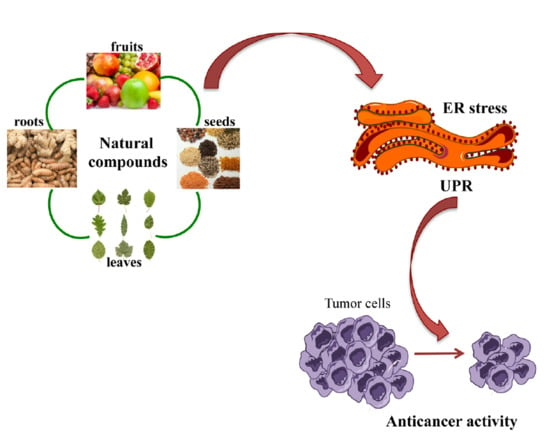
Role of Endoplasmic Reticulum Stress in the Anticancer Activity of Natural Compounds
Abstract
:1. Introduction
2. Endoplasmic Reticulum Stress Response Pathways
2.1. UPR
2.2. IRE1 Pathway
2.3. PERK Pathway
2.4. ATF6 Pathway
3. ER Stress Mediates UPR for Anticancer Strategies
3.1. Pro-Apoptotic Signals Involving IRE1α-XBP1
3.2. Pro-Apoptotic Signals Involving PERK-eIF2a-ATF4/CHOP
3.3. Pro-Apoptotic Signals Involving ER Ca2+ Release
3.4. The Role of UPR in Cancer Cells
4. Natural Compounds
4.1. Role of ER Stress in Curcumin-Induced Apoptosis in Cancer
4.2. Role of ER Stress in Resveratrol-Induced Apoptosis in Cancer
4.3. Role of ER Stress in Green Tea Polyphenols-Induced Apoptosis in Cancer
4.4. Role of ER stress in Tocotrienols-Induced Apoptosis in Cancer
4.5. Role of ER Stress in Garcinia-Induced Apoptosis in Cancer
4.6. Role of ER Stress in Other Natural Compound-Induced Apoptosis in Cancer
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 7-AB | Acetylsinumaximol B (7-AB) |
| AR | Androgen receptor |
| ASK | Apoptosis signal-regulating kinase |
| ATF6 | Activating transcription factor 6 |
| ATF4 | Activating transcription factor 4 |
| BiP | Binding immunoglobulin protein |
| BDMC | Bisdemethoxycurcumin |
| CaMKII | Calcium/calmodulin-dependent protein kinase II |
| C/EBP | CCAAT/enhancer binding protein family |
| CHOP | C/EBP homologous protein |
| COM | Cnidium officinale Makino |
| DMC | Demethoxycurcumin |
| GADD34 | Growth arrest and DNA-damage-inducible protein 34 |
| GRP78 | Glucose-regulated protein 78 |
| eIF2α | Eukaryotic initiation factor 2α |
| EGCG EMT | Epigallocatechin gallate Epithelial to mesenchimal transition |
| ER | Endoplasmic reticulum |
| ERAD | ER-associated degradation |
| ERO1α | Endoplasmic reticulum oxireductin 1α |
| ERSE | ER stress response elements |
| GA | Gambogic acid |
| GE | Garlic extract |
| IP3 | Inositol 1,4,5,-triphosphate |
| IP3R | Inositol 1,4,5,-triphosphate receptor |
| IRE1 | Inositol requiring enzyme1 |
| JNK | JUN N-terminal kinase |
| MFE | Mangosteen fruit extract |
| PERK | Protein kinase RNA(PKR)-like ER kinase |
| PDI | Protein disulfide isomerase |
| ROS | Reactive oxygene species |
| RIDD | Regulated IRE1-dependent decay |
| SERCA | Sarcoplasmic/endoplasmic Ca2+ ATPase |
| S1P | Site-1 protease |
| S2P | Site-2 protease |
| TPs | Tocopherols |
| TRAF2 | Tumor necrosis factor receptor-associated factor 2 |
| TTs | Tocotrienols |
| UPR | Unfolded protein response |
| XBP1 | X-box binding protein 1 |
References
- Porter, K.R.; Claude, A.; Fullam, E.F. A study of tissue culture cells by electron microscopy: Methods and preliminary observations. J. Exp. Med. 1945, 81, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Oakes, S.A.; Papa, F.R. The Role of Endoplasmic Reticulum Stress in Human Pathology. Annu. Rev. Pathol. Mech. Dis. 2015, 10, 173–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almanza, A.; Carlesso, A.; Chintha, C.; Creedican, S.; Doultsinos, D.; Leuzzi, B.; Luís, A.; McCarthy, N.; Montibeller, L.; More, S.; et al. Endoplasmic reticulum stress signalling - from basic mechanisms to clinical applications. FEBS J. 2018. [Google Scholar] [CrossRef]
- Kim, I.; Xu, W.; Reed, J.C. Cell death and endoplasmic reticulum stress: Disease relevance and therapeutic opportunities. Nat. Rev. Drug Discov. 2008, 7, 1013–1030. [Google Scholar] [CrossRef] [PubMed]
- Csala, M.; Kereszturi, É.; Mandl, J.; Bánhegyi, G. The Endoplasmic Reticulum As the Extracellular Space Inside the Cell: Role in Protein Folding and Glycosylation. Antioxid. Redox Signal. 2012, 16, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Maurel, M.; McGrath, E.P.; Mnich, K.; Healy, S.; Chevet, E.; Samali, A. Controlling the unfolded protein response-mediated life and death decisions in cancer. Semin. Cancer Biol. 2015, 33, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Mori, K. The unfolded protein response: The dawn of a new field. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2015, 91, 469–480. [Google Scholar] [CrossRef]
