Development of a Highly Efficient Hybrid Peptide That Increases Immunomodulatory Activity Via the TLR4-Mediated Nuclear Factor-κB Signaling Pathway
Abstract
:1. Introduction
2. Results
2.1. Selection of Immunomodulatory Peptides by Molecular Docking
2.2. Cytotoxicity to RAW264.7 Macrophage Cells
2.3. Ex Vivo Stability of LTAa in Plasma
2.4. Effect of LTAa on Body Weight and Immune Organs
2.5. Effects of LTAa on Peritoneal Macrophage Phagocytosis
2.6. Effects of LTAa on T cells in Mice Splenocytes
2.7. Effects of LTAa on Serum TNF-α, IL-6, and IL-1β Levels
2.8. Effects of LTAa on Serum Ig Contents
2.9. Specific Binding of LTAa to TLR4/MD-2
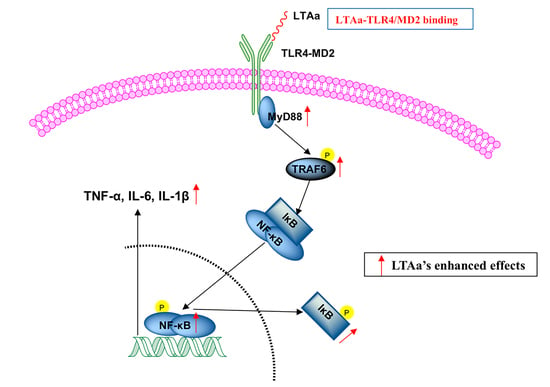
2.10. LTAa Activates the TLR4-NF-κB Pathway in CTX-Treated Mice
3. Discussion
4. Materials and Methods
4.1. Hybrid Peptide Design
4.2. Peptide Synthesis
4.3. Circular Dichroism Analysis
4.4. Hybrid Peptide Scan by Molecule Docking
4.5. Cell Culture
4.6. Cell Viability Assay
4.7. Immunomodulatory Activity in the RAW264.7 Cell Line
4.8. Ex Vivo Stability of LTAa in Plasma
4.9. Animal Model
4.10. Preparation of Peritoneal Macrophages
4.11. Peritoneal Macrophage Phagocytosis
4.12. Flow Cytometry
4.13. Serum Cytokine and Immunoglobulin (Ig) Measurements by ELISA
4.14. Binding Assay of LTAa to TLR4/MD-2
4.15. Western Blot Analysis
4.16. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hooper, L.V.; Littman, D.R.; Macpherson, A.J. Interactions Between the Microbiota and the Immune System. Science 2012, 336, 1268–1273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldszmid, R.S.; Dzutsev, A.; Trinchieri, G. Host Immune Response to Infection and Cancer: Unexpected Commonalities. Cell Host Microbe 2014, 15, 295–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, Y.; Xing, L.; Sun, M.M.; Su, F.C. Immunomodulatory effects of sulfated polysaccharides of pine pollen on mouse macrophages. Int. J. Biol. Macromol. 2016, 91, 846–855. [Google Scholar] [CrossRef]
- Li, X.Q.; Xu, W. TLR4-mediated activation of macrophages by the polysaccharide fraction from Polyporus umbellatus (pers.) Fries. J. Ethnopharmacol. 2011, 135, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Koo, J.E.; Seo, Y.J.; Tyagi, N.; Jeong, E.; Choi, J.; Lim, K.M.; Park, Z.Y.; Lee, J.Y. Suppression of Toll-like receptor 4 activation by caffeic acid phenethyl ester is mediated by interference of LPS binding to MD2. Br. J. Pharmacol. 2013, 168, 1933–1945. [Google Scholar] [CrossRef] [Green Version]
- Michaeli, A.; Mezan, S.; Kuhbacher, A.; Finkelmeier, D.; Elias, M.; Zatsepin, M.; Reed, S.G.; Duthie, M.S.; Rupp, S.; Lerner, I.; et al. Computationally Designed Bispecific MD2/CD14 Binding Peptides Show TLR4 Agonist Activity. J. Immunol. 2018, 201, 3383–3391. [Google Scholar] [CrossRef]
- Jung, J.Y.; Shin, J.S.; Lee, S.G.; Rhee, Y.K.; Cho, C.W.; Hong, H.D.; Lee, K.T. Lactobacillus sakei K040706 evokes immunostimulatory effects on macrophages through TLR 2-mediated activation. Int. Immunopharmacol. 2015, 28, 88–96. [Google Scholar] [CrossRef]
- Peng, G.Z.; Pan, X.; Hu, H.Y.; Xu, Y.H.; Wu, C.B. N-terminal site-specific PEGylation enhances the circulation half-life of Thymosin alpha 1. J. Drug Deliv. Sci. Technol. 2019, 49, 405–412. [Google Scholar] [CrossRef]
