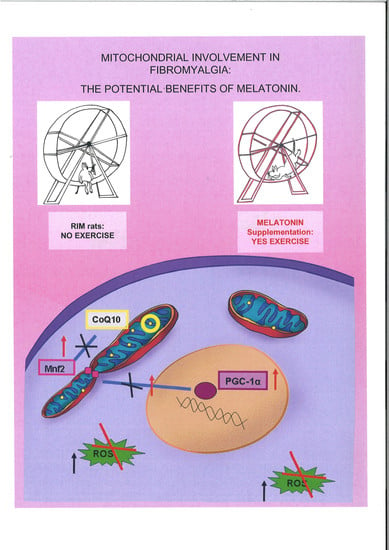
Mitochondrial Dysfunction in Skeletal Muscle of a Fibromyalgia Model: The Potential Benefits of Melatonin
Abstract
:1. Introduction
2. Results
2.1. Spontaneous Locomotor Activity Monitoring
2.2. Morphological Evaluations of Gastrocnemius Muscle
2.3. Mitochondrial Markers Evaluation
3. Discussion
4. Materials and Methods
4.1. Animal Treatment
- -
- control rats kept untreated;
- -
- control rats treated with melatonin for two months;
- -
- control rats treated with the vehicle of melatonin;
- -
- control rats treated with vehicle of reserpine;
- -
- reserpine-induced myalgic rats (RIM);
- -
- RIM rats plus tap water for two months (RIM + H2O);
- -
- RIM rats treated with melatonin for two months (RIM + MEL).
4.2. Morphometrical Analyses
4.3. Immunofluorescence Assay
4.4. Coenzyme Q10 ELISA Evaluation
4.5. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cordero, M.D.; Alcocer-Gómez, E.; Culic, O.; Carrión, A.M.; de Miguel, M.; Díaz-Parrado, E.; Pérez-Villegas, E.M.; Bullón, P.; Battino, M.; Sánchez-Alcazar, J.A. NLRP3 inflammasome is activated in fibromyalgia: The effect of coenzyme Q10. Antioxid. Redox Signal. 2014, 20, 1169–80. [Google Scholar] [CrossRef] [PubMed]
- Clauw, D.J.; D’Arcy, Y.; Gebke, K.; Semel, D.; Pauer, L.; Jones, K.D. Normalizing fibromyalgia as a chronic illness. Postgrad. Med. 2018, 130, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Arnold, L.M.; Bennett, R.M.; Crofford, L.J.; Dean, L.E.; Clauw, D.J.; Goldenberg, D.L.; Fitzcharles, M.A.; Paiva, E.S.; Staud, R.; Sarzi-Puttini, P.; et al. AAPT diagnostic criteria for fibromyalgia. J. Pain 2018, 1–18. [Google Scholar] [CrossRef]
- Alcocer-Gómez, E.; Culic, O.; Navarro-Pando, J.M.; Sánchez-Alcázar, J.A.; Bullón, P. Effect of coenzyme Q(10) on psychopathological symptoms in fibromyalgia patients. CNS Neurosci. Ther. 2017, 23, 188–189. [Google Scholar] [CrossRef] [PubMed]
- Basso, V.; Marchesan, E.; Peggion, C.; Chakraborty, J.; von Stockum, S.; Giacomello, M.; Ottolini, D.; Debattisti, V.; Caicci, F.; Tasca, E.; et al. Regulation of ER-mitochondria contacts by Parkin via Mfn2. Pharmacol. Res. 2018, 138, 43–56. [Google Scholar] [CrossRef]
- Mourier, A.; Motori, E.; Brandt, T.; Lagouge, M.; Atanassov, I.; Galinier, A.; Rappl, G.; Brodesser, S.; Hultenby, K.; Dieterich, C.; et al. Mitofusin 2 is required to maintain mitochondrial coenzyme Q levels. J. Cell Biol. 2015, 208, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Alcocer-Gómez, E.; Sánchez-Alcázar, J.A.; Cordero, M.D. Coenzyme q10 regulates serotonin levels and depressive symptoms in fibromyalgia patients: Results of a small clinical trial. J. Clin. Psychopharmacol. 2014, 34, 277–278. [Google Scholar] [CrossRef] [PubMed]
- Procaccio, V.; Bris, C.; Chao de la Barca, J.M.; Oca, F.; Chevrollier, A.; Amati-Bonneau, P.; Bonneau, D.; Reynier, P. Perspectives of drug-based neuroprotection targeting mitochondria. Rev. Neurol. (Paris) 2014, 170, 390–400. [Google Scholar] [CrossRef] [Green Version]