- Wang, M.; Law, M.E.; Castellano, R.K.; Law, B.K. The unfolded protein response as a target for anticancer therapeutics. Crit. Rev. Oncol. Hematol. 2018, 127, 66–79. [Google Scholar] [CrossRef]
- Martinon, F.; Chen, X.; Lee, A.-H.; Glimcher, L.H. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat. Immunol. 2010, 11, 411–418. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.-C.A.; Dougan, S.K.; McGehee, A.M.; Love, J.C.; Ploegh, H.L. XBP-1 regulates signal transduction, transcription factors and bone marrow colonization in B cells. EMBO J. 2009, 28, 1624–1636. [Google Scholar] [CrossRef] [Green Version]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Kingston, D.G.; Newman, D.J. Natural products as drug leads: An old process or the new hope for drug discovery? J. Med. Chem. 2008, 51, 2589–2599. [Google Scholar]
- Menezes, J.C.J.M.D.S.; Orlikova, B.; Morceau, F.; Diederich, M. Natural and Synthetic Flavonoids: Structure-Activity Relationship and Chemotherapeutic Potential for the Treatment of Leukemia. Crit. Rev. Food Sci. Nutr. 2016, 56 (Suppl. 1), S4–S28. [Google Scholar] [CrossRef]
- Kotecha, R.; Takami, A.; Espinoza, J.L. Dietary phytochemicals and cancer chemoprevention: A review of the clinical evidence. Oncotarget 2016, 7, 52517–52529. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Sharma, B.; Kanwar, S.S.; Kumar, A. Lead Phytochemicals for Anticancer Drug Development. Front. Plant Sci. 2016, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Júnior, R.G.; Christiane Adrielly, A.F.; da Silva Almeida, J.R.G.; Grougnet, R.; Thiéry, V.; Picot, L. Sensitization of tumor cells to chemotherapy by natural products: A systematic review of preclinical data and molecular mechanisms. Fitoterapia 2018, 129, 383–400. [Google Scholar] [CrossRef] [PubMed]
- Gardner, B.M.; Pincus, D.; Gotthardt, K.; Gallagher, C.M.; Walter, P. Endoplasmic Reticulum Stress Sensing in the Unfolded Protein Response. Cold Spring Harb. Perspect. Biol. 2013, 5, a013169. [Google Scholar] [CrossRef]
- Sun, H.; Lin, D.-C.; Guo, X.; Kharabi Masouleh, B.; Gery, S.; Cao, Q.; Alkan, S.; Ikezoe, T.; Akiba, C.; Paquette, R.; et al. Inhibition of IRE1α-driven pro-survival pathways is a promising therapeutic application in acute myeloid leukemia. Oncotarget 2016, 7, 18736–18749. [Google Scholar] [CrossRef]
- Lee, A.-H.; Glimcher, L.H. Intersection of the unfolded protein response and hepatic lipid metabolism. Cell. Mol. Life Sci. 2009, 66, 2835–2850. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Brandizzi, F. IRE1: ER stress sensor and cell fate executor. Trends Cell Biol. 2013, 23, 547–555. [Google Scholar] [CrossRef] [Green Version]
- Clarke, H.J.; Chambers, J.E.; Liniker, E.; Marciniak, S.J. Endoplasmic Reticulum Stress in Malignancy. Cancer Cell 2014, 25, 563–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishitoh, H.; Matsuzawa, A.; Tobiume, K.; Saegusa, K.; Takeda, K.; Inoue, K.; Hori, S.; Kakizuka, A.; Ichijo, H. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev. 2002, 16, 1345–1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DuRose, J.B.; Scheuner, D.; Kaufman, R.J.; Rothblum, L.I.; Niwa, M. Phosphorylation of Eukaryotic Translation Initiation Factor 2 Coordinates rRNA Transcription and Translation Inhibition during Endoplasmic Reticulum Stress. Mol. Cell. Biol. 2009, 29, 4295–4307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rozpedek, W.; Pytel, D.; Mucha, B.; Leszczynska, H.; Diehl, J.A.; Majsterek, I. The Role of the PERK/eIF2α/ATF4/CHOP Signaling Pathway in Tumor Progression During Endoplasmic Reticulum Stress. Curr. Mol. Med. 2016, 16, 533–544. [Google Scholar] [CrossRef]
