Human Embryonic Stem Cell-Derived Retinal Pigment Epithelium-Role in Dead Cell Clearance and Inflammation
Abstract
:1. Introduction
2. Results
2.1. Anoikis is Induced in hESC-RPE Cells
2.2. hESC-RPE Cells Die Due to Serum Deprivation and H2O2 Co-Treatment
2.3. Autophagy is Induced in hESC-RPE Cells Treated by Serum Deprivation and H2O2 Co-Treatment
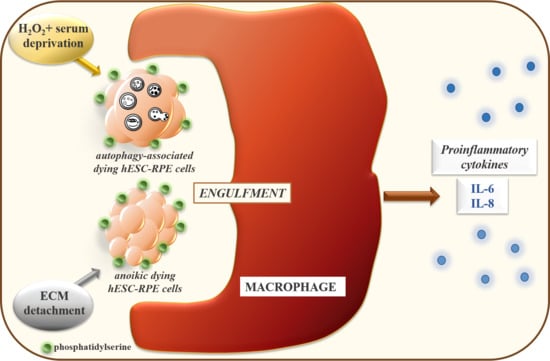
2.4. Macrophages can Efficiently Engulf Anokic and Autophagy-Associated Dying hESC-RPE Cells in vitro
2.5. The Phagocytosis of Anoikic and Autophagy-Associated Dying hESC-RPE Cells by Macrophages Induces Release of Pro-Inflammatory Cytokines
3. Discussion
4. Materials and Methods
4.1. Ethic Statement
4.2. Human ESC Culture, RPE Differentiation, and Treatment
4.3. Assays of Cell Death
4.4. Antibodies and Immunoblotting
4.5. Phagocytosis Assay
4.6. Quantification of IL-6 and IL-8 Release by ELISA
4.7. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Competing financial interests statement
Data availability statement
Conflicts of Interest
Abbreviations
| 2-MEA | 2-mercaptoethanol |
| 5(6)-CFDA-SE | 5-(and-6)-carboxyfluorescein diacetate, succinimidyl ester |
| CMTMR | 5-(and-6)-(((4-chloromethyl)benzoyl)amino)tetramethylrhodamine |
| AMD | age-related macular degeneration |
| AV | autophagic vacuole |
| CNV | choroideal neovascularization |
| DMEM | Dulbecco’s modified Eagle’s medium |
| EDTA | ethylenediaminetetraacetic acid |
| FCS | fetal calf serum |
| FITC | fluorescein isothio-cyanate |
| FACS | fluorescence-activated cell sorter |
| HRP | horseradish-peroxidase |
| hRPE | human retinal pigment epithelium |
| H2O2 | hydrogen peroxide |
| IL | interleukin |
| IMDM | Iscove’s Modified Dulbecco’s Medium |
| LC3 | light-chain-3 |
| MCSF | macrophage colony stimulating factor |
| PFA | paraformaldehyde |
| PBS | phosphate-buffered saline |
| PS | phosphatidyl serine |
| poly-HEMA | poly(2-hydroxyethylmethacrylate) |
| PI | propidium iodide |
| SD | standard deviation |
| TC | triamcinolone |
| TBS-T | tris buffered saline containing 0.05 % tween-20 |
References
- Strauss, O. The retinal pigment epithelium in visual function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef] [PubMed]
- Gehrs, K.M.; Anderson, D.H.; Johnson, L.V.; Hageman, G.S. Age-related macular degeneration—Emerging pathogenetic and therapeutic concepts. Ann. Med. 2006, 38, 450–471. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Klein, B.E. The prevalence of age-related eye diseases and visual impairment in aging: Current estimates. Investig. Ophthalmol. Vis. Sci. 2013, 54, ORSF5–ORSF13. [Google Scholar] [CrossRef]
- Dang, Y.; Zhang, C.; Zhu, Y. Stem cell therapies for age-related macular degeneration: The past, present, and future. Clin. Interv. Aging 2015, 10, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Lu, B.; Malcuit, C.; Wang, S.; Girman, S.; Francis, P.; Lemieux, L.; Lanza, R.; Lund, R. Long-term safety and function of RPE from human embryonic stem cells in preclinical models of macular degeneration. Stem Cells 2009, 27, 2126–2135. [Google Scholar] [CrossRef]
- Schwartz, S.D.; Hubschman, J.P.; Heilwell, G.; Franco-Cardenas, V.; Pan, C.K.; Ostrick, R.M.; Mickunas, E.; Gay, R.; Klimanskaya, I.; Lanza, R. Embryonic stem cell trials for macular degeneration: A preliminary report. Lancet 2012, 379, 713–720. [Google Scholar] [CrossRef]
