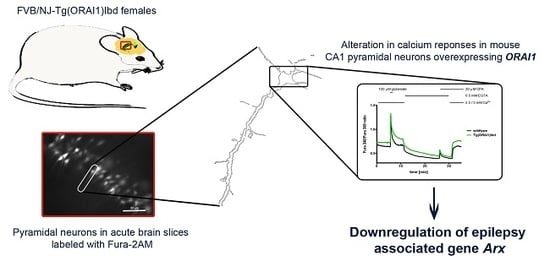
Changes in Calcium Homeostasis and Gene Expression Implicated in Epilepsy in Hippocampi of Mice Overexpressing ORAI1
Abstract
:1. Introduction
2. Results
2.1. Overexpression of ORAI1 in Neurons Leads to Altered Ca2+ Response in a Modified Ca2+ Addback Assay in CA1 Hippocampal Region
2.2. RNAseq Analysis of Transcripts from Hippocampi Indicates Up-Regulation of the Machinery Involved in Ribosome Biogenesis and Ribonucleoprotein Complex Assembly
2.3. Validation of RNAseq Data Using Customized Real-Time PCR Assays and ddPCR Technique Indicates Differential Expression of Dclk1, Cdkl5, and Arx Genes in the Hippocampi of Tg(ORAI1)Ibd Females
2.4. Expression of Hes-5, a Transcriptional Repressor That Is Involved in Neurogenesis, Is Up-Regulated in Tg(ORAI1) Ibd Females
3. Discussion
4. Materials and Methods
4.1. Animal Care
4.2. Generation of FVB/NJ–Tg(ORAI1)Ibd Transgenic Mice and Characterization of Transgene Expression
4.3. Brain Slice Preparation
4.4. Dye-Loading Procedure
4.5. Ca2+ Imaging of Neurons in Brain Slices
4.6. Data Analysis
4.7. Global Gene Expression Profiling
4.8. Real-Time Polymerase Chain Reaction Arrays and Quantitative Real-Time PCR (qPCR)
4.9. DdPCR Validation of Selected Genes
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| a.u. | arbitrary units |
| aCSF | artificial cerebrospinal fluid |
| AM | acetomethyl ester |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| Arx | aristaless related homeobox |
| CaMKII | Ca2+/calmodulin-dependent protein kinase II |
| Cdkl5 | cyclin-dependent kinase-like 5, also known as serine/threonine kinase 9 (STK9) |
| CPA | cyclopiazonic acid |
| CRAC | calcium release activated channel |
| EGTA | ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid |
| Dclk1 | doublecortin like kinase 1 |
| ddPCR | digital droplet PCR |
| DRG | dorsal root ganglion |
| ER | endoplasmic reticulum |
| FPKM | Fragments Per Kilobase Million |
| Fura-2 AM | Fura-2 acetomethyl ester |
| GO BP | gene ontology biological process |
| Hes-5 | hairy and enhancer of split-5 |
| Ibd | Institute of Experimental Biology, in Polish: Instytut Biologii Doświadczalnej |
| IP3R | inositol triphosphate receptor |
| NMDG | N-methyl-D-glucamine |
| nSOCE | neuronal store operated calcium entry |
| PMCA | plasma membrane calcium ATP-ase |
| RER | rough endoplasmic reticulum |
| RNASeq | RNA sequencing |
| SOCE | store operated calcium entry |
| STIM | stromal interaction molecule |
| Tg | transgenic |
| TRPCs | transient receptor potential cation channels |
| TTX | tetrodotoxin |
| VGCC | voltage-gated calcium channel |
References
- Putney, J.W., Jr. A model for receptor-regulated calcium entry. Cell Calcium 1986, 7, 1–12. [Google Scholar] [CrossRef]
- Majewski, L.; Kuznicki, J. SOCE in neurons: Signaling or just refilling? Biochim. Biophys. Acta 2015, 1853, 1940–1952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moccia, F.; Zuccolo, E.; Soda, T.; Tanzi, F.; Guerra, G.; Mapelli, L.; Lodola, F.; D’Angelo, E. Stim and Orai proteins in neuronal Ca(2+) signaling and excitability. Front. Cell. Neurosci. 2015, 9, 153. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Fivaz, M. Neuronal SOCE: Myth or Reality? Trends Cell Biol. 2016, 26, 890–893. [Google Scholar] [CrossRef]