- Zong, Z.-H.; Du, Z.-X.; Li, N.; Li, C.; Zhang, Q.; Liu, B.-Q.; Guan, Y.; Wang, H.-Q. Implication of Nrf2 and ATF4 in differential induction of CHOP by proteasome inhibition in thyroid cancer cells. Biochim. Biophys. Acta 2012, 1823, 1395–1404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabrera, E.; Hernández-Pérez, S.; Koundrioukoff, S.; Debatisse, M.; Kim, D.; Smolka, M.B.; Freire, R.; Gillespie, D.A. PERK inhibits DNA replication during the Unfolded Protein Response via Claspin and Chk1. Oncogene 2017, 36, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Hillary, R.F.; FitzGerald, U. A lifetime of stress: ATF6 in development and homeostasis. J. Biomed. Sci. 2018, 25, 48. [Google Scholar] [CrossRef] [Green Version]
- Thuerauf, D.J.; Marcinko, M.; Belmont, P.J.; Glembotski, C.C. Effects of the Isoform-specific Characteristics of ATF6α and ATF6β on Endoplasmic Reticulum Stress Response Gene Expression and Cell Viability. J. Biol. Chem. 2007, 282, 22865–22878. [Google Scholar] [CrossRef]
- Hirsch, I.; Weiwad, M.; Prell, E.; Ferrari, D.M. ERp29 deficiency affects sensitivity to apoptosis via impairment of the ATF6-CHOP pathway of stress response. Apoptosis 2014, 19, 801–815. [Google Scholar] [CrossRef]
- Kim, C.; Kim, B. Anti-Cancer Natural Products and Their Bioactive Compounds Inducing ER Stress-Mediated Apoptosis: A Review. Nutrients 2018, 10, 1021. [Google Scholar] [CrossRef]
- Madden, E.; Logue, S.E.; Healy, S.J.; Manie, S.; Samali, A. The role of the unfolded protein response in cancer progression: From oncogenesis to chemoresistance. Biol. Cell 2019, 111, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Iurlaro, R.; Muñoz-Pinedo, C. Cell death induced by endoplasmic reticulum stress. FEBS J. 2016, 283, 2640–2652. [Google Scholar] [CrossRef]
- Urano, F.; Bertolotti, A.; Ron, D. IRE1 and efferent signaling from the endoplasmic reticulum. J. Cell Sci. 2000, 113 Pt 21, 3697–3702. [Google Scholar]
- Hetz, C.; Bernasconi, P.; Fisher, J.; Lee, A.H.; Bassik, M.C.; Antonsson, B.; Brandt, G.S.; Iwakoshi, N.N.; Schrinzel, A.; Glimcher, L.H.; et al. Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1α. Science 2006, 321, 572–576. [Google Scholar] [CrossRef]
- Koong, A.C.; Chauhan, V.; Romero-Ramirez, L. Targeting XBP-1 as a novel anti-cancer strategy. Cancer Biol. Ther. 2006, 5, 756–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Liu, L.; Naik, I.; Braunstein, Z.; Zhong, J.; Ren, B. Transcription Factor C/EBP Homologous Protein in Health and Diseases. Front. Immunol. 2017, 8, 1612. [Google Scholar] [CrossRef] [PubMed]
- Oyadomari, S.; Mori, M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004, 11, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, L.; Tabas, I. Pivotal role of calcium/calmodulin-dependent protein kinase II in ER stress-induced apoptosis. Cell Cycle 2010, 9, 223–224. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, H.; Wang, H.-G. CHOP Is Involved in Endoplasmic Reticulum Stress-induced Apoptosis by Enhancing DR5 Expression in Human Carcinoma Cells. J. Biol. Chem. 2004, 279, 45495–45502. [Google Scholar] [CrossRef] [Green Version]
- Puthalakath, H.; O’Reilly, L.A.; Gunn, P.; Lee, L.; Kelly, P.N.; Huntington, N.D.; Hughes, P.D.; Michalak, E.M.; McKimm-Breschkin, J.; Motoyama, N.; et al. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell 2007, 129, 1337–1349. [Google Scholar] [CrossRef]
- Tan, B.; Jia, R.; Wang, G.; Yang, J. Astragaloside attenuates the progression of prostate cancer cells through endoplasmic reticulum stress pathways. Oncol. Lett. 2018, 16, 3901–3906. [Google Scholar] [CrossRef] [PubMed]
- Heath-Engel, H.M.; Wang, B.; Shore, G.C. Bcl2 at the endoplasmic reticulum protects against a Bax/Bak-independent paraptosis-like cell death pathway initiated via p20Bap31. Biochim. Biophys. Acta 2012, 1823, 335–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieto-Miguel, T.; Fonteriz, R.I.; Vay, L.; Gajate, C.; López-Hernández, S.; Mollinedo, F. Endoplasmic reticulum stress in the proapoptotic action of edelfosine in solid tumor cells. Cancer Res. 2007, 67, 10368–10378. [Google Scholar] [CrossRef] [PubMed]