- Nagakura, Y.; Oe, T.; Aoki, T.; Matsuoka, N. Biogenic amine depletion causes chronic muscular pain and tactile allodynia accompanied by depression: A putative animal model of fibromyalgia. Pain 2009, 146, 26–33. [Google Scholar] [CrossRef]
- Atzeni, F.; Gerardi, M.C.; Masala, I.F.; Alciati, A.; Batticciotto, A.; Sarzi-Puttini, P. An update on emerging drugs for fibromyalgia treatment. Expert Opin. Emerg. Drugs. 2017, 22, 357–367. [Google Scholar] [CrossRef]
- Macfarlane, G.J.; Kronisch, C.; Dean, L.E.; Atzeni, F.; Häuser, W.; Fluß, E.; Choy, E.; Kosek, E.; Amris, K.; Branco, J.; Dincer, F.; Leino-Arjas, P.; et al. EULAR revised recommendations for the management of fibromyalgia. Ann. Rheum. Dis. 2017, 76, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Higgs, J.B. Fibromyalgia in Primary Care. Prim. Care 2018, 45, 325–341. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, A.A.; Fatima, G.; Das, S.K.; Verma, N.S. Abnormality of circadian rhythm of serum melatonin and other biochemical parameters in fibromyalgia syndrome. Indian J. Biochem. Biophys. 2011, 48, 82–87. [Google Scholar] [PubMed]
- Citera, G.; Arias, M.A.; Maldonado-Cocco, J.A.; Lázaro, M.A.; Rosemffet, M.G.; Brusco, L.I.; Scheines, E.J.; Cardinalli, D.P. The effect of melatonin in patients with fibromyalgia: A pilot study. Clin Rheumatol. 2000, 19, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Acuna-Castroviejo, D.; Escames, G.; Reiter, R.J. Melatonin therapy in fibromyalgia. J Pineal Res. 2006, 40, 98–99. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Barceló, E.J.; Mediavilla, M.D.; Tan, D.X.; Reiter, R.J. Clinical uses of melatonin: Evaluation of human trials. Curr Med Chem. 2010, 17, 2070–2095. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Rosales-Corral, S.; Galano, A.; Zhou, X.J.; Xu, B. Mitochondria: Central organelles for melatonin’s antioxidant and anti-aging actions. Molecules 2018, 23, 509. [Google Scholar] [CrossRef]
- Rohr, U.D.; Herold, J. Melatonin deficiencies in women. Maturitas 2002, 41, 85–104. [Google Scholar] [CrossRef]
- Reiter, R.J.; Acuna-Castroviejo, D.; Tan, D.X. Melatonin therapy in fibromyalgia. Curr. Pain Headache Rep. 2007, 11, 339–342. [Google Scholar] [CrossRef]
- Hussain, S.A.; Al-Khalifa, I.I.; Jasim, N.A.; Gorial, F.I. Adjuvant use of melatonin for treatment of fibromyalgia. J. Pineal Res. 2011, 50, 267–271. [Google Scholar] [CrossRef]
- Sánchez-Domínguez, B.; Bullón, P.; Román-Malo, L.; Marín-Aguilar, F.; Alcocer-Gómez, E.; Carrión, A.M.; Sánchez-Alcazar, J.A.; Cordero, M.D. Oxidative stress, mitochondrial dysfunction and, inflammation common events in skin of patients with Fibromyalgia. Mitochondrion 2015, 21, 69–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danilov, A.; Kurganova, J. Melatonin in chronic pain syndromes. Pain Ther. 2016, 5, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Favero, G.; Trapletti, V.; Bonomini, F.; Stacchiotti, A.; Lavazza, A.; Rodella, L.F.; Rezzani, R. Oral supplementation of melatonin protects against fibromyalgia-related skeletal muscle alterations in reserpine-induced myalgia rats. Int. J. Mol. Sci. 2017, 18, 1389. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.G.; Acuña, M.J.; Cabrera, D.; Goldschmeding, R.; Brandan, E. The pro-fibrotic connective tissue growth factor (CTGF/CCN2) correlates with the number of necrotic-regenerative foci in dystrophic muscle. J. Cell. Commun. Signal. 2018, 12, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Cordero, M.D.; Alcocer-Gómez, E.; Marín-Aguilar, F.; Rybkina, T.; Cotán, D.; Pérez-Pulido, A.; Alvarez-Suarez, J.M.; Battino, M.; Sánchez-Alcazar, J.A.; Carrión, A.M.; et al. Mutation in cytochrome b gene of mitochondrial DNA in a family with fibromyalgia is associated with NLRP3-inflammasome activation. J. Med. Genet. 2016, 53, 113–122. [Google Scholar] [CrossRef]
- Blasco-Serra, A.; Escrihuela-Vidal, F.; González-Soler, E.M.; Martínez-Expósito, F.; Blasco-Ausina, M.C.; Martínez-Bellver, S.; Cervera-Ferri, A.; Teruel-Martí, V.; Valverde-Navarro, A.A. Depressive-like symptoms in a reserpine-induced model of fibromyalgia in rats. Physiol. Behav. 2015, 151, 456–462. [Google Scholar] [PubMed]
- Maeda, T.; Kudo, Y.; Horiuchi, T.; Makino, N. Clinical and anti-aging effect of mud-bathing therapy for patients with fibromyalgia. Mol. Cell. Biochem. 2018, 444, 87–92. [Google Scholar] [CrossRef]
- Segura-Jiménez, V.; Borges-Cosic, M.; Soriano-Maldonado, A.; Estévez-López, F.; Álvarez-Gallardo, I.C.; Herrador-Colmenero, M.; Delgado-Fernández, M.; Ruiz, J.R. Association of sedentary time and physical activity with pain, fatigue, and impact of fibromyalgia: The al-Ándalus study. Scand. J. Med. Sci. Sports 2017, 27, 83–92. [Google Scholar] [CrossRef]
- Asfour, H.A.; Allouh, M.Z.; Said, R.S. Myogenic regulatory factors: The orchestrators of myogenesis after 30 years of discovery. Exp. Biol. Med. (Maywood) 2018, 243, 118–128. [Google Scholar] [CrossRef]
- Siu, P.M.; Donley, D.A.; Bryner, R.W.; Always, S.E. Myogenin and oxidative enzyme gene expression levels are elevated in rat soleus muscles after endurance training. J. Appl. Physiol. (1985) 2004, 97, 277–285. [Google Scholar] [CrossRef]
- Bazgir, B.; Fathi, R.; Rezazadeh Valojerdi, M.; Mozdziak, P.; Asgari, A. Satellite cells contribution to exercise mediated muscle hypertrophy and repair. Cell. J. 2017, 18, 473–484. [Google Scholar] [PubMed]
- Chung, C.P.; Titova, D.; Oeser, A.; Randels, M.; Avalos, I.; Milne, G.L.; Morrow, J.D.; Stein, C.M. Oxidative stress in fibromyalgia and its relationship to symptoms. Clin. Rheumatol. 2009, 28, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Cannavino, J.; Brocca, L.; Sandri, M.; Bottinelli, R.; Pellegrino, M.A. PGC1-α over-expression prevents metabolic alterations and soleus muscle atrophy in hindlimb unloaded mice. J. Physiol. 2014, 592, 4575–4589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, M.L.; Robinson, M.M.; Nair, K.S. Skeletal muscle aging and the mitochondrion. Trends Endocrinol. Metab. 