- Wegierski, T.; Kuznicki, J. Neuronal calcium signaling via store-operated channels in health and disease. Cell Calcium 2018, 74, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Poggioli, J.; Mauger, J.P.; Guesdon, F.; Claret, M. A regulatory calcium-binding site for calcium channel in isolated rat hepatocytes. J. Biol. Chem. 1985, 260, 3289–3294. [Google Scholar] [PubMed]
- Lewis, R.S.; Cahalan, M.D. Mitogen-induced oscillations of cytosolic Ca2+ and transmembrane Ca2+ current in human leukemic T cells. Cell Regul. 1989, 1, 99–112. [Google Scholar] [CrossRef]
- Penner, R.; Matthews, G.; Neher, E. Regulation of calcium influx by second messengers in rat mast cells. Nature 1988, 334, 499–504. [Google Scholar] [CrossRef]
- Barak, P.; Parekh, A.B. Signaling through Ca(2+) Microdomains from Store-Operated CRAC Channels. Cold Spring Harb. Perspect. Biol. 2019. [Google Scholar] [CrossRef]
- Bird, G.S.; Putney, J.W., Jr. Capacitative calcium entry supports calcium oscillations in human embryonic kidney cells. J. Physiol. 2005, 562, 697–706. [Google Scholar] [CrossRef]
- Maus, M.; Cuk, M.; Patel, B.; Lian, J.; Ouimet, M.; Kaufmann, U.; Yang, J.; Horvath, R.; Hornig-Do, H.T.; Chrzanowska-Lightowlers, Z.M.; et al. Store-Operated Ca(2+) Entry Controls Induction of Lipolysis and the Transcriptional Reprogramming to Lipid Metabolism. Cell Metab. 2017, 25, 698–712. [Google Scholar] [CrossRef] [PubMed]
- Lacruz, R.S.; Feske, S. Diseases caused by mutations in ORAI1 and STIM1. Ann. N. Y. Acad. Sci. 2015, 1356, 45–79. [Google Scholar] [CrossRef] [PubMed]
- Liou, J.; Kim, M.L.; Heo, W.D.; Jones, J.T.; Myers, J.W.; Ferrell, J.E., Jr.; Meyer, T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr. Biol. 2005, 15, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Roos, J.; Di Gregorio, P.J.; Yeromin, A.V.; Ohlsen, K.; Lioudyno, M.; Zhang, S.; Safrina, O.; Kozak, J.A.; Wagner, S.L.; Cahalan, M.D.; et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J. Cell Biol. 2005, 169, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Feske, S.; Gwack, Y.; Prakriya, M.; Srikanth, S.; Puppel, S.H.; Tanasa, B.; Hogan, P.G.; Lewis, R.S.; Daly, M.; Rao, A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 2006, 441, 179–185. [Google Scholar] [CrossRef]
- Vig, M.; Peinelt, C.; Beck, A.; Koomoa, D.L.; Rabah, D.; Koblan-Huberson, M.; Kraft, S.; Turner, H.; Fleig, A.; Penner, R.; et al. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science 2006, 312, 1220–1223. [Google Scholar] [CrossRef]
- Prakriya, M.; Feske, S.; Gwack, Y.; Srikanth, S.; Rao, A.; Hogan, P.G. Orai1 is an essential pore subunit of the CRAC channel. Nature 2006, 443, 230–233. [Google Scholar] [CrossRef]
- Hou, X.; Pedi, L.; Diver, M.M.; Long, S.B. Crystal structure of the calcium release-activated calcium channel Orai. Science 2012, 338, 1308–1313. [Google Scholar] [CrossRef]
- Yen, M.; Lokteva, L.A.; Lewis, R.S. Functional Analysis of Orai1 Concatemers Supports a Hexameric Stoichiometry for the CRAC Channel. Biophys. J. 2016, 111, 1897–1907. [Google Scholar] [CrossRef] [Green Version]
