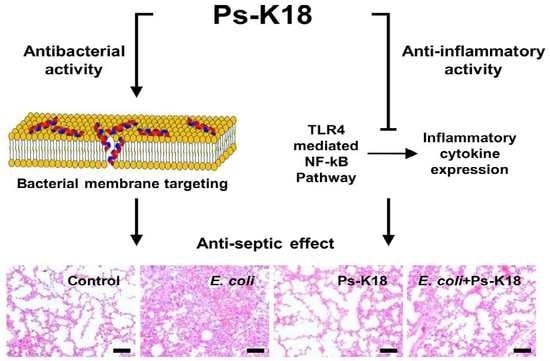
Antiseptic Effect of Ps-K18: Mechanism of Its Antibacterial and Anti-Inflammatory Activities
Abstract
:1. Introduction
2. Results
2.1. Properties of Ps-K18
2.2. Antibacterial Activity
2.3. Visualization of the Interaction Between E. coli and Ps Peptides based on Confocal Micrographs and Field Emission Scanning Electron Microscopic (FE-SEM) Micrographs
2.4. Cytotoxicity of Ps and Ps-K18 In Vitro and In Vivo
2.5. Specificity of Ps-K18 towards Various TLRs
2.6. Ps-K18 Suppresses Inflammation Response through TLR4-Mediated Signaling in RAW264.7 Cells and HEK-BlueTM hTLR4 Cells
2.7. Antisepsis Effect of Ps-K18 in LPS-Induced Endotoxemia Mouse Model
2.8. Antisepsis Effect of Ps-K18 Based on an E. coli K1-Induced Mouse Model of Septic Shock
2.9. Ps-K18 Treatment Effectively Suppresses Polymorphonuclear Lymphocyte Infiltration in LPS-Induced Endotoxemia Mouse Model and E. coli K1-Induced Mouse Model
3. Discussion
4. Materials and Methods
4.1. Peptide Synthesis
4.2. Antibacterial Activity
4.3. Confocal Microscope Analysis
4.4. FE-SEM Analysis
4.5. Cytotoxicity In Vitro
4.6. Specificity Against TLRs Selectively Activated by Agonists
4.7. Quantification of Inflammatory Cytokine Production in LPS-Stimulated RAW264.7 Cells
4.8. Inhibition Effect of Ps-K18 on TLR4-Mediated Inflammatory Response in LPS-Stimulated HEK-BlueTM hTLR4 and RAW264.7 Cells
4.9. Sepsis Mouse Model
4.10. Cytotoxicity In Vivo
4.11. Measurement of Antiseptic Activity of Peptides in LPS-Induced Endotoxemia and E. coli K1 Septic Shock Mouse Model
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chakrabarti, S.; Guha, S.; Majumder, K. Food-derived bioactive peptides in human health: Challenges and opportunities. Nutrients 2018, 10, 1738. [Google Scholar] [CrossRef] [PubMed]
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef]
- Hayashi, M.A.; Ducancel, F.; Konno, K. Natural peptides with potential applications in drug development, diagnosis, and/or biotechnology. Int. J. Pept. 2012, 2012, 757838. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.; Chapple, D.S. Peptide antibiotics. Antimicrob. Agents Chemother. 1999, 43, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Koppkubel, S. International nonproprietary names (inn) for pharmaceutical substances. Bull. World Health Organ. 1995, 73, 275–279. [Google Scholar]
- Kaspar, A.A.; Reichert, J.M. Future directions for peptide therapeutics development. Drug Discov. Today 2013, 18, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Iwaniak, A.; Darewicz, M.; Minkiewicz, P. Peptides derived from foods as supportive diet components in the prevention of metabolic syndrome. Compr. Rev. Food Sci. Food Saf. 2018, 17, 63–81. [Google Scholar] [CrossRef]
- Williams, K.J. The introduction of ’chemotherapy’ using arsphenamine-the first magic bullet. J. R. Soc. Med. 2009, 102, 343–348. [Google Scholar] [CrossRef]
- Drusano, G.L. An overview of the pharmacology of imipenem/cilastatin. J. Antimicrob. Chemother. 1986, 18 (Suppl. E), 79–92. [Google Scholar] [CrossRef]
