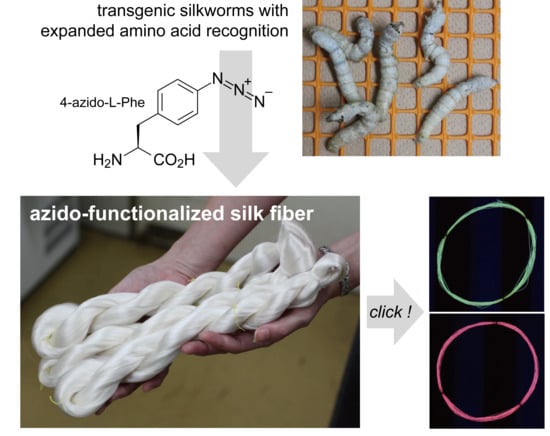
Characterization and Scaled-Up Production of Azido-Functionalized Silk Fiber Produced by Transgenic Silkworms with an Expanded Genetic Code
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fibroin Production and AzPhe Incorporation
2.2. Cocoon Reeling and Mechanical Property
2.3. Click Modification
3. Materials and Methods
3.1. Materials and Animals
3.2. Generation of the F1 Hybrid
3.3. Diet Preparation
3.4. AzPhe-Incorporation Assay
3.5. Urea Degumming
3.6. In-Gel Digestion and Mass Analysis
3.7. Production of Cocoons for the Reeling Experiment
3.8. Characterization of Raw Silk Fiber
3.9. Click Modification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AzPhe | 4-azido-L-phenylalanine |
| DBCO | dibenzocyclooctyne |
| FibH | fibroin heavy chain |
| FibL | fibroin light chain |
| MALDI-TOF-MS | matrix-assisted laser desorption/ionization time-of-flight mass spectrometry |
| PEG | polyethylene glycol |
| PheRS | phenylalanyl-tRNA synthetase |
| PSG | posterior silk gland |
| SPAAC | strain-promoted azido-alkyne cycloaddition |
References
- Silva, N.H.C.S.; Vilela, C.; Marrucho, I.M.; Freire, C.S.R.; Pascoal Neto, C.; Silvestre, A.J.D. Protein-based materials: From sources to innovative sustainable materials for biomedical applications. J. Mater. Chem. B 2014, 2, 3715–3740. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Vivekanandhan, S.; Pin, J.-M.; Misra, M. Composites from renewable and sustainable resources: Challenges and innovations. Science 2018, 362, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Eder, M.; Amini, S.; Fratzl, P. Biological composites—Complex structures for functional diversity. Science 2018, 362, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Holland, C.; Numata, K.; Rnjak-Kovacina, J.; Seib, F.P. The biomedical use of silk: Past, present, future. Adv. Healthc. Mater. 2018, 8, 1800465. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, D.; Tokareva, O.; Rim, N.G.; Wong, J.Y.; Kaplan, D.L.; Buehler, M.J. Silk–Its Mysteries, How It Is Made, and How It Is Used. ACS Biomater. Sci. Eng. 2015, 1, 864–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omenetto, F.G.; Kaplan, D.L. New opportunities for an ancient material. Science 2010, 329, 528–531. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, H.; Kojima, K. Production of Bombyx mori silk fibroin incorporated with unnatural amino acids. Biomacromolecules 2014, 15, 2682–2690. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, H.; Kojima, K. Incorporation of methionine analogues into Bombyx mori silk fibroin for click modifications. Macromol. Biosci. 2015, 15, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, H.; Nakajima, K.; Kojima, K. Azide-incorporated clickable silk fibroin materials with the ability to photopattern. ACS Biomater. Sci. Eng. 2016, 2, 251–258. [Google Scholar] [CrossRef]
- Teramoto, H.; Amano, Y.; Iraha, F.; Kojima, K.; Ito, T.; Sakamoto, K. Genetic code expansion of the silkworm Bombyx mori to functionalize silk fiber. ACS Synth. Biol. 2018, 7, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Borrmann, A.; van Hest, J.C.M. Bioorthogonal chemistry in living organisms. Chem. Sci. 2014, 5, 2123–2134. [Google Scholar] [CrossRef]
