytochemistry and pharmacological activities of the processed lateral root of Aconitum carmichaelii Debeaux. J. Ethnopharmacol. 2015, 160, 173–193. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.B.; Wang, J.L.; Yuan, J.; Quan, Q.H.; Ji, R.F.; Tan, P.; Han, J.; Liu, Y.G. Sugar Composition Analysis of Fuzi Polysaccharides by HPLC-MS(n) and Their Protective Effects on Schwann Cells Exposed to High Glucose. Molecules 2016, 21, 1496. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.X.; Zhang, Y.; Hu, X.Y.; Chen, G.; Liu, X.Y.; Nie, H.M.; Liu, J.L.; Wen, D.C. Aqueous extract from Aconitum carmichaelii Debeaux reduces liver injury in rats via regulation of HMGB1/TLR4/NF-KappaB/caspase-3 and PCNA signaling pathways. J. Ethnopharmacol. 2016, 183, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Gong, Y.; Lv, C.; Ye, L.; Liu, L.; Liu, Z. Pharmacokinetics of aconitine as the targeted marker of Fuzi (Aconitum carmichaeli) following single and multiple oral administrations of Fuzi extracts in rat by UPLC/MS/MS. J. Ethnopharmacol. 2012, 141, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, X.; Zhang, W.; Liu, Y.; Wang, S.; Liu, X.; He, H. Mechanism-based pharmacokinetic-pharmacodynamic modeling of salvianolic acid A effects on plasma xanthine oxidase activity and uric acid levels in acute myocardial infarction rats. Xenobiotica 2017, 47, 208–216. [Google Scholar] [CrossRef]
- Guzun, R.; Timohhina, N.; Tepp, K.; Gonzalez-Granillo, M.; Shevchuk, I.; Chekulayev, V.; Kuznetsov, A.V.; Kaambre, T.; Saks, V.A. Systems bioenergetics of creatine kinase networks: Physiological roles of creatine and phosphocreatine in regulation of cardiac cell function. Amino Acids 2011, 40, 1333–1348. [Google Scholar] [CrossRef]
- Takemura, G.; Kanamori, H.; Okada, H.; Miyazaki, N.; Watanabe, T.; Tsujimoto, A.; Goto, K.; Maruyama, R.; Fujiwara, T.; Fujiwara, H. Anti-apoptosis in nonmyocytes and pro-autophagy in cardiomyocytes: Two strategies against postinfarction heart failure through regulation of cell death/degeneration. Heart Fail. Rev. 2018, 23, 759–772. [Google Scholar] [CrossRef]
- Freigang, S. The regulation of inflammation by oxidized phospholipids. Eur. J. Immunol. 2016, 46, 1818–1825. [Google Scholar] [CrossRef] [Green Version]
- Tappia, P.S.; Yu, C.H.; Di Nardo, P.; Pasricha, A.K.; Dhalla, N.S.; Panagia, V. Depressed responsiveness of phospholipase C isoenzymes to phosphatidic acid in congestive heart failure. J. Mol. Cell Cardiol. 2001, 33, 431–440. [Google Scholar] [CrossRef]
- Serpi, R.; Tolonen, A.M.; Tenhunen, O.; Pievilainen, O.; Kubin, A.M.; Vaskivuo, T.; Soini, Y.; Kerkela, R.; Leskinen, H.; Ruskoaho, H. Divergent effects of losartan and metoprolol on cardiac remodeling, c-kit+ cells, proliferation and apoptosis in the left ventricle after myocardial infarction. Clin. Transl. Sci. 2009, 2, 422–430. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Zhang, Z.L.; Li, P.; Liang, W.Y.; Feng, X.R.; Liu, M.L. Shenyuan, an extract of American Ginseng and Corydalis Tuber formula, attenuates cardiomyocyte apoptosis via inhibition of endoplasmic reticulum stress and oxidative stress in a porcine model of acute myocardial infarction. J. Ethnopharmacol. 2013, 150, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, M.; Goldstein, S.; Li, Y.; Ge, J.; He, B.; Ruiz, G. The effect of c-fos on acute myocardial infarction and the significance of metoprolol intervention in a rat model. Cell Biochem. Biophys. 2013, 65, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, Z.; Gao, X.; Zhang, H.; Xiong, W.; Ju, J.; Xu, H. Meta-Analysis Comparing Metoprolol and Carvedilol on Mortality Benefits in Patients with Acute Myocardial Infarction. Am. J. Cardiol. 2017, 120, 1479–1486. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, R.; Wang, J.; Chen, S.; Xiong, C.; Wang, J.; Hou, J.; He, Q.; Zhang, N.; Nie, Z.; et al. 1,5-Diaminonaphthalene hydrochloride assisted laser desorption/ionization mass spectrometry imaging of small molecules in tissues following focal cerebral ischemia. Anal. Chem. 2014, 86, 10114–10121. [Google Scholar] [CrossRef] [PubMed]









© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Liu, X.; Gao, Z.-y.; Dai, Z.-f.; Lin, M.; Tian, F.; Zhao, X.; Sun, Y.; Pu, X.-p. Anti-Myocardial Infarction Effects of Radix Aconiti Lateralis Preparata Extracts and Their Influence on Small Molecules in the Heart Using Matrix-Assisted Laser Desorption/Ionization–Mass Spectrometry Imaging. Int. J. Mol. Sci. 2019, 20, 4837. https://doi.org/10.3390/ijms20194837
Wu H, Liu X, Gao Z-y, Dai Z-f, Lin M, Tian F, Zhao X, Sun Y, Pu X-p. Anti-Myocardial Infarction Effects of Radix Aconiti Lateralis Preparata Extracts and Their Influence on Small Molecules in the Heart Using Matrix-Assisted Laser Desorption/Ionization–Mass Spectrometry Imaging. International Journal of Molecular Sciences. 2019; 20(19):4837. https://doi.org/10.3390/ijms20194837
Chicago/Turabian StyleWu, Hao, Xi Liu, Ze-yu Gao, Zhen-feng Dai, Ming Lin, Fang Tian, Xin Zhao, Yi Sun, and Xiao-ping Pu. 2019. "Anti-Myocardial Infarction Effects of Radix Aconiti Lateralis Preparata Extracts and Their Influence on Small Molecules in the Heart Using Matrix-Assisted Laser Desorption/Ionization–Mass Spectrometry Imaging" International Journal of Molecular Sciences 20, no. 19: 4837. https://doi.org/10.3390/ijms20194837
APA StyleWu, H., Liu, X., Gao, Z. -y., Dai, Z. -f., Lin, M., Tian, F., Zhao, X., Sun, Y., & Pu, X. -p. (2019). Anti-Myocardial Infarction Effects of Radix Aconiti Lateralis Preparata Extracts and Their Influence on Small Molecules in the Heart Using Matrix-Assisted Laser Desorption/Ionization–Mass Spectrometry Imaging. International Journal of Molecular Sciences, 20(19), 4837. https://doi.org/10.3390/ijms20194837