- Lin, L.; Wagner, M.C.; Cocklin, R.; Kuzma, A.; Harrington, M.; Molitoris, B.A.; Goebl, M.G. The antibiotic gentamicin inhibits specific protein trafficking functions of the arf1/2 family of gtpases. Antimicrob. Agents Chemother. 2011, 55, 246–254. [Google Scholar] [CrossRef]
- Codjoe, F.S.; Donkor, E.S. Carbapenem resistance: A review. Med. Sci. (Basel) 2017, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Moellering, R.C., Jr.; Wennersten, C.; Kunz, L.J. Emergence of gentamicin-resistant bacteria: Experience with tobramycin therapy of infections due to gentamicin-resistant organisms. J. Infect. Dis. 1976, 134, S40–S49. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.V.; Yedery, R.D.; Aranha, C. Antimicrobial peptides: Premises and promises. Int. J. Antimicrob. Agents 2004, 24, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Grammatikos, A.P.; Michalopoulos, A. Potential of old-generation antibiotics to address current need for new antibiotics. Expert Rev. Anti Infect. Ther. 2008, 6, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Mahlapuu, M.; Hakansson, J.; Ringstad, L.; Bjorn, C. Antimicrobial peptides: An emerging category of therapeutic agents. Front. Cell. Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.K.; Kim, C.; Seo, C.H.; Park, Y. The therapeutic applications of antimicrobial peptides (amps): A patent review. J. Microbiol. 2017, 55, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Akin, A.; Alp, E.; Altindiş, M.; Azak, E.; Batirel, A.; Çağ, Y.; Durmuş, G.; Kepenek Kurt, E.; Sağiroğlu, P.; Türe, Z.; et al. Current diagnosis and treatment approach to sepsis. Mediterr. J. Infect. Microbes Antimicrob. 2018, 7, 17. [Google Scholar] [CrossRef]
- Fleischmann, C.; Scherag, A.; Adhikari, N.K.; Hartog, C.S.; Tsaganos, T.; Schlattmann, P.; Angus, D.C.; Reinhart, K. International Forum of Acute Care Trialists. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am. J. Respir. Crit. Care Med. 2016, 193, 259–272. [Google Scholar] [CrossRef]
- Xu, C.; Guo, Z.; Zhao, C.; Zhang, X.; Wang, Z. Potential mechanism and drug candidates for sepsis-induced acute lung injury. Exp. Ther. Med. 2018, 15, 4689–4696. [Google Scholar] [CrossRef] [PubMed]
- Park, B.S.; Lee, J.O. Recognition of lipopolysaccharide pattern by tlr4 complexes. Exp. Mol. Med. 2013, 45, e66. [Google Scholar] [CrossRef]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Moldawer, L.L.; Opal, S.M.; Reinhart, K.; Turnbull, I.R.; Vincent, J.L. Sepsis and septic shock. Nat. Rev. Dis. Prim. 2016, 2, 16045. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.; Vincent, J.L.; Adhikari, N.K.; Machado, F.R.; Angus, D.C.; Calandra, T.; Jaton, K.; Giulieri, S.; Delaloye, J.; Opal, S.; et al. Sepsis: A roadmap for future research. Lancet Infect. Dis. 2015, 15, 581–614. [Google Scholar] [CrossRef]