- Li, C.L.; Zhang, T.; Saibara, T.; Nemoto, Y.; Ono, M.; Akisawa, N.; Iwasaki, S.; Maeda, T.; Onishi, S. Thymosin alpha 1 accelerates restoration of T cell-mediated neutralizing antibody response in immunocompromised hosts. Int. Immunopharmacol. 2002, 2, 39–46. [Google Scholar] [CrossRef]
- Lau, G.K.K.; Nanji, A.; Hou, J.; Fong, D.Y.T.; Au, W.S.; Yuen, S.T.; Lin, M.; Kung, H.F.; Lam, S.K. Thymosin-alpha 1 and famciclovir combination therapy activates T-cell response in patients with chronic hepatitis B virus infection in immune-tolerant phase. J. Viral Hepat. 2002, 9, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Wolf, G.T.; Hudson, J.; Peterson, K.A.; Poore, J.A.; Mcclatchey, K.D. Interleukin-2 Receptor Expression in Patients with Head and Neck Squamous Carcinoma—Effects of Thymosin Alpha-1 Invitro. Arch. Otolaryngol. 1989, 115, 1345–1349. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Biswas, S.; Mathur, K.B.; Haq, W.; Garg, S.K.; Agarwal, S.S. Thymopentin and splenopentin as immunomodulators—Current status. Immunol. Res. 1998, 17, 345–368. [Google Scholar] [CrossRef] [PubMed]
- Cascinelli, N.; Belli, F.; Mascheroni, L.; Lenisa, L.; Clemente, C. Evaluation of clinical efficacy and tolerability of intravenous high dose thymopentin in advanced melanoma patients. Melanoma Res. 1998, 8, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Lee, J.; Um, S.H.; Ahn, S.H.; Chang, H.Y.; Park, Y.K.; Hong, S.P.; Moon, Y.M.; Han, K.H. Combination therapy of thymosin alpha-1 and lamivudine for HBeAg positive chronic hepatitis B: A prospective randomized, comparative pilot study. J. Gastroenterol. Hepatol. 2008, 23, 729–735. [Google Scholar] [CrossRef]
- Mansour, S.C.; Pena, O.M.; Hancock, R.E.W. Host defense peptides: Front-line immunomodulators. Trends Immunol. 2014, 35, 443–450. [Google Scholar] [CrossRef]
- Agier, J.; Rozalska, S.; Wiktorska, M.; Zelechowska, P.; Pastwinska, J.; Brzezinska-Blaszczyk, E. The RLR/NLR expression and pro-inflammatory activity of tissue mast cells are regulated by cathelicidin LL-37 and defensin hBD-2. Sci. Rep. UK 2018, 8, 11750. [Google Scholar] [CrossRef] [Green Version]
- Bowdish, D.M.E.; Davidson, D.J.; Speert, D.P.; Hancock, R.E.W. The human cationic peptide LL-37 induces activation of the extracellular signal-regulated kinase and p38 kinase pathways in primary human monocytes. J. Immunol. 2004, 172, 3758–3765. [Google Scholar] [CrossRef] [Green Version]
- Scott, M.G.; Davidson, D.J.; Gold, M.R.; Bowdish, D.; Hancock, R.E.W. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J. Immunol. 2002, 169, 3883–3891. [Google Scholar] [CrossRef] [Green Version]
- Tjabringa, G.S.; Aarbiou, J.; Ninaber, D.K.; Drijfhout, J.W.; Sorensen, O.E.; Borregaard, N.; Rabe, K.F.; Hiemstra, P.S. The antimicrobial peptide LL-37 activates innate immunity at the airway epithelial surface by transactivation of the epidermal growth factor receptor. J. Immunol. 2003, 171, 6690–6696. [Google Scholar] [CrossRef] [Green Version]