- Schwartz, S.D.; Regillo, C.D.; Lam, B.L.; Eliott, D.; Rosenfeld, P.J.; Gregori, N.Z.; Hubschman, J.P.; Davis, J.L.; Heilwell, G.; Spirn, M.; et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and stargardt’s macular dystrophy: Follow-up of two open-label phase 1/2 studies. Lancet 2015, 385, 509–516. [Google Scholar] [CrossRef]
- Kamao, H.; Mandai, M.; Okamoto, S.; Sakai, N.; Suga, A.; Sugita, S.; Kiryu, J.; Takahashi, M. Characterization of human induced pluripotent stem cell-derived retinal pigment epithelium cell sheets aiming for clinical application. Stem Cell Rep. 2014, 2, 205–218. [Google Scholar] [CrossRef]
- Subrizi, A.; Hiidenmaa, H.; Ilmarinen, T.; Nymark, S.; Dubruel, P.; Uusitalo, H.; Yliperttula, M.; Urtti, A.; Skottman, H. Generation of hesc-derived retinal pigment epithelium on biopolymer coated polyimide membranes. Biomaterials 2012, 33, 8047–8054. [Google Scholar] [CrossRef] [PubMed]
- Diniz, B.; Thomas, P.; Thomas, B.; Ribeiro, R.; Hu, Y.; Brant, R.; Ahuja, A.; Zhu, D.; Liu, L.; Koss, M.; et al. Subretinal implantation of retinal pigment epithelial cells derived from human embryonic stem cells: Improved survival when implanted as a monolayer. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5087–5096. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Zhang, Z.N.; Westenskow, P.D.; Todorova, D.; Hu, Z.; Lin, T.; Rong, Z.; Kim, J.; He, J.; Wang, M.; et al. Humanized mice reveal differential immunogenicity of cells derived from autologous induced pluripotent stem cells. Cell Stem Cell 2015, 17, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef] [PubMed]
- Kaarniranta, K.; Sinha, D.; Blasiak, J.; Kauppinen, A.; Vereb, Z.; Salminen, A.; Boulton, M.E.; Petrovski, G. Autophagy and heterophagy dysregulation leads to retinal pigment epithelium dysfunction and development of age-related macular degeneration. Autophagy 2013, 9, 973–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilmore, A.P. Anoikis. Cell Death Differ. 2005, 12 (Suppl. 2), 1473–1477. [Google Scholar] [CrossRef] [Green Version]
- Galluzzi, L.; Vitale, I.; Abrams, J.M.; Alnemri, E.S.; Baehrecke, E.H.; Blagosklonny, M.V.; Dawson, T.M.; Dawson, V.L.; El-Deiry, W.S.; Fulda, S.; et al. Molecular definitions of cell death subroutines: Recommendations of the nomenclature committee on cell death 2012. Cell Death Differ. 2012, 19, 107–120. [Google Scholar] [CrossRef]
- Hamidi, T.; Cano, C.E.; Grasso, D.; Garcia, M.N.; Sandi, M.J.; Calvo, E.L.; Dagorn, J.C.; Lomberk, G.; Goruppi, S.; Urrutia, R.; et al. Nupr1 works against the metabolic stress-induced autophagy-associated cell death in pancreatic cancer cells. Autophagy 2013, 9, 95–97. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, Y.S.; Kim, D.E.; Lee, J.S.; Song, J.H.; Kim, H.G.; Cho, D.H.; Jeong, S.Y.; Jin, D.H.; Jang, S.J.; et al. Bix-01294 induces autophagy-associated cell death via ehmt2/g9a dysfunction and intracellular reactive oxygen species production. Autophagy 2013, 9, 2126–2139. [Google Scholar] [CrossRef]
- Bursch, W.; Hochegger, K.; Torok, L.; Marian, B.; Ellinger, A.; Hermann, R.S. Autophagic and apoptotic types of programmed cell death exhibit different fates of cytoskeletal filaments. J. Cell Sci. 2000, 113 Pt 7, 1189–1198. [Google Scholar]
- Levine, B.; Yuan, J. Autophagy in cell death: An innocent convict? J. Clin. Investig. 2005, 115, 2679–2688. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J.; Abdelmohsen, K.; Abe, A.; Abedin, M.J.; Abeliovich, H.; Acevedo Arozena, A.; Adachi, H.; Adams, C.M.; Adams, P.D.; Adeli, K.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 2016, 12, 1–222. [Google Scholar] [CrossRef]