- Logue, S.E.; McGrath, E.P.; Cleary, P.; Greene, S.; Mnich, K.; Almanza, A.; Chevet, E.; Dwyer, R.M.; Oommen, A.; Legembre, P.; et al. Inhibition of IRE1 RNase activity modulates the tumor cell secretome and enhances response to chemotherapy. Nat. Commun. 2018, 9, 3267. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Iliopoulos, D.; Zhang, Q.; Tang, Q.; Greenblatt, M.B.; Hatziapostolou, M.; Lim, E.; Tam, W.L.; Ni, M.; Chen, Y.; et al. XBP1 promotes triple-negative breast cancer by controlling the HIF1α pathway. Nature 2014, 508, 103–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Y.-x.; Sokol, E.S.; Del Vecchio, C.A.; Sanduja, S.; Claessen, J.H.L.; Proia, T.A.; Jin, D.X.; Reinhardt, F.; Ploegh, H.L.; Wang, Q.; et al. Epithelial-to-Mesenchymal Transition Activates PERK-eIF2 and Sensitizes Cells to Endoplasmic Reticulum Stress. Cancer Discov. 2014, 4, 702–715. [Google Scholar] [CrossRef] [Green Version]
- Cuevas, E.P.; Eraso, P.; Mazón, M.J.; Santos, V.; Moreno-Bueno, G.; Cano, A.; Portillo, F. LOXL2 drives epithelial-mesenchymal transition via activation of IRE1-XBP1 signalling pathway. Sci. Rep. 2017, 7, 44988. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.K.; Kang, K.A.; Piao, M.J.; Ryu, Y.S.; Han, X.; Fernando, P.M.D.J.; Oh, M.C.; Park, J.E.; Shilnikova, K.; Boo, S.J.; et al. Endoplasmic reticulum stress induces 5-fluorouracil resistance in human colon cancer cells. Environ. Toxicol. Pharmacol. 2016, 44, 128–133. [Google Scholar] [CrossRef]
- Chen, O.I.; Bobak, Y.P.; Stasyk, O.V.; Kunz-Schughart, L.A. A Complex Scenario and Underestimated Challenge: The Tumor Microenvironment, ER Stress, and Cancer Treatment. Curr. Med. Chem. 2018, 25, 2465–2502. [Google Scholar] [CrossRef]
- Schüz, J.; Espina, C.; Villain, P.; Herrero, R.; Leon, M.E.; Minozzi, S.; Romieu, I.; Segnan, N.; Wardle, J.; Wiseman, M.; et al. European Code against Cancer 4th Edition: 12 ways to reduce your cancer risk. Cancer Epidemiol. 2015, 39, S1–S10. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.; Suman, S.; Shukla, Y. New enlightenment of skin cancer chemoprevention through phytochemicals: In vitro and in vivo studies and the underlying mechanisms. Biomed Res. Int. 2014, 2014, 243452. [Google Scholar] [CrossRef]
- Mao, X.-Y.; Jin, M.-Z.; Chen, J.-F.; Zhou, H.-H.; Jin, W.-L. Live or let die: Neuroprotective and anti-cancer effects of nutraceutical antioxidants. Pharmacol. Ther. 2018, 183, 137–151. [Google Scholar] [CrossRef]
- Leary, M.; Heerboth, S.; Lapinska, K.; Sarkar, S. Sensitization of drug resistant cancer cells: A matter of combination therapy. Cancers 2018, 10, 483. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, E.; Greco, G.; Potenza, L.; Calcabrini, C.; Fimognari, C. Natural products to fight cancer: A focus on Juglans regia. Toxins 2018, 10, 469. [Google Scholar] [CrossRef] [PubMed]
- Calcabrini, C.; Catanzaro, E.; Bishayee, A.; Turrini, E.; Fimognari, C. Marine Sponge Natural Products with Anticancer Potential: An Updated Review. Mar. Drugs 2017, 15, 310. [Google Scholar] [CrossRef]
- Cha, J.; Song, H.-S.; Kang, B.; Park, M.; Park, K.; Kim, S.-H.; Shim, B.-S.; Kim, B. miR-211 Plays a Critical Role in Cnidium officinale Makino Extract-Induced, ROS/ER Stress-Mediated Apoptosis in U937 and U266 Cells. Int. J. Mol. Sci. 2018, 19, 865. [Google Scholar] [CrossRef]
- Shehzad, A.; Wahid, F.; Lee, Y.S. Curcumin in cancer chemoprevention: Molecular targets, pharmacokinetics, bioavailability, and clinical trials. Arch. Pharm. 2010, 343, 489–499. [Google Scholar] [CrossRef]
- Garrido-Armas, M.; Corona, J.C.; Escobar, M.L.; Torres, L.; Ordóñez-Romero, F.; Hernández-Hernández, A.; Arenas-Huertero, F. Paraptosis in human glioblastoma cell line induced by curcumin. Toxicol. Vitr. 2018, 51, 63–73. [Google Scholar] [CrossRef]
- Rivera, M.; Ramos, Y.; Rodríguez-Valentín, M.; López-Acevedo, S.; Cubano, L.A.; Zou, J.; Zhang, Q.; Wang, G.; Boukli, N.M. Targeting multiple pro-apoptotic signaling pathways with curcumin in prostate cancer cells. PLoS One 2017, 12, e0179587. [Google Scholar] [CrossRef]