- Jeon, D.; Jeong, M.C.; Jacob, B.; Bang, J.K.; Kim, E.H.; Cheong, C.; Jung, I.D.; Park, Y.; Kim, Y. Investigation of cationicity and structure of pseudin-2 analogues for enhanced bacterial selectivity and anti-inflammatory activity. Sci. Rep. 2017, 7, 1455. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Vasil, A.I.; Hale, J.; Hancock, R.E.; Vasil, M.L.; Hodges, R.S. Effects of net charge and the number of positively charged residues on the biological activity of amphipathic alpha-helical cationic antimicrobial peptides. Adv. Exp. Med. Biol. 2009, 611, 561–562. [Google Scholar] [PubMed]
- Lee, E.; Shin, A.; Jeong, K.W.; Jin, B.; Jnawali, H.N.; Shin, S.; Shin, S.Y.; Kim, Y. Role of phenylalanine and valine10 residues in the antimicrobial activity and cytotoxicity of piscidin-1. PLoS ONE 2014, 9, e114453. [Google Scholar] [CrossRef]
- Bahar, A.A.; Ren, D. Antimicrobial peptides. Pharmaceuticals (Basel) 2013, 6, 1543–1575. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jacob, B.; Jang, M.; Kwak, C.; Lee, Y.; Son, K.; Lee, S.; Jung, I.D.; Jeong, M.S.; Kwon, S.H.; et al. Development of a novel short 12-meric papiliocin-derived peptide that is effective against gram-negative sepsis. Sci. Rep. 2019, 9, 3817. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef]
- Kim, J.; Durai, P.; Jeon, D.; Jung, I.D.; Lee, S.J.; Park, Y.M.; Kim, Y. Phloretin as a potent natural tlr2/1 inhibitor suppresses tlr2-induced inflammation. Nutrients 2018, 10, 868. [Google Scholar] [CrossRef]
- Lee, E.; Kim, J.K.; Jeon, D.; Jeong, K.W.; Shin, A.; Kim, Y. Functional roles of aromatic residues and helices of papiliocin in its antimicrobial and anti-inflammatory activities. Sci. Rep. 2015, 5, 12048. [Google Scholar] [CrossRef]
- Bronstein, I.; Fortin, J.J.; Voyta, J.C.; Juo, R.R.; Edwards, B.; Olesen, C.E.; Lijam, N.; Kricka, L.J. Chemiluminescent reporter gene assays: Sensitive detection of the gus and seap gene products. Biotechniques 1994, 17, 172–174, 176–177. [Google Scholar] [PubMed]
- Munford, R.S. Endotoxemia-menace, marker, or mistake? J. Leukoc. Biol. 2016, 100, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.; Guo, Y.; Ha, B.; Zen, K.; Liu, Y. Regulation of the inflammatory response: Enhancing neutrophil infiltration under chronic inflammatory conditions. J. Immunol. 2012, 188, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Bijli, K.M.; Kanter, B.G.; Minhajuddin, M.; Leonard, A.; Xu, L.; Fazal, F.; Rahman, A. Regulation of endothelial cell inflammation and lung polymorphonuclear lymphocyte infiltration by transglutaminase 2. Shock 2014, 42, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.R. Neutrophils and lung injury: Getting it right. J. Clin. Investig. 2002, 110, 1603–1605. [Google Scholar] [CrossRef] [PubMed]
- Van der Poll, T.; van de Veerdonk, F.L.; Scicluna, B.P.; Netea, M.G. The immunopathology of sepsis and potential therapeutic targets. Nat. Rev. Immunol. 2017, 17, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gong, S.; Wang, F.; Niu, M.; Wei, G.; He, Z.; Gu, T.; Jiang, Y.; Liu, A.; Chen, P. Granisetron protects polymicrobial sepsis-induced acute lung injury in mice. Biochem. Biophys. Res. Commun. 2019, 508, 1004–1010. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. P T 2015, 40, 277–283. [Google Scholar]