- Wang, A.L.; Lukas, T.J.; Yuan, M.; Du, N.; Tso, M.O.; Neufeld, A.H. Autophagy and exosomes in the aged retinal pigment epithelium: Possible relevance to drusen formation and age-related macular degeneration. PLoS ONE 2009, 4, e4160. [Google Scholar] [CrossRef] [PubMed]
- Majai, G.; Petrovski, G.; Fesus, L. Inflammation and the apopto-phagocytic system. Immunol. Lett. 2006, 104, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Bowes Rickman, C.; Farsiu, S.; Toth, C.A.; Klingeborn, M. Dry age-related macular degeneration: Mechanisms, therapeutic targets, and imaging. Investig. Ophthalmol. Vis. Sci. 2013, 54, ORSF68–ORSF80. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.D.; Kuppermann, B.D. Wet age-related macular degeneration. Adv. Drug Deliv. Rev. 2005, 57, 1994–2009. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.G.; Kaiser, P.K. Emerging treatments for wet age-related macular degeneration. Expert Opin. Emerg. Drugs 2014, 19, 157–164. [Google Scholar] [CrossRef]
- Elliott, M.R.; Ravichandran, K.S. Clearance of apoptotic cells: Implications in health and disease. J. Cell Boil. 2010, 189, 1059–1070. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, K.S. Find-me and eat-me signals in apoptotic cell clearance: Progress and conundrums. J. Exp. Med. 2010, 207, 1807–1817. [Google Scholar] [CrossRef] [PubMed]
- Danis, R.P.; Bingaman, D.P.; Yang, Y.; Ladd, B. Inhibition of preretinal and optic nerve head neovascularization in pigs by intravitreal triamcinolone acetonide. Ophthalmology 1996, 103, 2099–2104. [Google Scholar] [CrossRef]
- Jonas, J.B.; Kreissig, I.; Hugger, P.; Sauder, G.; Panda-Jonas, S.; Degenring, R. Intravitreal triamcinolone acetonide for exudative age related macular degeneration. Br. J. Ophthalmol. 2003, 87, 462–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonas, J.B.; Kreissig, I.; Degenring, R.F. Factors influencing visual acuity after intravitreal triamcinolone acetonide as treatment of exudative age related macular degeneration. Br. J. Ophthalmol. 2004, 88, 1557–1562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, J.I.; Niec, M.; Wong, V. One year results of a phase 1 study of the safety and tolerability of combination therapy using sustained release intravitreal triamcinolone acetonide and ranibizumab for subfoveal neovascular amd. Br. J. Ophthalmol. 2015, 99, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Albert, R.; Kristof, E.; Zahuczky, G.; Szatmari-Toth, M.; Vereb, Z.; Olah, B.; Moe, M.C.; Facsko, A.; Fesus, L.; Petrovski, G. Triamcinolone regulated apopto-phagocytic gene expression patterns in the clearance of dying retinal pigment epithelial cells. A key role of mertk in the enhanced phagocytosis. Biochim. Biophys. Acta 2014, 1850, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Petrovski, G.; Zahuczky, G.; Majai, G.; Fesus, L. Phagocytosis of cells dying through autophagy evokes a pro-inflammatory response in macrophages. Autophagy 2007, 3, 509–511. [Google Scholar] [CrossRef] [PubMed]
- Stanton, C.M.; Wright, A.F. Inflammatory biomarkers for amd. Adv. Exp. Med. Boil. 2014, 801, 251–257. [Google Scholar]
- Marmorstein, A.D.; Finnemann, S.C.; Bonilha, V.L.; Rodriguez-Boulan, E. Morphogenesis of the retinal pigment epithelium: Toward understanding retinal degenerative diseases. Ann. N. Y. Acad. Sci. 1998, 857, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.; Temple, S. Retinal pigment epithelial cell proliferation. Exp. Boil. Med. 2015, 240, 1079–1086. [Google Scholar] [CrossRef] [Green Version]