2013, 24, 247–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hood, D.A.; Tryon, L.D.; Carter, H.N.; Kim, Y.; Chen, C.C. Unravelling the mechanisms regulating muscle mitochondrial biogenesis. Biochem. J. 2016, 473, 2295–2314. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.H.; Hancock, C.R.; Terada, S.; Higashida, K.; Holloszy, J.O.; Han, D.H. PPARβ is essential for maintaining normal levels of PGC-1α and mitochondria and for the increase in muscle mitochondria induced by exercise. Cell. Metab. 2017, 25, 1176–1185. [Google Scholar] [CrossRef] [PubMed]
- Triolo, M.; Hood, D.A. Mitochondrial breakdown in skeletal muscle and the emerging role of the lysosomes. Arch. Biochem. Biophys. 2018, 661, 66–73. [Google Scholar] [CrossRef]
- Hyatt, H.; Deminice, R.; Yoshihara, T.; Powers, S.K. Mitochondrial dysfunction induces muscle atrophy during prolonged T inactivity: A review of the causes and effects. Arch. Biochem. Biophys. 2019, 662, 49–60. [Google Scholar] [CrossRef]
- Filadi, R.; Pendin, D.; Pizzo, P. Mitofusin 2: From functions to disease. Cell. Death. Dis. 2018, 9, 330. [Google Scholar] [CrossRef]
- Zorzano, A. Regulation of mitofusin-2 expression in skeletal muscle. Appl. Physiol. Nutr. Metab. 2009, 34, 433–439. [Google Scholar] [CrossRef]
- Marino Gammazza, A.; Macaluso, F.; Di Felice, V.; Cappello, F.; Barone, R. Hsp60 in skeletal muscle fiber biogenesis and homeostasis: From physical exercise to skeletal muscle pathology. Cells 2018, 7, 224. [Google Scholar] [CrossRef] [PubMed]
- Cordero, M.D.; Alcocer-Gómez, E.; de Miguel, M.; Cano-García, F.J.; Luque, C.M.; Fernández-Riejo, P.; Fernández, A.M.; Sánchez-Alcazar, J.A. Coenzyme Q(10): A novel therapeutic approach for fibromyalgia? case series with 5 patients. Mitochondrion 2011, 11, 623–625. [Google Scholar] [CrossRef] [PubMed]
- Cordero, M.D.; Cotán, D.; del-Pozo-Martín, Y.; Carrión, A.M.; de Miguel, M.; Bullón, P.; Sánchez-Alcazar, J.A. Oral coenzyme Q10 supplementation improves clinical symptoms and recovers pathologic alterations in blood mononuclear cells in a fibromyalgia patient. Nutrition 2012, 28, 1200–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyamae, T.; Seki, M.; Naga, T.; Uchino, S.; Asazuma, H.; Yoshida, T.; Iizuka, Y.; Kikuchi, M.; Imagawa, T.; Natsumeda, Y.; et al. Increased oxidative stress and coenzyme Q10 deficiency in juvenile fibromyalgia: Amelioration of hypercholesterolemia and fatigue by ubiquinol-10 supplementation. Redox Rep. 2013, 18, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Dadar, M.; Chirumbolo, S.; Aaseth, J. Fibromyalgia and nutrition: Therapeutic possibilities? Biomed. Pharmacother. 2018, 103, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Kleszczyński, K.; Bilska, B.; Stegemann, A.; Flis, D.J.; Ziolkowski, W.; Pyza, E.; Luger, T.A.; Reiter, R.J.; Böhm, M.; Slominski, A.T. Melatonin and its metabolites ameliorate UVR-induced mitochondrial oxidative stress in human MNT-1 melanoma cells. Int. J. Mol. Sci. 2018, 19, 3786. [Google Scholar] [CrossRef] [PubMed]
