A Miniaturized Pump Out Method for Characterizing Molecule Interaction with ABC Transporters
Abstract
:1. Introduction
2. Results
2.1. Caco-2 Cell Culture
2.2. Concentration-Dependent Permeability of Quinidine to the Caco-2
2.3. Drug Characterization: Caco-2 Pump Out Assay
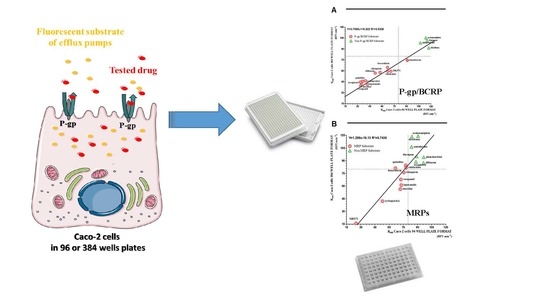
2.4. Miniaturization of the Caco-2 Pump Out Assay
3. Material and Methods
3.1. Cell Culture
3.2. Mycoplasma Detection
3.3. Caco-2 Pump Out Assay
3.4. TEER Measurements
3.5. mRNA Extraction and RT qPCR
3.6. Transport Studies
3.7. Immunostaining
3.8. MTT Tetrazolium Viability Assay
3.9. Quenching Test
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pedersen, J.M.; Matsson, P.; Bergström, C.A.S.; Norinder, U.; Hoogstraate, J.; Artursson, P. Prediction and Identification of Drug Interactions with the Human ATP-Binding Cassette Transporter Multidrug-Resistance Associated Protein 2 (MRP2; ABCC2). J. Med. Chem. 2008, 51, 3275–3287. [Google Scholar] [CrossRef]
- Matsson, P.; Pedersen, J.M.; Norinder, U.; Bergström, C.A.S.; Artursson, P. Identification of Novel Specific and General Inhibitors of the Three Major Human ATP-Binding Cassette Transporters P-gp, BCRP and MRP2 Among Registered Drugs. Pharm. Res. 2009, 26, 1816–1831. [Google Scholar] [CrossRef]
- Poirier, A.; Cascais, A.-C.; Bader, U.; Portmann, R.; Brun, M.-E.; Walter, I.; Hillebrecht, A.; Ullah, M.; Funk, C. Calibration of In Vitro Multidrug Resistance Protein 1 Substrate and Inhibition Assays as a Basis to Support the Prediction of Clinically Relevant Interactions In Vivo. Drug Metab. Dispos. 2014, 42, 1411–1422. [Google Scholar] [CrossRef] [PubMed]
- Hubatsch, I.; Ragnarsson, E.G.E.; Artursson, P. Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nat. Protoc. 2007, 2, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Sevin, E.; Dehouck, L.; Fabulas-da Costa, A.; Cecchelli, R.; Dehouck, M.P.; Lundquist, S.; Culot, M. Accelerated Caco-2 cell permeability model for drug discovery. J. Pharmacol. Toxicol. Methods 2013, 68, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Brück, S.; Strohmeier, J.; Busch, D.; Drozdzik, M.; Oswald, S. Caco-2 cells - expression, regulation and function of drug transporters compared with human jejunal tissue. Biopharm. Drug Dispos. 2017, 38, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Gameiro, M.; Silva, R.; Rocha-Pereira, C.; Carmo, H.; Carvalho, F.; Bastos, M.D.L.; Remião, F. Cellular Models and In Vitro Assays for the Screening of modulators of P-gp, MRP1 and BCRP. Molecules 2017, 22, 600. [Google Scholar] [CrossRef] [PubMed]
- Kerns, E.H.; Di, L.; Petusky, S.; Farris, M.; Ley, R.; Jupp, P. Combined application of parallel artificial membrane permeability assay and Caco-2 permeability assays in drug discovery. J. Pharm. Sci. 2004, 93, 1440–1453. [Google Scholar] [CrossRef] [PubMed]