- Huang, A.-C.; Chang, C.-L.; Yu, C.-S.; Chen, P.-Y.; Yang, J.-S.; Ji, B.-C.; Lin, T.-P.; Chiu, C.-F.; Yeh, S.-P.; Huang, Y.-P.; et al. Induction of apoptosis by curcumin in murine myelomonocytic leukemia WEHI-3 cells is mediated via endoplasmic reticulum stress and mitochondria-dependent pathways. Environ. Toxicol. 2013, 28, 255–266. [Google Scholar] [CrossRef]
- Roberts, J.L.; Poklepovic, A.; Booth, L. Curcumin interacts with sildenafil to kill GI tumor cells via endoplasmic reticulum stress and reactive oxygen/ nitrogen species. Oncotarget 2017, 8, 99451–99469. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-F.; Zhu, D.-J.; Chen, X.-W.; Chen, Q.-K.; Luo, Z.-T.; Liu, C.-C.; Wang, G.-X.; Zhang, W.-J.; Liao, N.-Z. Curcumin enhances the effects of irinotecan on colorectal cancer cells through the generation of reactive oxygen species and activation of the endoplasmic reticulum stress pathway. Oncotarget 2017, 8, 40264. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-T.; Huang, A.-C.; Tang, N.-Y.; Liu, H.-C.; Liao, C.-L.; Ji, B.-C.; Chou, Y.-C.; Yang, M.-D.; Lu, H.-F.; Chung, J.-G. Bisdemethoxycurcumin-induced S phase arrest through the inhibition of cyclin A and E and induction of apoptosis via endoplasmic reticulum stress and mitochondria-dependent pathways in human lung cancer NCI H460 cells. Environ. Toxicol. 2016, 31, 1899–1908. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.-C.; Lien, J.-C.; Liu, H.-C.; Hsu, S.-C.; Ji, B.-C.; Yang, M.-D.; Hsu, W.-H.; Chung, J.-G. Demethoxycurcumin induces the apoptosis of human lung cancer NCI-H460 cells through the mitochondrial-dependent pathway. Oncol. Rep. 2015, 33, 2429–2437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, A.; Li, H.; Wang, X.; Feng, Z.; Xu, J.; Cao, K.; Zhou, B.; Wu, J.; Liu, J. Anticancer Effect of a Curcumin Derivative B63: ROS Production and Mitochondrial Dysfunction. Curr. Cancer Drug Targets 2014, 14, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, H.Q.; Zhu, G.H.; Wang, Y.H.; Yu, X.C.; Zhu, X.B.; Liang, G.; Xiao, J.; Li, X.K. A novel mono-carbonyl analogue of curcumin induces apoptosis in ovarian oxygen species production. Mol Med Rep. 2012, 5, 739–744. [Google Scholar] [PubMed]
- Dai, X.; Zhang, J.; Guo, G.; Cai, Y.; Cui, R.; Yin, C.; Liu, W.; Vinothkumar, R.; Zhang, T.; Liang, G.; et al. A mono-carbonyl analog of curcumin induces apoptosis in drug-resistant EGFR-mutant lung cancer through the generation of oxidative stress and mitochondrial dysfunction. Cancer Manag. Res. 2018, 10, 3069–3082. [Google Scholar] [CrossRef]
- Chang, L.-C.; Hsieh, M.-T.; Yang, J.-S.; Lu, C.-C.; Tsai, F.-J.; Tsao, J.-W.; Chiu, Y.-J.; Kuo, S.-C.; Lee, K.-H. Effect of bis(hydroxymethyl) alkanoate curcuminoid derivative MTH-3 on cell cycle arrest, apoptotic and autophagic pathway in triple-negative breast adenocarcinoma MDA-MB-231 cells: An in vitro study. Int. J. Oncol. 2017, 52, 67–76. [Google Scholar] [CrossRef]
- Biagi, M.; Bertelli, A.A.E. Wine, alcohol and pills: What future for the French paradox? Life Sci. 2015, 131, 19–22. [Google Scholar] [CrossRef]
- Elshaer, M.; Chen, Y.; Wang, X.J.; Tang, X. Resveratrol: An overview of its anti-cancer mechanisms. Life Sci. 2018, 207, 340–349. [Google Scholar] [CrossRef]
- Wang, F.-M.; Galson, D.L.; Roodman, G.D.; Ouyang, H. Resveratrol triggers the pro-apoptotic endoplasmic reticulum stress response and represses pro-survival XBP1 signaling in human multiple myeloma cells. Exp. Hematol. 2011, 39, 999–1006. [Google Scholar] [CrossRef] [Green Version]
- Heo, J.; Kim, S.; Hwang, K.; Kang, J.; Choi, K. Resveratrol induced reactive oxygen species and endoplasmic reticulum stress-mediated apoptosis, and cell cycle arrest in the A375SM malignant melanoma cell line. Int. J. Mol. Med. 2018, 42, 1427–1435. [Google Scholar] [CrossRef] [PubMed]
- Rojas, C.; Pan-Castillo, B.; Valls, C.; Pujadas, G.; Garcia-Vallve, S.; Arola, L.; Mulero, M. Resveratrol enhances palmitate-induced ER stress and apoptosis in cancer cells. PLoS One 2014, 9, e113929. [Google Scholar] [CrossRef] [PubMed]