- Suofu, Y.; Li, W.; Jean-Alphonse, F.G.; Jia, J.; Khattar, N.K.; Li, J.; Baranov, S.V.; Leronni, D.; Mihalik, A.C.; He, Y.; et al. Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. PNAS 2017, 114, 7997–8006. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Manchester, L.C.; El-Sawi, M.R. Melatonin reduces oxidant damage and promotes mitochondrial respiration: Implications for aging. Ann. N. Y. Acad. Sci. 2002, 959, 238–250. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, S.; Pei, Z.; Drozda, M.; Stavrovskaya, I.G.; Del Signore, S.J.; Cormier, K.; Shimony, E.M.; Wang, H.; Ferrante, R.J.; et al. Inhibitors of cytochrome c release with therapeutic potential for Huntington’s disease. J. Neurosci. 2008, 28, 9473–9485. [Google Scholar] [CrossRef]
- Wang, X.; Sirianni, A.; Pei, Z.; Cormier, K.; Smith, K.; Jiang, J.; Zhou, S.; Wang, H.; Zhao, R.; Yano, H.; et al. The melatonin MT1 receptor axis modulates mutant Huntingtin-mediated toxicity. J. Neurosci. 2011, 31, 14496–14507. [Google Scholar] [CrossRef]
- Zhang, Y.; Cook, A.; Kim, J.; Baranov, S.V.; Jiang, J.; Smith, K.; Cormier, K.; Bennett, E.; Browser, R.P.; Day, A.L.; et al. Melatonin inhibits the caspase 1/cytochrome c/caspase-3 cell death pathway, inhibits MT1 receptor loss and delays disease progression in a mouse model of amyotrophic lateral sclerosis. Neurobiol. Dis. 2013, 55, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, A.; Calpena, A.C.; Clares, B. Evaluating the oxidative stress in inflammation: Role of melatonin. Int. J. Mol. Sci. 2015, 16, 16981–17004. [Google Scholar] [CrossRef] [PubMed]
- Ramis, M.R.; Esteban, S.; Miralles, A.; Tan, D.X.; Reiter, R.J. Protective Effects of Melatonin and Mitochondria-targeted Antioxidants Against Oxidative Stress: A Review. Curr. Med. Chem. 2015, 22, 2690–2711. [Google Scholar] [CrossRef] [PubMed]
- Kiso, T.; Moriyama, A.; Furutani, M.; Matsuda, R.; Funatsu, Y. Effects of pregabalin and duloxetine on neurotransmitters in the dorsal horn of the spinal cord in a rat model of fibromyalgia. Eur. J. Pharmacol. 2018, 827, 117–124. [Google Scholar] [CrossRef]
- Ogino, S.; Nagakura, Y.; Tsukamoto, M.; Watabiki, T.; Ozawa, T.; Oe, T.; Shimizu, Y.; Ito, H. Systemic administration of 5-HT(2C) receptor agonists attenuates muscular hyperalgesia in reserpine-induced myalgia model. Pharmacol. Biochem. Behav. 2013, 108, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Favero, G.; Stacchiotti, A.; Castrezzati, S.; Bonomini, F.; Albanese, M.; Rezzani, R.; Rodella, L.F. Melatonin reduces obesity and restores adipokine patterns and metabolism in obese (ob/ob) mice. Nutr. Res. 2015, 35, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Rezzani, R.; Favero, G.; Stacchiotti, A.; Rodella, L.F. Endothelial and vascular smooth muscle cell dysfunction mediated by cyclophylin A and the atheroprotective effects of melatonin. Life Sci. 2013, 92, 875–882. [Google Scholar] [CrossRef]