- Vaeth, M.; Yang, J.; Yamashita, M.; Zee, I.; Eckstein, M.; Knosp, C.; Kaufmann, U.; Karoly Jani, P.; Lacruz, R.S.; Flockerzi, V.; et al. ORAI2 modulates store-operated calcium entry and T cell-mediated immunity. Nat. Commun. 2017, 8, 14714. [Google Scholar] [CrossRef] [Green Version]
- Tsvilovskyy, V.; Solis-Lopez, A.; Schumacher, D.; Medert, R.; Roers, A.; Kriebs, U.; Freichel, M. Deletion of Orai2 augments endogenous CRAC currents and degranulation in mast cells leading to enhanced anaphylaxis. Cell Calcium 2018, 71, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, M.; Vaeth, M.; Aulestia, F.J.; Costiniti, V.; Kassam, S.N.; Bromage, T.G.; Pedersen, P.; Issekutz, T.; Idaghdour, Y.; Moursi, A.M.; et al. Differential regulation of Ca(2+) influx by ORAI channels mediates enamel mineralization. Sci. Signal. 2019, 12, eaav4663. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Mei, Y.; Xia, J.; Hu, H. Orai1 and Orai3 Mediate Store-Operated Calcium Entry Contributing to Neuronal Excitability in Dorsal Root Ganglion Neurons. Front. Cell. Neurosci. 2017, 11, 400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schindl, R.; Frischauf, I.; Bergsmann, J.; Muik, M.; Derler, I.; Lackner, B.; Groschner, K.; Romanin, C. Plasticity in Ca2+ selectivity of Orai1/Orai3 heteromeric channel. Proc. Natl. Acad. Sci. USA 2009, 106, 19623–19628. [Google Scholar] [CrossRef] [PubMed]
- Feske, S.; Skolnik, E.Y.; Prakriya, M. Ion channels and transporters in lymphocyte function and immunity. Nat. Rev. Immunol. 2012, 12, 532–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen-Engerer, H.J.; Hartmann, J.; Karl, R.M.; Yang, J.; Feske, S.; Konnerth, A. Two types of functionally distinct Ca(2+) stores in hippocampal neurons. Nat. Commun. 2019, 10, 3223. [Google Scholar] [CrossRef]
- Vashisht, A.; Tanwar, J.; Motiani, R.K. Regulation of proto-oncogene Orai3 by miR18a/b and miR34a. Cell Calcium 2018, 75, 101–111. [Google Scholar] [CrossRef]
- Hoth, M.; Niemeyer, B.A. The neglected CRAC proteins: Orai2, Orai3, and STIM2. Curr. Top. Membr. 2013, 71, 237–271. [Google Scholar]
- Prakriya, M.; Lewis, R.S. Store-Operated Calcium Channels. Physiol. Rev. 2015, 95, 1383–1436. [Google Scholar] [CrossRef] [Green Version]
- Vaeth, M.; Feske, S. Ion channelopathies of the immune system. Curr. Opin. Immunol. 2018, 52, 39–50. [Google Scholar] [CrossRef]
- Feske, S. CRAC channels and disease—From human CRAC channelopathies and animal models to novel drugs. Cell Calcium 2019, 80, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Maciag, F.; Majewski, L.; Boguszewski, P.M.; Gupta, R.K.; Wasilewska, I.; Wojtas, B.; Kuznicki, J. Behavioral and electrophysiological changes in female mice overexpressing ORAI1 in neurons. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 1137–1150. [Google Scholar] [CrossRef] [PubMed]
- Kipanyula, M.J.; Contreras, L.; Zampese, E.; Lazzari, C.; Wong, A.K.; Pizzo, P.; Fasolato, C.; Pozzan, T. Ca2+ dysregulation in neurons from transgenic mice expressing mutant presenilin 2. Aging Cell 2012, 11, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Amrhein, V.; Greenland, S.; McShane, B. Scientists rise up against statistical significance. Nature 2019, 567, 305–307. [Google Scholar] [CrossRef] [Green Version]