- Bai, T.; Dong, D.-S.; Pei, L. Synergistic antitumor activity of resveratrol and miR-200c in human lung cancer. Oncol. Rep. 2014, 31, 2293–2297. [Google Scholar] [CrossRef] [PubMed]
- Chow, S.-E.; Kao, C.-H.; Liu, Y.-T.A.; Cheng, M.-L.; Yang, Y.-W.; Huang, Y.-K.; Hsu, C.-C.; Wang, J.-S. Resveratrol induced ER expansion and ER caspase-mediated apoptosis in human nasopharyngeal carcinoma cells. Apoptosis 2014, 19, 527–541. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Chen, C.; Jiang, X.; Zhang, Z. ROS-mediated endoplasmic reticulum stress and mitochondrial dysfunction underlie apoptosis induced by resveratrol and arsenic trioxide in A549 cells. Chem. Biol. Interact. 2016, 245, 100–109. [Google Scholar] [CrossRef]
- Gwak, H.; Kim, S.; Dhanasekaran, D.N.; Song, Y.S. Resveratrol triggers ER stress-mediated apoptosis by disrupting N -linked glycosylation of proteins in ovarian cancer cells. Cancer Lett. 2016, 371, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Sang, S. Metabolism and pharmacokinetics of resveratrol and pterostilbene. Biofactors 2018, 44, 16–25. [Google Scholar] [CrossRef]
- Fan, X.-X.; Yao, X.-J.; Xu, S.W.; Wong, V.K.-W.; He, J.-X.; Ding, J.; Xue, W.-W.; Mujtaba, T.; Michelangeli, F.; Huang, M.; et al. (Z)3,4,5,4′-trans-tetramethoxystilbene, a new analogue of resveratrol, inhibits gefitinb-resistant non-small cell lung cancer via selectively elevating intracellular calcium level. Sci. Rep. 2015, 5, 16348. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Yang, Y.; Fan, C.; Di, S.; Hu, W.; Jiang, S.; Li, T.; Ma, Z.; Chao, D.; Feng, X.; et al. Pterostilbene Inhibits the Growth of Human Esophageal Cancer Cells by Regulating Endoplasmic Reticulum Stress. Cell. Physiol. Biochem. 2016, 38, 1226–1244. [Google Scholar] [CrossRef]
- Park, J.; Choi, W.; Lee, P.; Chung, S.; Kim, B.; Chung, H.; Cho, S.; Kim, J.; Kang, B.; Kim, H.; et al. The novel resveratrol derivative 3,5-diethoxy-3′,4′-dihydroxy-trans-stilbene induces mitochondrial ROS-mediated ER stress and cell death in human hepatoma cells in vitro. Acta Pharmacol. Sin. 2017, 38, 1486–1500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Révész, K.; Tütto, A.; Szelényi, P.; Konta, L. Tea flavan-3-ols as modulating factors in endoplasmic reticulum function. Nutr. Res. 2011, 31, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Davalli, P.; Rizzi, F.; Caldara, G.F.; Davoli, S.; Corti, A.; Silva, A.; Astancolle, S.; Vitale, M.; Bettuzzi, S.; Arcari, M.; et al. Chronic administration of green tea extract to TRAMP mice induces the collapse of Golgi apparatus in prostate secretory cells and results in alterations of protein post-translational processing. Int. J. Oncol. 2011, 39, 1521–1527. [Google Scholar] [PubMed]
- Rizzi, F.; Naponelli, V.; Silva, A.; Modernelli, A.; Ramazzina, I.; Bonacini, M.; Tardito, S.; Gatti, R.; Uggeri, J.; Bettuzzi, S. Polyphenon E®, a standardized green tea extract, induces endoplasmic reticulum stress, leading to death of immortalized PNT1a cells by anoikis and tumorigenic PC3 by necroptosis. Carcinogenesis 2014, 35, 828–839. [Google Scholar] [CrossRef] [PubMed]
- Modernelli, A.; Naponelli, V.; Giovanna Troglio, M.; Bonacini, M.; Ramazzina, I.; Bettuzzi, S.; Rizzi, F. EGCG antagonizes Bortezomib cytotoxicity in prostate cancer cells by an autophagic mechanism. Sci. Rep. 2015, 5, 15270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinotti, S.; Ranzato, E.; Burlando, B. (−)- Epigallocatechin-3-gallate induces GRP78 accumulation in the ER and shifts mesothelioma constitutive UPR into proapoptotic ER stress. J. Cell. Physiol. 2018. [Google Scholar] [CrossRef]
- Xie, J.; Yun, J.; Yang, Y.; Hua, F.; Zhang, X.; Lin, H.; Lv, X.; Li, K.; Zhang, P.; Hu, Z. A novel ECG analog 4-(S)-(2,4,6-trimethylthiobenzyl)-epigallocatechin gallate selectively induces apoptosis of B16-F10 melanoma via activation of autophagy and ROS. Sci. Rep. 2017, 7, 42194. [Google Scholar] [CrossRef] [Green Version]