- Agabiti-Rosei, C.; De Ciuceis, C.; Rossini, C.; Porteri, E.; Rodella, L.F.; Withers, S.B.; Heagerty, A.M.; Favero, G.; Agabiti-Rosei, E.; Rizzoni, D.; et al. Anticontractile activity of perivascular fat in obese mice and the effect of long-term treatment with melatonin. J. Hypertens. 2014, 32, 1264–1274. [Google Scholar] [CrossRef] [Green Version]
- Agabiti-Rosei, C.; Favero, G.; De Ciuceis, C.; Rossini, C.; Porteri, E.; Rodella, L.F.; Franceschetti, L.; Maria Sarkar, A.; Agabiti-Rosei, E.; Rizzoni, D.; et al. Effect of long-term treatment with melatonin on vascular markers of oxidative stress/inflammation and on the anticontractile activity of perivascular fat in aging mice. Hypertens. Res. 2017, 40, 41–50. [Google Scholar] [CrossRef]
- Rodella, L.F.; Rossini, C.; Favero, G.; Foglio, E.; Loreto, C.; Rezzani, R. Nicotine-induced morphological changes in rat aorta: The protective role of melatonin. Cells Tissues Organs 2012, 195, 252–259. [Google Scholar] [CrossRef]
- Favero, G.; Paini, A.; De Ciuceis, C.; Rodella, L.F.; Moretti, E.; Porteri, E.; Rossini, C.; Ministrini, S.; Solaini, L.; Stefano, C.; et al. Changes in extracellular matrix in subcutaneous small resistance arteries of patients with essential hypertension. Blood Press. 2018, 27, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.A.; Oliveira, C.S.; Ineu, R.P.; Moraes-Silva, L.; de Siqueira, L.F.; Pereira, M.E. Lactating and non-lactating rats differ in sensitivity to HgCl(2): Protective effect of ZnCl(2). J. Trace Elem. Med. Biol. 2014, 28, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Bonomini, F.; Favero, G.; Rodella, L.F.; Moghadasian, M.H.; Rezzani, R. Melatonin modulation of sirtuin-1 attenuates liver injury in a hypercholesterolemic mouse model. BioMed Res. Int. 2018, 2018, 7968452. [Google Scholar] [CrossRef] [PubMed]
- Rodella, L.F.; Favero, G.; Boninsegna, R.; Borgonovo, A.; Rezzani, R.; Santoro, F. TGF-beta1 and VEGF after fresh frozen bone allograft insertion in oral-maxillo-facial surgery. Histol. Histopathol. 2010, 25, 463–471. [Google Scholar] [PubMed]




© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Favero, G.; Bonomini, F.; Franco, C.; Rezzani, R. Mitochondrial Dysfunction in Skeletal Muscle of a Fibromyalgia Model: The Potential Benefits of Melatonin. Int. J. Mol. Sci. 2019, 20, 765. https://doi.org/10.3390/ijms20030765
Favero G, Bonomini F, Franco C, Rezzani R. Mitochondrial Dysfunction in Skeletal Muscle of a Fibromyalgia Model: The Potential Benefits of Melatonin. International Journal of Molecular Sciences. 2019; 20(3):765. https://doi.org/10.3390/ijms20030765
Chicago/Turabian StyleFavero, Gaia, Francesca Bonomini, Caterina Franco, and Rita Rezzani. 2019. "Mitochondrial Dysfunction in Skeletal Muscle of a Fibromyalgia Model: The Potential Benefits of Melatonin" International Journal of Molecular Sciences 20, no. 3: 765. https://doi.org/10.3390/ijms20030765
APA StyleFavero, G., Bonomini, F., Franco, C., & Rezzani, R. (2019). Mitochondrial Dysfunction in Skeletal Muscle of a Fibromyalgia Model: The Potential Benefits of Melatonin. International Journal of Molecular Sciences, 20(3), 765. https://doi.org/10.3390/ijms20030765