- De Duve, C.; Wattiaux, R. Functions of lysosomes. Annu. Rev. Physiol. 1966, 28, 435–492. [Google Scholar] [CrossRef]
- Baudhuin, P. Lysosomes and cellular autophagy. Brux. Med. 1966, 45, 1059–1070. [Google Scholar]
- Levine, B.; Kroemer, G. Autophagy in the pathogenesis of disease. Cell 2008, 132, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Codogno, P.; Meijer, A.J. Autophagy and signaling: Their role in cell survival and cell death. Cell Death Differ. 2005, 12 (Suppl. 2), 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Baehrecke, E.H. Autophagy: Dual roles in life and death? Nat. Rev. Mol. Cell Biol. 2005, 6, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Kiffin, R.; Bandyopadhyay, U.; Cuervo, A.M. Oxidative stress and autophagy. Antioxid. Redox Signal. 2006, 8, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.N.; Allen, J.I.; Somerfield, P.J. Autophagy: Role in surviving environmental stress. Mar. Environ. Res. 2006, 62, S420–S425. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Yamamoto, A.; Matsui, M.; Yoshimori, T.; Ohsumi, Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol. Boil. Cell 2004, 15, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Finn, P.F.; Dice, J.F. Proteolytic and lipolytic responses to starvation. Nutrition 2006, 22, 830–844. [Google Scholar] [CrossRef]
- Krysko, D.V.; D’Herde, K.; Vandenabeele, P. Clearance of apoptotic and necrotic cells and its immunological consequences. Apoptosis Int. J. Program. Cell Death 2006, 11, 1709–1726. [Google Scholar] [CrossRef]
- Erwig, L.P.; Henson, P.M. Clearance of apoptotic cells by phagocytes. Cell Death Differ. 2008, 15, 243–250. [Google Scholar] [CrossRef]
- Fadeel, B.; Xue, D.; Kagan, V. Programmed cell clearance: Molecular regulation of the elimination of apoptotic cell corpses and its role in the resolution of inflammation. Biochem. Biophys. Res. Commun. 2010, 396, 7–10. [Google Scholar] [CrossRef] [Green Version]
- Morescalchi, F.; Duse, S.; Gambicorti, E.; Romano, M.R.; Costagliola, C.; Semeraro, F. Proliferative vitreoretinopathy after eye injuries: An overexpression of growth factors and cytokines leading to a retinal keloid. Mediat. Inflamm. 2013, 2013, 269787. [Google Scholar] [CrossRef]
- Petrovski, G.; Ayna, G.; Majai, G.; Hodrea, J.; Benko, S.; Madi, A.; Fesus, L. Phagocytosis of cells dying through autophagy induces inflammasome activation and il-1beta release in human macrophages. Autophagy 2011, 7, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Klimanskaya, I.; Hipp, J.; Rezai, K.A.; West, M.; Atala, A.; Lanza, R. Derivation and comparative assessment of retinal pigment epithelium from human embryonic stem cells using transcriptomics. Cloning Stem Cells 2004, 6, 217–245. [Google Scholar] [CrossRef] [PubMed]
- Vugler, A.; Carr, A.J.; Lawrence, J.; Chen, L.L.; Burrell, K.; Wright, A.; Lundh, P.; Semo, M.; Ahmado, A.; Gias, C.; et al. Elucidating the phenomenon of hesc-derived RPE: Anatomy of cell genesis, expansion and retinal transplantation. Exp. Neurol. 2008, 214, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.S.; Shearer, R.L.; Capowski, E.E.; Wright, L.S.; Wallace, K.A.; McMillan, E.L.; Zhang, S.C.; Gamm, D.M. Modeling early retinal development with human embryonic and induced pluripotent stem cells. Proc. Natl. Acad. Sci. USA 2009, 106, 16698–16703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Idelson, M.; Alper, R.; Obolensky, A.; Ben-Shushan, E.; Hemo, I.; Yachimovich-Cohen, N.; Khaner, H.; Smith, Y.; Wiser, O.; Gropp, M.; et al. Directed differentiation of human embryonic stem cells into functional retinal pigment epithelium cells. Cell Stem Cell 2009, 5, 396–408. [Google Scholar] [CrossRef] [PubMed]