- Balimane, P.V; Patel, K.; Marino, A.; Chong, S. Utility of 96 well Caco-2 cell system for increased throughput of P-gp screening in drug discovery. Eur. J. Pharm. Biopharm. 2004, 58, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Hellinger, E.; Bakk, M.L.; Pócza, P.; Tihanyi, K.; Vastag, M. Drug penetration model of vinblastine-treated Caco-2 cultures. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2010, 41, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Anderle, P.; Niederer, E.; Rubas, W.; Hilgendorf, C.; Spahn-Langguth, H.; Wunderli-Allenspach, H.; Merkle, H.P.; Langguth, P. P-Glycoprotein (P-gp) mediated efflux in Caco-2 cell monolayers: the influence of culturing conditions and drug exposure on P-gp expression levels. J. Pharm. Sci. 1998, 87, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Hayeshi, R.; Hilgendorf, C.; Artursson, P.; Augustijns, P.; Brodin, B.; Dehertogh, P.; Fisher, K.; Fossati, L.; Hovenkamp, E.; Korjamo, T.; et al. Comparison of drug transporter gene expression and functionality in Caco-2 cells from 10 different laboratories. Eur. J. Pharm. Sci. 2008, 35, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Young, L.; Sung, J.; Stacey, G.; Masters, J.R. Detection of Mycoplasma in cell cultures. Nat. Protoc. 2010, 5, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Pamies, D.; Bal-Price, A.; Simeonov, A.; Tagle, D.; Allen, D.; Gerhold, D.; Yin, D.; Pistollato, F.; Inutsuka, T.; Sullivan, K.; et al. Good Cell Culture Practice for stem cells and stem-cell-derived models. ALTEX 2016, 34, 95–132. [Google Scholar] [CrossRef]
- Yamashita, S.; Konishi, K.; Yamazaki, Y.; Taki, Y.; Sakane, T.; Sezaki, H.; Furuyama, Y. New and better protocols for a short-term Caco-2 cell culture system. J. Pharm. Sci. 2002, 91, 669–679. [Google Scholar] [CrossRef]
- Balimane, P. V; Chong, S. Cell culture-based models for intestinal permeability: a critique. Drug Discov. Today 2005, 10, 335–343. [Google Scholar] [CrossRef]
- Forster, S.; Thumser, A.E.; Hood, S.R.; Plant, N. Characterization of Rhodamine-123 as a Tracer Dye for Use In In vitro Drug Transport Assays. PLoS ONE 2012, 7, e33253. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Zhao, Y.L.; Nadai, M.; Yoshizumi, H.; Cen, X.; Torita, S.; Takagi, K.; Takagi, K.; Hasegawa, T. Involvement of the drug transporters p glycoprotein and multidrug resistance-associated protein Mrp2 in telithromycin transport. Antimicrob. Agents Chemother. 2006, 50, 80–87. [Google Scholar] [CrossRef]
- Bui, K.; She, F.; Zhou, D.; Butler, K.; Al-Huniti, N.; Sostek, M. The effect of quinidine, a strong P-glycoprotein inhibitor, on the pharmacokinetics and central nervous system distribution of naloxegol. J. Clin. Pharmacol. 2016, 56, 497–505. [Google Scholar] [CrossRef]