- Taylor, S.C.; Laperriere, G.; Germain, H. Droplet Digital PCR versus qPCR for gene expression analysis with low abundant targets: From variable nonsense to publication quality data. Sci. Rep. 2017, 7, 2409. [Google Scholar] [CrossRef]
- Hartmann, J.; Karl, R.M.; Alexander, R.P.; Adelsberger, H.; Brill, M.S.; Ruhlmann, C.; Ansel, A.; Sakimura, K.; Baba, Y.; Kurosaki, T.; et al. STIM1 controls neuronal Ca(2+) signaling, mGluR1-dependent synaptic transmission, and cerebellar motor behavior. Neuron 2014, 82, 635–644. [Google Scholar] [CrossRef]
- Dittmer, P.J.; Wild, A.R.; Dell’Acqua, M.L.; Sather, W.A. STIM1 Ca(2+) Sensor Control of L-type Ca(2+)-Channel-Dependent Dendritic Spine Structural Plasticity and Nuclear Signaling. Cell Rep. 2017, 19, 321–334. [Google Scholar] [CrossRef]
- Garcia-Alvarez, G.; Lu, B.; Yap, K.A.; Wong, L.C.; Thevathasan, J.V.; Lim, L.; Ji, F.; Tan, K.W.; Mancuso, J.J.; Tang, W.; et al. STIM2 regulates PKA-dependent phosphorylation and trafficking of AMPARs. Mol. Biol. Cell 2015, 26, 1141–1159. [Google Scholar] [CrossRef]
- Gruszczynska-Biegala, J.; Sladowska, M.; Kuznicki, J. AMPA Receptors Are Involved in Store-Operated Calcium Entry and Interact with STIM Proteins in Rat Primary Cortical Neurons. Front. Cell. Neurosci. 2016, 10, 251. [Google Scholar] [CrossRef] [Green Version]
- Majewski, L.; Maciag, F.; Boguszewski, P.M.; Wasilewska, I.; Wiera, G.; Wojtowicz, T.; Mozrzymas, J.; Kuznicki, J. Overexpression of STIM1 in neurons in mouse brain improves contextual learning and impairs long-term depression. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1071–1087. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, H.; Liu, J.; Popugaeva, E.; Xu, N.J.; Feske, S.; White, C.L., III; Bezprozvanny, I. Reduced synaptic STIM2 expression and impaired store-operated calcium entry cause destabilization of mature spines in mutant presenilin mice. Neuron 2014, 82, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sun, S.; Wu, L.; Pchitskaya, E.; Zakharova, O.; Fon Tacer, K.; Bezprozvanny, I. Store-Operated Calcium Channel Complex in Postsynaptic Spines: A New Therapeutic Target for Alzheimer’s Disease Treatment. J. Neurosci. 2016, 36, 11837–11850. [Google Scholar] [CrossRef]
- Wojda, U.; Kuznicki, J. Alzheimer’s disease modeling: Ups, downs, and perspectives for human induced pluripotent stem cells. J. Alzheimer Dis. 2013, 34, 563–588. [Google Scholar] [CrossRef] [PubMed]
- Greget, R.; Pernot, F.; Bouteiller, J.M.; Ghaderi, V.; Allam, S.; Keller, A.F.; Ambert, N.; Legendre, A.; Sarmis, M.; Haeberle, O.; et al. Simulation of postsynaptic glutamate receptors reveals critical features of glutamatergic transmission. PLoS ONE 2011, 6, e28380. [Google Scholar] [CrossRef] [PubMed]
- Weiler, I.J.; Childers, W.S.; Greenough, W.T. Calcium ion impedes translation initiation at the synapse. J. Neurochem. 1996, 66, 197–202. [Google Scholar] [CrossRef]
- Lur, G.; Haynes, L.P.; Prior, I.A.; Gerasimenko, O.V.; Feske, S.; Petersen, O.H.; Burgoyne, R.D.; Tepikin, A.V. Ribosome-free terminals of rough ER allow formation of STIM1 puncta and segregation of STIM1 from IP(3) receptors. Curr. Biol. 2009, 19, 1648–1653. [Google Scholar] [CrossRef]