- Savolainen, V.; Juuti-Uusitalo, K.; Onnela, N.; Vaajasaari, H.; Narkilahti, S.; Suuronen, R.; Skottman, H.; Hyttinen, J. Impedance spectroscopy in monitoring the maturation of stem cell-derived retinal pigment epithelium. Ann. Biomed. Eng. 2011, 39, 3055–3069. [Google Scholar] [CrossRef] [PubMed]
- Vaajasaari, H.; Ilmarinen, T.; Juuti-Uusitalo, K.; Rajala, K.; Onnela, N.; Narkilahti, S.; Suuronen, R.; Hyttinen, J.; Uusitalo, H.; Skottman, H. Toward the defined and xeno-free differentiation of functional human pluripotent stem cell-derived retinal pigment epithelial cells. Mol. Vis. 2011, 17, 558–575. [Google Scholar]
- Onnela, N.; Savolainen, V.; Juuti-Uusitalo, K.; Vaajasaari, H.; Skottman, H.; Hyttinen, J. Electric impedance of human embryonic stem cell-derived retinal pigment epithelium. Med. Boil. Eng. Comput. 2012, 50, 107–116. [Google Scholar] [CrossRef]
- Juuti-Uusitalo, K.; Vaajasaari, H.; Ryhanen, T.; Narkilahti, S.; Suuronen, R.; Mannermaa, E.; Kaarniranta, K.; Skottman, H. Efflux protein expression in human stem cell-derived retinal pigment epithelial cells. PLoS ONE 2012, 7, e30089. [Google Scholar] [CrossRef]
- Abouzeid, H.; Wolfensberger, T.J. Macular recovery after retinal detachment. Acta Ophthalmol. Scand. 2006, 84, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Zayit-Soudry, S.; Moroz, I.; Loewenstein, A. Retinal pigment epithelial detachment. Surv. Ophthalmol. 2007, 52, 227–243. [Google Scholar] [CrossRef] [PubMed]
- Petrovski, G.; Berenyi, E.; Moe, M.C.; Vajas, A.; Fesus, L.; Berta, A.; Facsko, A. Clearance of dying arpe-19 cells by professional and nonprofessional phagocytes in vitro-implications for age-related macular degeneration (AMD). Acta Ophthalmol. 2011, 89, e30–e34. [Google Scholar] [CrossRef] [PubMed]
- Emoto, K.; Umeda, M. Apoptosis by phosphatidylserine in mammalian cells. Sub-Cell. Biochem. 2002, 36, 61–77. [Google Scholar]
- Schlegel, R.A.; Williamson, P. Phosphatidylserine, a death knell. Cell Death Differ. 2001, 8, 551–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vermes, I.; Haanen, C.; Steffens-Nakken, H.; Reutelingsperger, C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled annexin V. J. Immunol. Methods 1995, 184, 39–51. [Google Scholar] [CrossRef]
- Yao, X.; Gray, S.; Pham, T.; Delgardo, M.; Nguyen, A.; Do, S.; Ireland, S.K.; Chen, R.; Abdel-Mageed, A.B.; Biliran, H. Downregulation of bit1 expression promotes growth, anoikis resistance, and transformation of immortalized human bronchial epithelial cells via erk activation-dependent suppression of e-cadherin. Biochem. Biophys. Res. Commun. 2018, 495, 1240–1248. [Google Scholar] [CrossRef]
- Van Engeland, M.; Nieland, L.J.; Ramaekers, F.C.; Schutte, B.; Reutelingsperger, C.P. Annexin V-affinity assay: A review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry 1998, 31, 1–9. [Google Scholar] [CrossRef]
- Sgonc, R.; Gruber, J. Apoptosis detection: An overview. Exp. Gerontol. 1998, 33, 525–533. [Google Scholar] [CrossRef]
- Nicoletti, I.; Migliorati, G.; Pagliacci, M.C.; Grignani, F.; Riccardi, C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods 1991, 139, 271–279. [Google Scholar] [CrossRef]
- Blasiak, J.; Petrovski, G.; Vereb, Z.; Facsko, A.; Kaarniranta, K. Oxidative stress, hypoxia, and autophagy in the neovascular processes of age-related macular degeneration. BioMed Res. Int. 2014, 2014, 768026. [Google Scholar] [CrossRef] [PubMed]