- Boutureira, O.; Bernardes, G.J.L. Advances in chemical protein modification. Chem. Rev. 2015, 115, 2174–2195. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, H. Intermolecular crosslinking of silk fibroin by click chemistry. J. Silk Sci. Technol. Jpn. 2017, 25, 17–25. [Google Scholar] [CrossRef]
- Tsukada, M.; Obo, M.; Kato, H.; Freddi, G.; Zanetti, F. Structure and dyeability of Bombyx mori silk fibers with different filament sizes. J. Appl. Polym. Sci. 1996, 60, 1619–1627. [Google Scholar] [CrossRef]
- Zhou, C.Z.; Confalonieri, F.; Medina, N.; Zivanovic, Y.; Esnault, C.; Yang, T.; Jacquet, M.; Janin, J.; Duguet, M.; Perasso, R.; et al. Fine organization of Bombyx mori fibroin heavy chain gene. Nucleic Acids Res. 2000, 28, 2413–2419. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, D.N.; Preda, R.C.; Yücel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 2011, 6, 1612–1631. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, Y.; Morikawa, H.; Yamanaka, S.; Tamada, Y. Electrospinning of silk fibroin from all aqueous solution at low concentration. Mater. Sci. Eng. C 2017, 73, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Wongpinyochit, T.; Johnston, B.F.; Seib, F.P. Degradation behavior of silk nanoparticles—Enzyme responsiveness. ACS Biomater. Sci. Eng. 2018, 4, 942–951. [Google Scholar] [CrossRef]
- Kai, H.; Nishi, K. Diapause development in Bombyx eggs in relation to ‘esterase A’ activity. J. Insect Physiol. 1976, 22, 1315–1320. [Google Scholar] [CrossRef]




| AzPhe (wt%) | Fiber Length 1 (m) | Raw Silk Ratio 2 (%) | Fiber Size 3 (D) | Degumming Ratio 4 (%) |
|---|---|---|---|---|
| 0 | 714 ± 146 | 14.8 ± 1.6 | 3.73 ± 0.25 | 25.6 ± 0.3 |
| 0.05 | 631 ± 81 | 11.9 ± 0.1 | 3.55 ± 0.26 | 24.3 ± 8.7 |
| N.S. | p < 0.05 | N.S. | N.S. |
| AzPhe (wt%) | Young’s Modulus (GPa) | Maximum Stress (MPa) | Maximum Strain (%) |
|---|---|---|---|
| 0 | 14.1 ± 0.2 | 455 ± 20 | 20.5 ± 0.8 |
| 0.05 | 14.2 ± 0.5 | 454 ± 33 | 21.2 ± 1.2 |
| N.S. | N.S. | N.S. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teramoto, H.; Iga, M.; Tsuboi, H.; Nakajima, K. Characterization and Scaled-Up Production of Azido-Functionalized Silk Fiber Produced by Transgenic Silkworms with an Expanded Genetic Code. Int. J. Mol. Sci. 2019, 20, 616. https://doi.org/10.3390/ijms20030616
Teramoto H, Iga M, Tsuboi H, Nakajima K. Characterization and Scaled-Up Production of Azido-Functionalized Silk Fiber Produced by Transgenic Silkworms with an Expanded Genetic Code. International Journal of Molecular Sciences. 2019; 20(3):616. https://doi.org/10.3390/ijms20030616
Chicago/Turabian StyleTeramoto, Hidetoshi, Masatoshi Iga, Hiromi Tsuboi, and Kenichi Nakajima. 2019. "Characterization and Scaled-Up Production of Azido-Functionalized Silk Fiber Produced by Transgenic Silkworms with an Expanded Genetic Code" International Journal of Molecular Sciences 20, no. 3: 616. https://doi.org/10.3390/ijms20030616
APA StyleTeramoto, H., Iga, M., Tsuboi, H., & Nakajima, K. (2019). Characterization and Scaled-Up Production of Azido-Functionalized Silk Fiber Produced by Transgenic Silkworms with an Expanded Genetic Code. International Journal of Molecular Sciences, 20(3), 616. https://doi.org/10.3390/ijms20030616