- Myers, K.A.; Johnstone, D.L.; Dyment, D.A. Epilepsy genetics: Current knowledge, applications, and future directions. Clin. Genet. 2019, 95, 95–111. [Google Scholar] [CrossRef]
- Sherr, E.H. The ARX story (epilepsy, mental retardation, autism, and cerebral malformations): One gene leads to many phenotypes. Curr. Opin. Pediatr. 2003, 15, 567–571. [Google Scholar] [CrossRef]
- Abedini, S.S.; Kahrizi, K.; Behjati, F.; Banihashemi, S.; Ghasemi Firoozabadi, S.; Najmabadi, H. Mutational screening of ARX gene in Iranian families with X-linked intellectual disability. Arch. Iran. Med. 2012, 15, 361–365. [Google Scholar]
- Bonneau, D.; Toutain, A.; Laquerriere, A.; Marret, S.; Saugier-Veber, P.; Barthez, M.A.; Radi, S.; Biran-Mucignat, V.; Rodriguez, D.; Gelot, A. X-linked lissencephaly with absent corpus callosum and ambiguous genitalia (XLAG): Clinical, magnetic resonance imaging, and neuropathological findings. Ann. Neurol. 2002, 51, 340–349. [Google Scholar] [CrossRef]
- Cossee, M.; Faivre, L.; Philippe, C.; Hichri, H.; de Saint-Martin, A.; Laugel, V.; Bahi-Buisson, N.; Lemaitre, J.F.; Leheup, B.; Delobel, B.; et al. ARX polyalanine expansions are highly implicated in familial cases of mental retardation with infantile epilepsy and/or hand dystonia. Am. J. Med. Genet. A 2011, 155a, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Olivetti, P.R.; Noebels, J.L. Interneuron, interrupted: Molecular pathogenesis of ARX mutations and X-linked infantile spasms. Curr. Opin. Neurobiol. 2012, 22, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Stalpers, X.L.; Spruijt, L.; Yntema, H.G.; Verrips, A. Clinical phenotype of 5 females with a CDKL5 mutation. J. Child Neurol. 2012, 27, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Nemos, C.; Lambert, L.; Giuliano, F.; Doray, B.; Roubertie, A.; Goldenberg, A.; Delobel, B.; Layet, V.; N’Guyen, M.A.; Saunier, A.; et al. Mutational spectrum of CDKL5 in early-onset encephalopathies: A study of a large collection of French patients and review of the literature. Clin. Genet. 2009, 76, 357–371. [Google Scholar] [CrossRef]
- Liang, J.S.; Shimojima, K.; Takayama, R.; Natsume, J.; Shichiji, M.; Hirasawa, K.; Imai, K.; Okanishi, T.; Mizuno, S.; Okumura, A.; et al. CDKL5 alterations lead to early epileptic encephalopathy in both genders. Epilepsia 2011, 52, 1835–1842. [Google Scholar] [CrossRef]
- Saitsu, H.; Osaka, H.; Nishiyama, K.; Tsurusaki, Y.; Doi, H.; Miyake, N.; Matsumoto, N. A girl with early-onset epileptic encephalopathy associated with microdeletion involving CDKL5. Brain Dev. 2012, 34, 364–367. [Google Scholar] [CrossRef]
- Kilstrup-Nielsen, C.; Rusconi, L.; La Montanara, P.; Ciceri, D.; Bergo, A.; Bedogni, F.; Landsberger, N. What we know and would like to know about CDKL5 and its involvement in epileptic encephalopathy. Neural Plast. 2012, 2012, 728267. [Google Scholar] [CrossRef]
- Stouffer, M.A.; Golden, J.A.; Francis, F. Neuronal migration disorders: Focus on the cytoskeleton and epilepsy. Neurobiol. Dis. 2016, 92, 18–45. [Google Scholar] [CrossRef]
- Srivastava, S.; Cohen, J.; Pevsner, J.; Aradhya, S.; McKnight, D.; Butler, E.; Johnston, M.; Fatemi, A. A novel variant in GABRB2 associated with intellectual disability and epilepsy. Am. J. Med. Genet. A 2014, 164a, 2914–2921. [Google Scholar] [CrossRef]