- Rose, R.C.; Richer, S.P.; Bode, A.M. Ocular oxidants and antioxidant protection. Proc. Soc. Exp. Boil. Med. 1998, 217, 397–407. [Google Scholar] [CrossRef]
- Winkler, B.S.; Boulton, M.E.; Gottsch, J.D.; Sternberg, P. Oxidative damage and age-related macular degeneration. Mol. Vis. 1999, 5, 32. [Google Scholar] [PubMed]
- Mao, H.; Seo, S.J.; Biswal, M.R.; Li, H.; Conners, M.; Nandyala, A.; Jones, K.; Le, Y.Z.; Lewin, A.S. Mitochondrial oxidative stress in the retinal pigment epithelium leads to localized retinal degeneration. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4613–4627. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T. Signal transduction by reactive oxygen species. J. Cell Boil. 2011, 194, 7–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scandalios, J.G. Oxidative stress: Molecular perception and transduction of signals triggering antioxidant gene defenses. Braz. J. Med. Boil. Res. 2005, 38, 995–1014. [Google Scholar] [CrossRef]
- Coyle, C.H.; Martinez, L.J.; Coleman, M.C.; Spitz, D.R.; Weintraub, N.L.; Kader, K.N. Mechanisms of H2O2-induced oxidative stress in endothelial cells. Free Radic. Boil. Med. 2006, 40, 2206–2213. [Google Scholar] [CrossRef]
- Ha, J.H.; Noh, H.S.; Shin, I.W.; Hahm, J.R.; Kim, D.R. Mitigation of H2O2-induced autophagic cell death by propofol in H9C2 cardiomyocytes. Cell Boil. Toxicol. 2012, 28, 19–29. [Google Scholar] [CrossRef]
- Levonen, A.L.; Hill, B.G.; Kansanen, E.; Zhang, J.; Darley-Usmar, V.M. Redox regulation of antioxidants, autophagy, and the response to stress: Implications for electrophile therapeutics. Free Radic. Boil. Med. 2014, 71, 196–207. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.H.; Li, B.; Zheng, X.F.; Chen, J.W.; Chen, K.; Jiang, S.D.; Jiang, L.S. Oxidative damage to osteoblasts can be alleviated by early autophagy through the endoplasmic reticulum stress pathway—Implications for the treatment of osteoporosis. Free Radic. Boil. Med. 2014, 77, 10–20. [Google Scholar] [CrossRef]
- Klettner, A.; Kauppinen, A.; Blasiak, J.; Roider, J.; Salminen, A.; Kaarniranta, K. Cellular and molecular mechanisms of age-related macular degeneration: From impaired autophagy to neovascularization. Int. J. Biochem. Cell Biol. 2013, 45, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Kunchithapautham, K.; Rohrer, B. Apoptosis and autophagy in photoreceptors exposed to oxidative stress. Autophagy 2007, 3, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shen, H.; Shi, J.; Tang, L. The effects of alpha lipoic acid in preventing oxidative stress-induced retinal pigment epithelial cell injury. Can. J. Physiol. Pharmacol. 2014, 92, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Hsieh, M.C.; Wu, H.J.; Wu, W.C.; Kao, Y.H. Methylglyoxal, a reactive glucose metabolite, enhances autophagy flux and suppresses proliferation of human retinal pigment epithelial arpe-19 cells. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2015, 29, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Szatmári-Tóth, M.; Kristóf, E.; Veréb, Z.; Akhtar, S.; Facskó, A.; Fésüs, L.; Kauppinen, A.; Kaarniranta, K.; Petrovski, G. Clearance of autophagy-associated dying retinal pigment epithelial cells—A possible source for inflammation in age-related macular degeneration. Cell Death Dis. 2016, 7, e2367. [Google Scholar]