- Lebedeva, I. V; Pande, P.; Patton, W.F. Sensitive and specific fluorescent probes for functional analysis of the three major types of mammalian ABC transporters. PLoS ONE 2011, 6, e22429. [Google Scholar] [CrossRef]
- Nakanishi, T.; Shibue, Y.; Fukuyama, Y.; Yoshida, K.; Fukuda, H.; Shirasaka, Y.; Tamai, I. Quantitative time-lapse imaging-based analysis of drug-drug interaction mediated by hepatobiliary transporter, multidrug resistance-associated protein 2, in sandwich-cultured rat hepatocytes. Drug Metab. Dispos. 2011, 39, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Bakos, É.; Homolya, L. Portrait of multifaceted transporter, the multidrug resistance-associated protein 1 (MRP1/ABCC1). Pflügers Arch. Eur. J. Physiol. 2007, 453, 621–641. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wu, T.; Qi, Y.; Zhang, Z. Recent Advances in the Application of Vitamin E TPGS for Drug Delivery. Theranostics 2018, 8, 464. [Google Scholar] [CrossRef] [PubMed]
- Montanari, F.; Ecker, G.F. Prediction of drug–ABC-transporter interaction — Recent advances and future challenges. Adv. Drug Deliv. Rev. 2015, 86, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Schinkel, A.H.; Jonker, J.W. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overview. Adv. Drug Deliv. Rev. 2003, 55, 3–29. [Google Scholar] [CrossRef]
- Mao, Q.; Unadkat, J.D. Role of the breast cancer resistance protein (ABCG2) in drug transport. AAPS J. 2005, 7, E118–E133. [Google Scholar] [CrossRef] [Green Version]






| 96 Well Plate Format | 384 Well Plate Format | Correlation 96/384 Well Plate Format | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Working Conc. | kout Rho 123 | kout CMFDA | kout Rho 123 | kout CMFDA | Ratio Kout | Transporter Interaction | ||||||||||
| pmol.min−1.mg−1 Protein | pmol.min−1.mg−1 Protein | 96/384 Wells | 96 Wells | 384 Wells | ||||||||||||
| Compounds | µM | n | mean | n | mean | n | mean | n | mean | P-gp/BCRP | MRPs | P-gp/BCRP | MRPs | P-gp/BCRP | MRPs | |
| 1 | verapamil | 50 | 34 | 33.9 ± 7.0 | 8 | 72.4 ± 6.6 | 16 | 39.3 ± 6.8 | 43 | 67.8 ± 16.5 | 0.86 | 1.07 | + | + | + | + |
| 2 | elacridar | 10 | 29 | 34.6 ± 8.9 | 8 | 71.8 ± 6.6 | 16 | 42.5 ± 9.0 | 44 | 56.4 ± 14.3 | 0.81 | 1.27 | + | + | + | + |
| 3 | MK571 | 50 | 24 | 70.9 ± 10.6 | 24 | 25.0 ± 6.6 | 16 | 59.5 ± 9.9 | 44 | 15.7 ± 5.8 | 1.19 | 1.59 | + | + | + | + |
| 4 | diazepam | 50 | 29 | 115.2 ± 18.9 | 8 | 89.2 ± 8.6 | 16 | 101.7 ± 14.6 | 44 | 95.1 ± 14.1 | 1.13 | 0.94 | − | − | − | − |
| 5 | acetanomiphen | 50 | 8 | 113.7 ± 12.6 | 8 | 90.6 ± 6.8 | 16 | 105.4 ± 8.2 | 16 | 119.1 ± 29.1 | 1.08 | 0.76 | − | − | − | − |
| 6 | amiodarone | 50 | 8 | 91.1 ± 10.2 | 8 | 83.7 ± 12.7 | 16 | 74.5 ± 12.4 | 16 | 106.5 ± 17.6 | 1.22 | 0.79 | − | − | + | − |