- Urak, L.; Feucht, M.; Fathi, N.; Hornik, K.; Fuchs, K. A GABRB3 promoter haplotype associated with childhood absence epilepsy impairs transcriptional activity. Hum. Mol. Genet. 2006, 15, 2533–2541. [Google Scholar] [CrossRef] [Green Version]
- Macdonald, R.L.; Kang, J.Q.; Gallagher, M.J. Mutations in GABAA receptor subunits associated with genetic epilepsies. J. Physiol. 2010, 588, 1861–1869. [Google Scholar] [CrossRef] [PubMed]
- Doty, C.N. SCN9A: Another sodium channel excited to play a role in human epilepsies. Clin. Genet. 2010, 77, 326–328. [Google Scholar] [CrossRef] [PubMed]
- Meisler, M.H.; O’Brien, J.E.; Sharkey, L.M. Sodium channel gene family: Epilepsy mutations, gene interactions and modifier effects. J. Physiol. 2010, 588, 1841–1848. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.A.; Pappas, C.; Dahle, E.J.; Claes, L.R.; Pruess, T.H.; De Jonghe, P.; Thompson, J.; Dixon, M.; Gurnett, C.; Peiffer, A.; et al. A role of SCN9A in human epilepsies, as a cause of febrile seizures and as a potential modifier of Dravet syndrome. PLoS Genet. 2009, 5, e1000649. [Google Scholar] [CrossRef] [PubMed]
- Heron, S.E.; Hernandez, M.; Edwards, C.; Edkins, E.; Jansen, F.E.; Scheffer, I.E.; Berkovic, S.F.; Mulley, J.C. Neonatal seizures and long QT syndrome: A cardiocerebral channelopathy? Epilepsia 2010, 51, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Vidal, M.; Morris, R.; Grosveld, F.; Spanopoulou, E. Tissue-specific control elements of the Thy-1 gene. EMBO J. 1990, 9, 833–840. [Google Scholar] [CrossRef]
- Caroni, P. Overexpression of growth-associated proteins in the neurons of adult transgenic mice. J. Neurosci. Methods 1997, 71, 3–9. [Google Scholar] [CrossRef]
- Murphy, D.; Carter, D.A. An overview of transgenic mouse production. Methods Mol. Biol. 1993, 18, 111–114. [Google Scholar]
- Ting, J.T.; Lee, B.R.; Chong, P.; Soler-Llavina, G.; Cobbs, C.; Koch, C.; Zeng, H.; Lein, E. Preparation of Acute Brain Slices Using an Optimized N-Methyl-D-glucamine Protective Recovery Method. J. Vis. Exp. 2018. [Google Scholar] [CrossRef]
- Tarazona, S.; Furio-Tari, P.; Turra, D.; Pietro, A.D.; Nueda, M.J.; Ferrer, A.; Conesa, A. Data quality aware analysis of differential expression in RNA-seq with NOISeq R/Bioc package. Nucleic Acids Res. 2015, 43, e140. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package, for comparing biological themes among gene clusters. Omics 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.; Domrachev, M.; Lash, A.E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002, 30, 207–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Bio-Rad Assays | qPCR | |||||
|---|---|---|---|---|---|---|
| Gene Name | ENSEMBL ID | Description | Cat. No. | 2−∆∆Ct | p-Value Tukey | |
| Genes associated with epilepsy | Kcne2 | ENSMUSG00000039672 | potassium voltage-gated channel, Isk-related subfamily, gene 2 | qMmuCID0011027 | 0.487 | 0.670 |
| Arx | ENSMUSG00000035277 | aristaless related homeobox | qMmuCED0004831 | 0.787 | 0.046 | |
| Dclk1 | ENSMUSG00000027797 | serine/threonine-protein kinase DCLK1 | qMmuCID0022222 | 0.827 | 0.015 | |