- Zhou, R.; Caspi, R.R. Ocular immune privilege. F1000 Biol. Rep. 2010, 212, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Ambati, J.; Atkinson, J.P.; Gelfand, B.D. Immunology of age-related macular degeneration. Nat. Rev. Immunol. 2013, 13, 438–451. [Google Scholar] [CrossRef] [Green Version]
- Kauppinen, A.; Paterno, J.J.; Blasiak, J.; Salminen, A.; Kaarniranta, K. Inflammation and its role in age-related macular degeneration. Cell. Mol. Life Sci. CMLS 2016, 7, 1765–1786. [Google Scholar] [CrossRef]
- Patel, M.; Chan, C.C. Immunopathological aspects of age-related macular degeneration. Semin. Immunopathol. 2008, 30, 97–110. [Google Scholar] [CrossRef] [Green Version]
- Lauber, K.; Blumenthal, S.G.; Waibel, M.; Wesselborg, S. Clearance of apoptotic cells: Getting rid of the corpses. Mol. Cell 2004, 14, 277–287. [Google Scholar] [CrossRef]
- Li, W. Eat-me signals: Keys to molecular phagocyte biology and “appetite” control. J. Cell. Physiol. 2012, 227, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Kay, J.G.; Grinstein, S. Phosphatidylserine-mediated cellular signaling. Adv. Exp. Med. Boil. 2013, 991, 177–193. [Google Scholar]
- Segawa, K.; Nagata, S. An apoptotic ‘eat me’ signal: Phosphatidylserine exposure. Trends Cell Boil. 2015, 25, 639–650. [Google Scholar] [CrossRef]
- Lemke, G.; Rothlin, C.V. Immunobiology of the tam receptors. Nat. Rev. Immunol. 2008, 8, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Rothlin, C.V.; Carrera-Silva, E.A.; Bosurgi, L.; Ghosh, S. Tam receptor signaling in immune homeostasis. Annu. Rev. Immunol. 2015, 33, 355–391. [Google Scholar] [CrossRef] [PubMed]
- Van der Meer, J.H.; van der Poll, T.; van’t Veer, C. Tam receptors, gas6, and protein s: Roles in inflammation and hemostasis. Blood 2014, 123, 2460–2469. [Google Scholar] [CrossRef]
- Fernandez-Fernandez, L.; Bellido-Martin, L.; Garcia de Frutos, P. Growth arrest-specific gene 6 (gas6). An outline of its role in haemostasis and inflammation. Thromb. Haemost. 2008, 100, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Savill, J.; Fadok, V. Corpse clearance defines the meaning of cell death. Nature 2000, 407, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Savill, J.; Dransfield, I.; Gregory, C.; Haslett, C. A blast from the past: Clearance of apoptotic cells regulates immune responses. Nat. Rev. Immunol. 2002, 2, 965–975. [Google Scholar] [CrossRef]
- Byrne, A.; Reen, D.J. Lipopolysaccharide induces rapid production of il-10 by monocytes in the presence of apoptotic neutrophils. J. Immunol. 2002, 168, 1968–1977. [Google Scholar] [CrossRef]
- Gregory, C.D.; Devitt, A. The macrophage and the apoptotic cell: An innate immune interaction viewed simplistically? Immunology 2004, 113, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Fadok, V.A.; Bratton, D.L.; Konowal, A.; Freed, P.W.; Westcott, J.Y.; Henson, P.M. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J. Clin. Investig. 1998, 101, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Fadok, V.A.; Bratton, D.L.; Guthrie, L.; Henson, P.M. Differential effects of apoptotic versus lysed cells on macrophage production of cytokines: Role of proteases. J. Immunol. 2001, 166, 6847–6854. [Google Scholar] [CrossRef] [PubMed]
- Poon, I.K.; Hulett, M.D.; Parish, C.R. Molecular mechanisms of late apoptotic/necrotic cell clearance. Cell Death Differ. 2010, 17, 381–397. [Google Scholar] [CrossRef]