- Mookherjee, N.; Brown, K.L.; Bowdish, D.M.E.; Doria, S.; Falsafi, R.; Hokamp, K.; Roche, F.M.; Mu, R.X.; Doho, G.H.; Pistolic, J.; et al. Modulation of the TLR-mediated inflammatory response by the endogenous human host defense peptide LL-37. J. Immunol. 2006, 176, 2455–2464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molhoek, E.M.; den Hertog, A.L.; de Vries, A.M.B.C.; Nazmi, K.; Veerman, E.C.I.; Hartgers, F.C.; Yazdanbakhsh, M.; Bikker, F.J.; van der Kleij, D. Structure-function relationship of the human antimicrobial peptide LL-37 and LL-37 fragments in the modulation of TLR responses. Biol. Chem. 2009, 390, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Anders, E.; Dahl, S.; Svensson, D.; Nilsson, B.O. LL-37-induced human osteoblast cytotoxicity and permeability occurs independently of cellular LL-37 uptake through clathrin-mediated endocytosis. Biochem. Biophys. Res. Commun. 2018, 501, 280–285. [Google Scholar] [CrossRef]
- Li, Y.Q.; Smith, C.; Wu, H.F.; Teng, P.; Shi, Y.; Padhee, S.; Jones, T.; Nguyen, A.M.; Cao, C.H.; Yin, H.; et al. Short Antimicrobial Lipo-alpha/gamma-AA Hybrid Peptides. ChemBioChem 2014, 15, 2275–2280. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.B.; Wu, R.J.; Zhang, L.L.; Ahmad, B.; Si, D.Y.; Zhang, R.J. Expression, Purification, and Characterization of a Novel Hybrid Peptide with Potent Antibacterial Activity. Molecules 2018, 23, 1491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grottesi, A.; Sette, M.; Palamara, A.T.; Rotilio, G.; Garaci, E.; Paci, M. The conformation of peptide thymosin alpha 1 in solution and in a membrane-like environment by circular dichroism and NMR spectroscopy. A possible model for its interaction with the lymphocyte membrane. Peptides 1998, 19, 1731–1738. [Google Scholar] [CrossRef]
- Mandaliti, W.; Nepravishta, R.; Vallebona, P.S.; Pica, F.; Garaci, E.; Paci, M. Thymosin alpha 1 Interacts with Exposed Phosphatidylserine in Membrane Models and in Cells and Uses Serum Albumin as a Carrier. Biochemistry US 2016, 55, 1462–1472. [Google Scholar] [CrossRef]
- Gao, D.; Zhang, X.; Zhang, J.; Cao, J.; Wang, F. Expression of thymosin alpha1-thymopentin fusion peptide in Pichia pastoris and its characterization. Arch. Pharm. Res. 2008, 31, 1471–1476. [Google Scholar] [CrossRef]
- Giavasis, I. Bioactive fungal polysaccharides as potential functional ingredients in food and nutraceuticals. Curr. Opin. Biotechnol. 2014, 26, 162–173. [Google Scholar] [CrossRef]
- Wang, H.; Xu, L.; Yu, M.M.; Wang, Y.H.; Jiang, T.F.; Yang, S.; Lv, Z.H. Glycosaminoglycan from Apostichopus japonicus induces immunomodulatory activity in cyclophosphamide-treated mice and in macrophages. Int. J. Biol. Macromol. 2019, 130, 229–237. [Google Scholar] [CrossRef]
- Romani, L.; Oikonomou, V.; Moretti, S.; Iannitti, R.G.; D’Adamo, M.C.; Villella, V.R.; Pariano, M.; Sforna, L.; Borghi, M.; Bellet, M.M.; et al. Thymosin alpha1 represents a potential potent single-molecule-based therapy for cystic fibrosis. Nat. Med. 2017, 23, 590–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Zheng, L.; Li, P.L.; Wang, F.S. Intein-mediated expression, purification, and characterization of thymosin alpha 1-thymopentin fusion peptide in Escherichia coli. Protein Expr. Purif. 2012, 84, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Zhang, X.G.; Jiang, Y.T.; Yan, L.Y.; Tang, L.; Yin, Y.W.; Cheng, D.S.; Chen, J.; Wang, M. Bioactivity and pharmacokinetics of two human serum albumin-thymosin alpha 1-fusion proteins, rHSA-T alpha 1 and rHSA-L-T alpha 1, expressed in recombinant Pichia pastoris. Cancer Immunol. Immunother. 2010, 59, 1335–1345. [Google Scholar] [CrossRef] [PubMed]