| Cdkl5 | ENSMUSG00000031292 | cyclin-dependent kinase-like 5 | qMmuCID0013381 | 0.868 | 0.064 | |
| Gabrb2 | ENSMUSG00000007653 | gamma-aminobutyric acid (GABA) A receptor, subunit beta 2 | qMmuCID0005334 | 0.886 | 0.224 | |
| Scn9a | ENSMUSG00000075316 | sodium channel protein type 9 subunit alpha | qMmuCID0015354 | 1.283 | 0.131 | |
| Folr1 | ENSMUSG00000001827 | folate receptor 1 | qMmuCID0016330 | 0.5262 | 0.688 | |
| Clic6 | ENSMUSG00000022949 | chloride intracellular channel 6 | qMmuCID0011099 | 0.615 | 0.678 | |
| Kl | ENSMUSG00000058488 | klotho, the Klotho peptide generated by cleavage of the membrane-bound isoform may be an anti-aging circulating hormone | qMmuCID0007154 | 0.663 | 0.648 | |
| Trpm3 | ENSMUSG00000052387 | transient receptor potential cation channel, subfamily M, member 3 | qMmuCID0040163 | 0.8 | 0.747 | |
| Fam26e | ENSMUSG00000049872 | calcium homeostasis modulator protein 5 | qMmuCID0008059 | 0.882 | 0.213 | |
| Cul4b | ENSMUSG00000031095 | cullin 4B, Core component of multiple cullin-RING-based E3 ubiquitin-protein ligase complexes | qMmuCID0011478 | 0.884 | 0.667 | |
| Mmp14 | ENSMUSG00000000957 | matrix metallopeptidase 14 (membrane-inserted) | qMmuCID0006120 | 0.889 | 0.473 | |
| Ston2 | ENSMUSG00000020961 | stonin 2, adapter protein involved in endocytic machinery | qMmuCID0014604 | 0.936 | 0.462 | |
| Shisa9 | ENSMUSG00000022494 | regulator of short-term neuronal synaptic plasticity in the dentate gyrus | qMmuCID0022663 | 0.940 | 0.523 | |
| Akap9 | ENSMUSG00000040407 | A-kinase anchor protein 9, scaffolding protein | qMmuCID0023460 | 0.958 | 0.542 | |
| Enah | ENSMUSG00000022995 | ENAH actin regulator; actin-associated proteins involved in a range of processes dependent on cytoskeleton remodeling and cell polarity such as axon guidance | qMmuCID0026581 | 0.985 | 0.593 | |
| Nrp2 | ENSMUSG00000025969 | Neuropilin-2, High affinity receptor for semaphorins | qMmuCID0005743 | 1.021 | 0.835 | |
| Mdm2 | ENSMUSG00000020184 | E3 ubiquitin-protein ligase | qMmuCID0025320 | 1.044 | 0.676 | |
| Prex2 | ENSMUSG00000048960 | phosphatidylinositol-3,4,5-trisphosphate-dependent Rac exchange factor 2 | qMmuCID0039899 | 1.070 | 0.187 | |
| Primer sequence | qPCR | |||||
|---|---|---|---|---|---|---|
| Gene Name | ENSEMBL ID | Description | Forward | Revers | 2−∆∆Ct | p-Value Tukey |
| Ddb1 | ENSMUSG00000024740 | damage specific DNA binding protein 1 | TCAAAGAGGTGGGAATGTACG | GATGTCAATGCTTTCGCCAC | 0.147 | 0.351 |
| Vdac2 | ENSMUSG00000021771 | voltage-dependent anion channel 2 | TGGAACACCGATAACACTCTG | CACTCCCTCTTGTAAGCAGAC | 0.18 | 0.317 |
| Cul1 | ENSMUSG00000029686 | cullin 1 | ACCGATTCTCCAGCAAAGTG | TCTCCATGTCACCAATGCAAG | 0.204 | 0.400 |
| Nol3 | ENSMUSG00000014776 | nucleolar protein 3 (apoptosis repressor with CARD domain) | TCCAAGAAGAGGATGAATCTGAAG | ATTTGGCAGTAGGTGTCTCG | 0.298 | 0.255 |
| Itmbl2 | ENSMUSG00000022108 | integral membrane protein 2B | TTTGAGGAAGACGCAGTGG | TGTTCAGAGGAATCACGTAG | 0.396 | 0.272 |
| Clnx | ENSMUSG00000020368 | calnexin | CTTTGCCAGTGTTCCTTGTG | CTTCCTCTTCATCCCTCTTGTTC | 0.495 | 0.534 |
| Sv2a | ENSMUSG00000038486 | synaptic vesicle glycoprotein 2 a | GTGGACACTCTACTTCGTGC | ATGCCCAGGTACACAATGAG | 0.502 | 0.395 |