- Diamond, G.; Beckloff, N.; Weinberg, A.; Kisich, K.O. The roles of antimicrobial peptides in innate host defense. Curr. Pharm. Des. 2009, 15, 2377–2392. [Google Scholar] [CrossRef]
- Matsuzaki, K. Control of cell selectivity of antimicrobial peptides. Biochim. Biophys. Acta 2009, 1788, 1687–1692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zelezetsky, I.; Tossi, A. Alpha-helical antimicrobial peptides--using a sequence template to guide structure-activity relationship studies. Biochim. Biophys. Acta 2006, 1758, 1436–1449. [Google Scholar] [CrossRef] [PubMed]
- Al-Lawama, M.; Aljbour, H.; Tanash, A.; Badran, E. Intravenous colistin in the treatment of multidrug-resistant acinetobacter in neonates. Ann. Clin. Microbiol. Antimicrob. 2016, 15, 8. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Zhao, X.; Huang, J.; Liu, W.; Zheng, Y.; Yang, X.; Zhang, Y.; Lamy de la Chapelle, M.; Fu, W. Rapid screening of colistin-resistant escherichia coli, acinetobacter baumannii and pseudomonas aeruginosa by the use of raman spectroscopy and hierarchical cluster analysis. Analyst 2019, 144, 2803–2810. [Google Scholar] [CrossRef] [PubMed]
- Jenssen, H.; Hamill, P.; Hancock, R.E. Peptide antimicrobial agents. Clin. Microbiol. Rev. 2006, 19, 491–511. [Google Scholar] [CrossRef] [PubMed]
- Pal, T.; Sonnevend, A.; Galadari, S.; Conlon, J.M. Design of potent, non-toxic antimicrobial agents based upon the structure of the frog skin peptide, pseudin-2. Regul. Pept. 2005, 129, 85–91. [Google Scholar] [CrossRef]
- Tossi, A.; Sandri, L.; Giangaspero, A. Amphipathic, alpha-helical antimicrobial peptides. Biopolymers 2000, 55, 4–30. [Google Scholar] [CrossRef]
- Kang, H.K.; Seo, C.H.; Luchian, T.; Park, Y. Pse-t2, an antimicrobial peptide with high-level, broad-spectrum antimicrobial potency and skin biocompatibility against multidrug-resistant pseudomonas aeruginosa infection. Antimicrob. Agents Chemother. 2018, 62, e01493-18. [Google Scholar] [CrossRef]
- Angus, D.C.; van der Poll, T. Severe sepsis and septic shock. N. Engl. J. Med. 2013, 369, 840–851. [Google Scholar] [CrossRef]
- Martin, L.; van Meegern, A.; Doemming, S.; Schuerholz, T. Antimicrobial peptides in human sepsis. Front. Immunol. 2015, 6, 404. [Google Scholar] [CrossRef]
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. Lps/tlr4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Shin, A.; Kim, Y. Anti-inflammatory activities of cecropin a and its mechanism of action. Arch. Insect Biochem. Physiol. 2015, 88, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Cheon, D.; Kim, J.; Jeon, D.; Shin, H.C.; Kim, Y. Target proteins of phloretin for its anti-inflammatory and antibacterial activities against propionibacterium acnes-induced skin infection. Molecules 2019, 24, 1319. [Google Scholar] [CrossRef] [PubMed]
- Jnawali, H.N.; Lee, E.; Jeong, K.W.; Shin, A.; Heo, Y.S.; Kim, Y. Anti-inflammatory activity of rhamnetin and a model of its binding to c-jun nh2-terminal kinase 1 and p38 mapk. J. Nat. Prod. 2014, 77, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Huang, M.; Zhang, Y.; Li, H.; Zheng, H.; Yu, L.; Chu, K.; Lin, Y.; Chen, L. Extracts of bauhinia championii (benth.) benth. Attenuate the in fl ammatory response in a rat model of collagen-induced arthritis. Mol. Med. Rep. 2016, 13, 4167–4174. [Google Scholar] [CrossRef]