- Garaci, E.; Pica, F.; Sinibaldi-Vallebona, P.; Pierimarchi, P.; Mastino, A.; Matteucci, C.; Rasi, G. Thymosin α1 in combination with cytokines and chemotherapy for the treatment of cancer. Int. Immunopharmacol. 2003, 3, 1145–1150. [Google Scholar] [CrossRef]
- Nijnik, A.; Hancock, R.E.W. The roles of cathelicidin LL-37 in immune defences and novel clinical applications. Curr. Opin. Hematol. 2009, 16, 41–47. [Google Scholar] [CrossRef]
- Liu, Y.F.; Xia, X.; Xu, L.; Wang, Y.Z. Design of hybrid beta-hairpin peptides with enhanced cell specificity and potent anti-inflammatory activity. Biomaterials 2013, 34, 237–250. [Google Scholar] [CrossRef]
- Ma, Z.; Wei, D.D.; Yan, P.; Zhu, X.; Shan, A.S.; Bi, Z.P. Characterization of cell selectivity, physiological stability and endotoxin neutralization capabilities of alpha-helix-based peptide amphiphiles. Biomaterials 2015, 52, 517–530. [Google Scholar] [CrossRef]
- Fan, Y.P.; Lu, Y.; Wang, D.Y.; Liu, J.G.; Song, X.P.; Zhang, W.M.; Zhao, X.J.; Luong, N.Y.; Hu, Y.L. Effect of epimedium polysaccharide-propolis flavone immunopotentiator on immunosuppression induced by cyclophosphamide in chickens. Cell. Immunol. 2013, 281, 37–43. [Google Scholar] [CrossRef]
- Ren, Z.; He, C.H.; Fan, Y.H.; Guo, L.W.; Si, H.M.; Wang, Y.W.; Shi, Z.Y.; Zhang, H.B. Immuno-enhancement effects of ethanol extract from Cyrtomium macrophyllum (Makino) Tagawa on cyclophosphamide-induced immunosuppression in BALB/c mice. J. Ethnopharmacol. 2014, 155, 769–775. [Google Scholar] [CrossRef]
- Gong, Y.; Wu, J.; Li, S.T. Immuno-enhancement effects of Lycium ruthenicum Murr. polysaccharide on cyclophosphamide-induced immunosuppression in mice. Int. J. Clin. Exp. Med. 2015, 8, 20631–20637. [Google Scholar]
- Mei, Y.X.; Chen, H.X.; Zhang, J.; Zhang, X.D.; Liang, Y.X. Protective effect of chitooligosaccharides against cyclophosphamide-induced immunosuppression in mice. Int. J. Biol. Macromol. 2013, 62, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Dong, Z.H.; Zhu, X.S.; Xu, H.Y.; Zhao, Z.X. Characterization and protective effect of Polygonatum sibiricum polysaccharide against cyclophosphamide-induced immunosuppression in Balb/c mice. Int. J. Biol. Macromol. 2018, 107, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.W.; Han, C.J.; Rhee, Y.K.; Lee, Y.C.; Shin, K.S.; Shin, J.S.; Lee, K.T.; Hong, H.D. Cheonggukjang polysaccharides enhance immune activities and prevent cyclophosphamide-induced immunosuppression. Int. J. Biol. Macromol. 2015, 72, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Yun, L.Y.; Wu, T.; Li, Q.; Zhang, M. Dietary supplementation with purified wheat germ glycoprotein improve immunostimulatory activity in cyclophosphamide induced Balb/c mice. Int. J. Biol. Macromol. 2018, 118, 1267–1275. [Google Scholar] [CrossRef]
- Fan, H.J.; Xie, Z.P.; Lu, Z.W.; Tan, Z.B.; Bi, Y.M.; Xie, L.P.; Wu, Y.T.; Zhang, W.T.; Liu-Kot, K.; Liu, B.; et al. Anti-inflammatory and immune response regulation of Si-Ni-San in 2,4-dinitrochlorobenzene-induced atopic dermatitis-like skin dysfunction. J. Ethnopharmacol. 2018, 222, 1–10. [Google Scholar] [CrossRef]