- Huang, K.-H.; Kuo, K.-L.; Chen, S.-C.; Weng, T.-I.; Chuang, Y.-T.; Tsai, Y.-C.; Pu, Y.-S.; Chiang, C.-K.; Liu, S.-H. Down-regulation of glucose-regulated protein (GRP) 78 potentiates cytotoxic effect of celecoxib in human urothelial carcinoma cells. PLoS One 2012, 7, e33615. [Google Scholar] [CrossRef]
- Huang, X.; Li, L.; Zhang, L.; Zhang, Z.; Wang, X.; Zhang, X.; Hou, L.; Wu, K. Crosstalk between endoplasmic reticulum stress and oxidative stress in apoptosis induced by α-tocopheryl succinate in human gastric carcinoma cells. Br. J. Nutr. 2013, 109, 727–735. [Google Scholar] [CrossRef]
- Marzagalli, M.; Moretti, R.M.; Messi, E.; Marelli, M.M.; Fontana, F.; Anastasia, A.; Bani, M.R.; Beretta, G.; Limonta, P. Targeting melanoma stem cells with the Vitamin E derivative δ-tocotrienol. Sci. Rep. 2018, 8, 587. [Google Scholar] [CrossRef] [Green Version]
- Montagnani Marelli, M.; Marzagalli, M.; Fontana, F.; Raimondi, M.; Moretti, R.M.; Limonta, P. Anticancer properties of tocotrienols: A review of cellular mechanisms and molecular targets. J. Cell. Physiol. 2019, 234, 1147–1164. [Google Scholar] [CrossRef]
- Wali, V.B.; Bachawal, S.V.; Sylvester, P.W. Endoplasmic reticulum stress mediates gamma-tocotrienol-induced apoptosis in mammary tumor cells. Apoptosis 2009, 14, 1366–1377. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Sanders, B.G.; Kline, K. Tocotrienols induce apoptosis in breast cancer cell lines via an endoplasmic reticulum stress-dependent increase in extrinsic death receptor signaling. Breast Cancer Res. Treat. 2010, 124, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Patacsil, D.; Tran, A.T.; Cho, Y.S.; Suy, S.; Saenz, F.; Malyukova, I.; Ressom, H.; Collins, S.P.; Clarke, R.; Kumar, D. Gamma-tocotrienol induced apoptosis is associated with unfolded protein response in human breast cancer cells. J. Nutr. Biochem. 2012, 23, 93–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiwari, R.V.; Parajuli, P.; Sylvester, P.W. γ-Tocotrienol-induced endoplasmic reticulum stress and autophagy act concurrently to promote breast cancer cell death. Biochem. Cell Biol. 2015, 93, 306–320. [Google Scholar] [CrossRef] [PubMed]
- Comitato, R.; Guantario, B.; Leoni, G.; Nesaretnam, K.; Ronci, M.B.; Canali, R.; Virgili, F. Tocotrienols induce endoplasmic reticulum stress and apoptosis in cervical cancer cells. Genes Nutr. 2016, 11, 32. [Google Scholar] [CrossRef]
- Montagnani Marelli, M.; Marzagalli, M.; Moretti, R.M.; Beretta, G.; Casati, L.; Comitato, R.; Gravina, G.L.; Festuccia, C.; Limonta, P. Vitamin E δ-tocotrienol triggers endoplasmic reticulum stress-mediated apoptosis in human melanoma cells. Sci. Rep. 2016, 6, 30502. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Sintara, M.; Chang, T.; Ou, B. Daily consumption of a mangosteen-based drink improves in vivo antioxidant and anti-inflammatory biomarkers in healthy adults: A randomized, double-blind, placebo-controlled clinical trial. Food Sci. Nutr. 2015, 3, 342–348. [Google Scholar] [CrossRef]
- Kritsanawong, S.; Innajak, S.; Imoto, M.; Watanapokasin, R. Antiproliferative and apoptosis induction of α-mangostin in T47D breast cancer cells. Int. J. Oncol. 2016, 48, 2155–2165. [Google Scholar] [CrossRef]
- Sato, A.; Fujiwara, H.; Oku, H.; Ishiguro, K.; Ohizumi, Y. Alpha-mangostin induces Ca2+-ATPase-dependent apoptosis via mitochondrial pathway in PC12 cells. J. Pharmacol. Sci. 2004, 95, 33–40. [Google Scholar] [CrossRef]
- Li, G.; Petiwala, S.M.; Pierce, D.R.; Nonn, L.; Johnson, J.J. Selective modulation of endoplasmic reticulum stress markers in prostate cancer cells by a standardized mangosteen fruit extract. PLoS One 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Petiwala, S.M.; Nonn, L.; Johnson, J.J. Inhibition of CHOP accentuates the apoptotic effect of α-mangostin from the mangosteen fruit (Garcinia mangostana) in 22Rv1 prostate cancer cells. Biochem. Biophys. Res. Commun. 2014, 453, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-H.; Liu, Q.-Y.; Li, T.; Liu, J.-L.; Chen, X.; Huang, L.; Qiang, W.-A.; Chen, X.; Wang, Y.; Lin, L.