- Mushtaq, N.; Redpath, M.B.; Luzio, J.P.; Taylor, P.W. Prevention and cure of systemic escherichia coli k1 infection by modification of the bacterial phenotype. Antimicrob. Agents Chemother. 2004, 48, 1503–1508. [Google Scholar] [CrossRef]
- Kaczmarek, A.; Budzynska, A.; Gospodarek, E. Detection of k1 antigen of escherichia coli rods isolated from pregnant women and neonates. Folia Microbiol. (Praha) 2014, 59, 419–422. [Google Scholar] [CrossRef]
- Jnawali, H.N.; Jeon, D.; Jeong, M.C.; Lee, E.; Jin, B.; Ryoo, S.; Yoo, J.; Jung, I.D.; Lee, S.J.; Park, Y.M.; et al. Antituberculosis activity of a naturally occurring flavonoid, isorhamnetin. J. Nat. Prod. 2016, 79, 961–969. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, S.J.; Cho, J.; Gharbi, A.; Han, H.D.; Kang, T.H.; Kim, Y.; Lee, Y.; Park, W.S.; Jung, I.D.; et al. Tamarixetin exhibits anti-inflammatory activity and prevents bacterial sepsis by increasing il-10 production. J. Nat. Prod. 2018, 81, 1435–1443. [Google Scholar] [CrossRef]
- Otvos, L., Jr.; Wade, J.D. Current challenges in peptide-based drug discovery. Front. Chem. 2014, 2, 62. [Google Scholar] [CrossRef]







| Peptide | Sequence | Molecular Weighta | Net Charge | Hydrophobicityb <H> |
|---|---|---|---|---|
| Ps | GLNALKKVFQGIHEAIKLINNHVQ | 2685 | +2 | 0.407 |
| Ps-K18 | GLNALKKVFQGIHEAIKKINNHVQ | 2702 | +3 | 0.295 |
| MIC (µM) | Ps | Ps-K18 | Melittin |
|---|---|---|---|
| Standard Gram-negative bacteria | |||
| E. coli KCTC1682 | 4 | 4 | 4 |
| E. coli K1 | 2 | 2 | 2 |
| P. aeruginosa KCCM11328 | 4 | 4 | 8 |
| A. baumannii KCCM40203 | 2 | 2 | 2 |
| MDR Gram-negative bacteria | |||
| E. coli CCARM 1229 | 2 | 2 | 1 |
| P. aeruginosa CCARM 2003 | 2 | 2 | 2 |
| A. baumannii CCARM 12010 | 2 | 2 | 1 |
| GMa | 2.57 | 2.57 | 2.86 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, M.; Kim, J.; Choi, Y.; Bang, J.; Kim, Y. Antiseptic Effect of Ps-K18: Mechanism of Its Antibacterial and Anti-Inflammatory Activities. Int. J. Mol. Sci. 2019, 20, 4895. https://doi.org/10.3390/ijms20194895
Jang M, Kim J, Choi Y, Bang J, Kim Y. Antiseptic Effect of Ps-K18: Mechanism of Its Antibacterial and Anti-Inflammatory Activities. International Journal of Molecular Sciences. 2019; 20(19):4895. https://doi.org/10.3390/ijms20194895
Chicago/Turabian StyleJang, Mihee, Jieun Kim, Yujin Choi, JeongKyu Bang, and Yangmee Kim. 2019. "Antiseptic Effect of Ps-K18: Mechanism of Its Antibacterial and Anti-Inflammatory Activities" International Journal of Molecular Sciences 20, no. 19: 4895. https://doi.org/10.3390/ijms20194895
APA StyleJang, M., Kim, J., Choi, Y., Bang, J., & Kim, Y. (2019). Antiseptic Effect of Ps-K18: Mechanism of Its Antibacterial and Anti-Inflammatory Activities. International Journal of Molecular Sciences, 20(19), 4895. https://doi.org/10.3390/ijms20194895