- Liu, X.C.; Zhu, Z.Y.; Tang, Y.L.; Wang, M.F.; Wang, Z.; Liu, A.J.; Zhang, Y.M. Structural properties of polysaccharides from cultivated fruit bodies and mycelium of Cordyceps militaris. Carbohydr. Polym. 2016, 142, 63–72. [Google Scholar] [CrossRef]
- Yu, Q.; Nie, S.P.; Wang, J.Q.; Liu, X.Z.; Yin, P.F.; Huang, D.F.; Li, W.J.; Gong, D.M.; Xie, M.Y. Chemoprotective effects of Ganoderma atrum polysaccharide in cyclophosphamide-induced mice. Int. J. Biol. Macromol. 2014, 64, 395–401. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. Signaling to NF-kappa B. Gene. Dev. 2004, 18, 2195–2224. [Google Scholar] [CrossRef] [Green Version]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Guo, S.T.; Huang, Y.Y.; Zhang, W.D.; Wang, W.W.; Wei, T.; Lin, D.S.; Xing, J.F.; Deng, L.D.; Du, Q.; Liang, Z.C.; et al. Ternary complexes of amphiphilic polycaprolactone-graft-poly (N,N-dimethylaminoethyl methaaylate), DNA and polyglutamic acid-graft-poly (ethylene glycol) for gene delivery. Biomaterials 2011, 32, 4283–4292. [Google Scholar] [CrossRef]
- Amoscato, A.A.; Balasubramaniam, A.; Alexander, J.W.; Babcock, G.F. Degradation of Thymopentin by Human-Lymphocytes—Evidence for Aminopeptidase Activity. Biochim. Biophys. Acta 1988, 955, 164–174. [Google Scholar] [CrossRef]
- Tang, J.Q.; Zhen, H.M.; Wang, N.N.; Yan, Q.J.; Jing, H.; Jiang, Z.Q. Curdlan oligosaccharides having higher immunostimulatory activity than curdlan in mice treated with cyclophosphamide. Carbohyd. Polym. 2019, 207, 131–142. [Google Scholar] [CrossRef] [PubMed]













| Peptide | Sequence | H a | Net Charge |
|---|---|---|---|
| LL-37 | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES | −0.559 | +6 |
| Tα1 | SDAAVDTSSEITTKDLKEKKEVVEEAEN | −1.029 | −5 |
| LTAa | IGKEFKRIVQRIKDFLRNLVPRTEKEKKEVVE | −0.894 | +4 |
| LTAb | IGKEFKRIVQRIKDFLRNLVPRTEEVVEEA | −0.503 | +1 |
| LTAc | IGKEFKRIVQRIKDFLRNLVPRTEEVVEEAEN | −0.691 | 0 |
| Peptide | LL-37 | Tα1 | LTAa |
|---|---|---|---|
| t1/2 (h) | 3.1 ± 0.87 b | 1.8 ± 0.24 c | 4.2 ± 1.13 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Wei, X.; Zhang, R.; Koci, M.; Si, D.; Ahmad, B.; Cheng, J.; Wang, J. Development of a Highly Efficient Hybrid Peptide That Increases Immunomodulatory Activity Via the TLR4-Mediated Nuclear Factor-κB Signaling Pathway. Int. J. Mol. Sci. 2019, 20, 6161. https://doi.org/10.3390/ijms20246161
Zhang L, Wei X, Zhang R, Koci M, Si D, Ahmad B, Cheng J, Wang J. Development of a Highly Efficient Hybrid Peptide That Increases Immunomodulatory Activity Via the TLR4-Mediated Nuclear Factor-κB Signaling Pathway. International Journal of Molecular Sciences. 2019; 20(24):6161. https://doi.org/10.3390/ijms20246161
Chicago/Turabian StyleZhang, Lulu, Xubiao Wei, Rijun Zhang, Matthew Koci, Dayong Si, Baseer Ahmad, Junhao Cheng, and Junyong Wang. 2019. "Development of a Highly Efficient Hybrid Peptide That Increases Immunomodulatory Activity Via the TLR4-Mediated Nuclear Factor-κB Signaling Pathway" International Journal of Molecular Sciences 20, no. 24: 6161. https://doi.org/10.3390/ijms20246161
APA StyleZhang, L., Wei, X., Zhang, R., Koci, M., Si, D., Ahmad, B., Cheng, J., & Wang, J. (2019). Development of a Highly Efficient Hybrid Peptide That Increases Immunomodulatory Activity Via the TLR4-Mediated Nuclear Factor-κB Signaling Pathway. International Journal of Molecular Sciences, 20(24), 6161. https://doi.org/10.3390/ijms20246161