| 7 | cyclosporinA | 10 | 8 | 39.6 ± 9.9 | 8 | 53.1 ± 7.6 | 16 | 46.4 ± 8.1 | 16 | 42.2 ± 10.1 | 0.85 | 1.26 | + | + | + | + |
| 8 | diltiazem | 50 | 8 | 51.7 ± 5.9 | 8 | 96.4 ± 8.7 | 16 | 56.9 ± 7.1 | 16 | 87.6 ± 24.1 | 0.91 | 1.10 | + | − | + | − |
| 9 | diclofenac | 50 | 8 | 117.3 ± 15.6 | 8 | 84.1 ± 7.5 | 16 | 91.5 ± 17.4 | 16 | 118.7 ± 18.0 | 1.28 | 0.71 | − | − | − | − |
| 10 | loperamide | 50 | 8 | 42.0 ± 4.9 | 8 | 72.9 ± 4.0 | 16 | 41.6 ± 6.2 | 16 | 61.2± 16.2 | 1.01 | 1.19 | + | + | + | + |
| 11 | doxorubicin | 20 | 8 | 66.7 ± 11.0 | 8 | 66.6 ± 5.6 | 16 | 64.7 ± 6.8 | 16 | 81.3 ± 16.6 | 1.03 | 0.82 | + | + | + | − |
| 12 | phenobarbital | 50 | 8 | 107.1 ± 10.0 | 16 | 96.6 ± 11.6 | 16 | 98.5 ± 7.4 | 16 | 94.2 ± 22.4 | 1.09 | 1.03 | − | − | − | − |
| 13 | quinidine | 50 | 8 | 36.4 ± 4.2 | 8 | 77.6 ± 8.9 | 16 | 45.8 ± 6.7 | 32 | 84.7 ± 16.9 | 0.79 | 0.92 | + | +/− | + | − |
| 14 | rifampicin | 50 | 8 | 66.8 ± 4.4 | 8 | 75.5 ± 7.9 | 16 | 59.5 ± 8.0 | 16 | 76.3 ± 21.5 | 1.12 | 0.99 | + | +/− | + | +/− |
| 15 | vinblastine | 50 | 8 | 57.6 ± 3.8 | 8 | 77.6 ± 10.6 | 16 | 57.9 ± 7.6 | 32 | 81.9 ± 17.5 | 0.99 | 0.95 | + | +/− | + | − |
| 16 | tocopherol | 10 | 8 | 33.7 ± 2.7 | 8 | 88.4 ± 6.9 | 16 | 44.6 ± 6.2 | 32 | 88.8 ± 15.33 | 0.76 | 1.00 | + | − | + | − |
| mRNA | F/R | Sequences | Accession Number |
|---|---|---|---|
| ABCB1 | F | 5′-TCTGCTGTGGAAAAATTACA-3′ | NM_013850.1 |
| R | 5′-GACAGCCACTAGGATGAAGA-3′ | ||
| ABCG2 | F | 5′-CAAGCATCTTCAGTTCATCAGC-3′ | NM_013454.3 |
| R | 5′-GAGTGTAGCAGGGACCACATAA-3′ | ||
| ABCC2 | F | 5′-CTCCCAAGTCACACAAGAACTG-3′ | NM_009696.3 |
| R | 5′-TCCTCCAGCTCCTTTTTGTAAG-3′ | ||
| ACTIN | F | 5′-CTGAGGACCTTCCGCAAGATGT-3′ | NM_138955 |
| R | 5′-GCTTCAGGTTGGCAGAGACCAT-3′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sevin, E.; Dehouck, L.; Versele, R.; Culot, M.; Gosselet, F. A Miniaturized Pump Out Method for Characterizing Molecule Interaction with ABC Transporters. Int. J. Mol. Sci. 2019, 20, 5529. https://doi.org/10.3390/ijms20225529
Sevin E, Dehouck L, Versele R, Culot M, Gosselet F. A Miniaturized Pump Out Method for Characterizing Molecule Interaction with ABC Transporters. International Journal of Molecular Sciences. 2019; 20(22):5529. https://doi.org/10.3390/ijms20225529
Chicago/Turabian StyleSevin, Emmanuel, Lucie Dehouck, Romain Versele, Maxime Culot, and Fabien Gosselet. 2019. "A Miniaturized Pump Out Method for Characterizing Molecule Interaction with ABC Transporters" International Journal of Molecular Sciences 20, no. 22: 5529. https://doi.org/10.3390/ijms20225529
APA StyleSevin, E., Dehouck, L., Versele, R., Culot, M., & Gosselet, F. (2019). A Miniaturized Pump Out Method for Characterizing Molecule Interaction with ABC Transporters. International Journal of Molecular Sciences, 20(22), 5529. https://doi.org/10.3390/ijms20225529