- Fernandez-Boyanapalli, R.; McPhillips, K.A.; Frasch, S.C.; Janssen, W.J.; Dinauer, M.C.; Riches, D.W.; Henson, P.M.; Byrne, A.; Bratton, D.L. Impaired phagocytosis of apoptotic cells by macrophages in chronic granulomatous disease is reversed by ifn-gamma in a nitric oxide-dependent manner. J. Immunol. 2010, 185, 4030–4041. [Google Scholar] [CrossRef] [PubMed]
- Poon, I.K.; Lucas, C.D.; Rossi, A.G.; Ravichandran, K.S. Apoptotic cell clearance: Basic biology and therapeutic potential. Nat. Rev. Immunol. 2014, 14, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Medina, C.B.; Ravichandran, K.S. Do not let death do us part: ‘Find-me’ signals in communication between dying cells and the phagocytes. Cell Death Differ. 2016, 23, 979. [Google Scholar] [CrossRef] [PubMed]
- Idelson, M.; Alper, R.; Obolensky, A.; Yachimovich-Cohen, N.; Rachmilewitz, J.; Ejzenberg, A.; Beider, E.; Banin, E.; Reubinoff, B. Immunological properties of human embryonic stem cell-derived retinal pigment epithelial cells. Stem Cell Rep. 2018, 11, 681–695. [Google Scholar] [CrossRef] [PubMed]
- Skottman, H. Derivation and characterization of three new human embryonic stem cell lines in finland. Vitr. Cell. Dev. Boil. Anim. 2010, 46, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Ilmarinen, T.; Hiidenmaa, H.; Koobi, P.; Nymark, S.; Sorkio, A.; Wang, J.H.; Stanzel, B.V.; Thieltges, F.; Alajuuma, P.; Oksala, O.; et al. Ultrathin polyimide membrane as cell carrier for subretinal transplantation of human embryonic stem cell derived retinal pigment epithelium. PLoS ONE 2015, 10, e0143669. [Google Scholar] [CrossRef] [PubMed]
- Petrovski, G.; Zahuczky, G.; Katona, K.; Vereb, G.; Martinet, W.; Nemes, Z.; Bursch, W.; Fesus, L. Clearance of dying autophagic cells of different origin by professional and non-professional phagocytes. Cell Death Differ. 2007, 14, 1117–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinnunen, K.; Petrovski, G.; Moe, M.C.; Berta, A.; Kaarniranta, K. Molecular mechanisms of retinal pigment epithelium damage and development of age-related macular degeneration. Acta Ophthalmol. 2012, 90, 299–309. [Google Scholar] [CrossRef] [PubMed]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szatmári-Tóth, M.; Ilmarinen, T.; Mikhailova, A.; Skottman, H.; Kauppinen, A.; Kaarniranta, K.; Kristóf, E.; Lytvynchuk, L.; Veréb, Z.; Fésüs, L.; et al. Human Embryonic Stem Cell-Derived Retinal Pigment Epithelium-Role in Dead Cell Clearance and Inflammation. Int. J. Mol. Sci. 2019, 20, 926. https://doi.org/10.3390/ijms20040926
Szatmári-Tóth M, Ilmarinen T, Mikhailova A, Skottman H, Kauppinen A, Kaarniranta K, Kristóf E, Lytvynchuk L, Veréb Z, Fésüs L, et al. Human Embryonic Stem Cell-Derived Retinal Pigment Epithelium-Role in Dead Cell Clearance and Inflammation. International Journal of Molecular Sciences. 2019; 20(4):926. https://doi.org/10.3390/ijms20040926
Chicago/Turabian StyleSzatmári-Tóth, Mária, Tanja Ilmarinen, Alexandra Mikhailova, Heli Skottman, Anu Kauppinen, Kai Kaarniranta, Endre Kristóf, Lyubomyr Lytvynchuk, Zoltán Veréb, László Fésüs, and et al. 2019. "Human Embryonic Stem Cell-Derived Retinal Pigment Epithelium-Role in Dead Cell Clearance and Inflammation" International Journal of Molecular Sciences 20, no. 4: 926. https://doi.org/10.3390/ijms20040926
APA StyleSzatmári-Tóth, M., Ilmarinen, T., Mikhailova, A., Skottman, H., Kauppinen, A., Kaarniranta, K., Kristóf, E., Lytvynchuk, L., Veréb, Z., Fésüs, L., & Petrovski, G. (2019). Human Embryonic Stem Cell-Derived Retinal Pigment Epithelium-Role in Dead Cell Clearance and Inflammation. International Journal of Molecular Sciences, 20(4), 926. https://doi.org/10.3390/ijms20040926