| Gpr17 | ENSMUSG00000052229 | G protein-coupled receptor 17 | TCTCCTGTCCTTTCCTTCCT | TCTCTTGTCCGCATTGCTC | 0.587 | 0.117 |
| Arc | ENSMUSG00000022602 | activity regulated cytoskeletal-associated protein | CTACAGAGCCAGGAGAATGAC | GTGTCTTGGAACCCATGTAGG | 0.752 | 0.668 |
| Rab5 | ENSMUSG00000000711 | Rab5B. member RAS oncogene family | AGGGAACAAAGCTGACCTTG | TGCCAGGAAGAGATCATTCAC | 0.820 | 0.505 |
| Atxn | ENSMUSG00000074748 | ataxin 7-like 3B | TGTGTACCCAGCCTATACAATTC | CCTGACCATCAACACCATCTAA | 0.906 | 0.865 |
| TIMP1 | ENSMUSG00000001131 | tissue inhibitor of metalloproteinase 1 | CTCAAAGACCTATAGTGCTGGC | CAAAGTGACGGCTCTGGTAG | 0.913 | 0.890 |
| Dok3 | ENSMUSG00000035711 | docking protein 3 | ACTGGTGCCTTCCTGATTAC | GATCCTGACGAACATTCTCCG | 0.961 | 0.949 |
| Strap | ENSMUSG00000030224 | serine/threonine kinase receptor associated protein | CCAGGGAGATACAGGAGACT | AGACCGCATCCCATACTTTG | 1.568 | 0.186 |
| Hes-5 | ENSMUSG00000048001 | hes family bHLH transcription factor 5 | CTACCTGAAACACAGCAAAGC | AGCTTCATCTGCGTGTCG | 2.932 | 0.003 |
| Gene Name | Encoded Protein Function | Phenotype in Human | Reference |
|---|---|---|---|
| Arx | aristaless related homeobox gene- regulator of gene transcription important forbrain development | Early infantile epileptic encephalopathy (EIEE); X-linked lissencephaly with abnormal genitalia (XLAG) | [48,49,50,51,52] |
| Cdkl5 | X-linked serine/threonine kinase cyclin-dependent kinase-like 5 | Early infantile epileptic encephalopathy (EIEE) | [53,54,55,56,57] |
| Dclk1 | doublecortin-like kinase 1 | Focal seizures | [58] |
| Gabrb2 | subunit of γ-aminobutyric acid type A (GABAA) receptor | Generalized epilepsy with febrile seizures plus (GEFS+); Childhood absence epilepsy (CAE); Lennox-Gastaut syndrome | [59,60,61] |
| Scn9a | sodium channel | Dravet syndrome (DS); Familial febrile seizures (FFS); Generalized epilepsy with febrile seizures plus (GEFS+) | [62,63,64] |
| Kcne | a family of single-helix transmembrane proteins with 5 known members that modulate the function of several ion channels | Familial neonatal seizures | [65] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majewski, L.; Wojtas, B.; Maciąg, F.; Kuznicki, J. Changes in Calcium Homeostasis and Gene Expression Implicated in Epilepsy in Hippocampi of Mice Overexpressing ORAI1. Int. J. Mol. Sci. 2019, 20, 5539. https://doi.org/10.3390/ijms20225539
Majewski L, Wojtas B, Maciąg F, Kuznicki J. Changes in Calcium Homeostasis and Gene Expression Implicated in Epilepsy in Hippocampi of Mice Overexpressing ORAI1. International Journal of Molecular Sciences. 2019; 20(22):5539. https://doi.org/10.3390/ijms20225539
Chicago/Turabian StyleMajewski, Lukasz, Bartosz Wojtas, Filip Maciąg, and Jacek Kuznicki. 2019. "Changes in Calcium Homeostasis and Gene Expression Implicated in Epilepsy in Hippocampi of Mice Overexpressing ORAI1" International Journal of Molecular Sciences 20, no. 22: 5539. https://doi.org/10.3390/ijms20225539
APA StyleMajewski, L., Wojtas, B., Maciąg, F., & Kuznicki, J. (2019). Changes in Calcium Homeostasis and Gene Expression Implicated in Epilepsy in Hippocampi of Mice Overexpressing ORAI1. International Journal of Molecular Sciences, 20(22), 5539. https://doi.org/10.3390/ijms20225539