-G.; et al. Garcinone E induces apoptosis and inhibits migration and invasion in ovarian cancer cells. Sci. Rep. 2017, 7, 10718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Antalek, M.; Nguyen, L.; Li, X.; Tian, X.; Le, A.; Zi, X. The Effect of Gartanin, a Naturally Occurring Xanthone in Mangosteen Juice, on the mTOR Pathway, Autophagy, Apoptosis, and the Growth of Human Urinary Bladder Cancer Cell Lines. Nutr. Cancer 2013, 65, 68–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Petiwala, S.M.; Yan, M.; Won, J.H.; Petukhov, P.A.; Johnson, J.J. Gartanin, an isoprenylated xanthone from the mangosteen fruit (Garcinia mangostana), is an androgen receptor degradation enhancer. Mol. Nutr. Food Res. 2016, 60, 1458–1469. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.-C.; Tsai, M.-L.; Liu, C.-M.; Lee, M.-F.; Nagabhushanam, K.; Ho, C.-T.; Pan, M.-H. Garcinol inhibits cell growth in hepatocellular carcinoma Hep3B cells through induction of ROS-dependent apoptosis. Food Funct. 2010, 1, 301. [Google Scholar] [CrossRef] [PubMed]
- Nie, F.; Zhang, X.; Qi, Q.; Yang, L.; Yang, Y.; Liu, W.; Lu, N.; Wu, Z.; You, Q.; Guo, Q. Reactive oxygen species accumulation contributes to gambogic acid-induced apoptosis in human hepatoma SMMC-7721 cells. Toxicology 2009, 260, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Chen, Y.; Zeng, L.; Shu, W.; Zhao, F.; Wen, L.; Liu, Y. Gambogic acid induces G0/G1 arrest and apoptosis involving inhibition of SRC-3 and inactivation of Akt pathway in K562 leukemia cells. Toxicology 2009, 262, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Krajarng, A.; Imoto, M.; Tashiro, E.; Fujimaki, T.; Shinjo, S.; Watanapokasin, R. Apoptosis induction associated with the ER stress response through up-regulation of JNK in HeLa cells by gambogic acid. BMC Complement. Altern. Med. 2015, 15, 26. [Google Scholar] [CrossRef] [PubMed]
- Aghaei, M.; Ghanadian, M.; Sajjadi, S.E.; Saghafian, R.; Keyvanloo shahrestanaki, M. Pimpinelol, a novel atypical Sesquiterpene lactone from Pimpinella haussknechtii fruits with evaluation of endoplasmic reticulum stress in breast cancer cells. Fitoterapia 2018, 129, 198–202. [Google Scholar] [CrossRef]
- Cevatemre, B.; Erkısa, M.; Aztopal, N.; Karakas, D.; Alper, P.; Tsimplouli, C.; Sereti, E.; Dimas, K.; Armutak, E.I.I.; Gurevin, E.G.; et al. A promising natural product, pristimerin, results in cytotoxicity against breast cancer stem cells in vitro and xenografts in vivo through apoptosis and an incomplete autopaghy in breast cancer. Pharmacol. Res. 2018, 129, 500–514. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Song, H.-S.; Park, H.; Kim, B. Activation of ER Stress-Dependent miR-216b Has a Critical Role in Salviamiltiorrhiza Ethanol-Extract-Induced Apoptosis in U266 and U937 Cells. Int. J. Mol. Sci. 2018, 19, 1240. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Yao, X. Methyl protogracillin (NSC-698792): The spectrum of cytotoxicity against 60 human cancer cell lines in the National Cancer Instituteʼs anticancer drug screen panel. Anticancer. Drugs 2001, 12, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-L.; Lee, C.-H.; Chen, C.-M.; Cheng, C.-W.; Chen, P.-N.; Ying, T.-H.; Hsieh, Y.-H. Protodioscin Induces Apoptosis Through ROS-Mediated Endoplasmic Reticulum Stress via the JNK/p38 Activation Pathways in Human Cervical Cancer Cells. Cell. Physiol. Biochem. 2018, 46, 322–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.-H.; Weng, Y.-P.; Tsai, H.-Y.; Chen, C.-J.; Lee, D.-Y.; Hsieh, C.-L.; Wu, Y.-C.; Lin, J.-Y. Aqueous extracts of Paeonia suffruticosa modulates mitochondrial proteostasis by reactive oxygen species-induced endoplasmic reticulum stress in pancreatic cancer cells. Phytomedicine 2018, 46, 184–192. [Google Scholar] [CrossRef]
- Lu, M.-C.; Li, T.-Y.; Hsieh, Y.-C.; Hsieh, P.-C.; Chu, Y.-L. Chemical evaluation and cytotoxic mechanism investigation of Clinacanthus nutans extract in lymphoma SUP-T1 cells. Environ. Toxicol. 2018, 33, 1229–1236. [Google Scholar] [CrossRef]
- Chen, K.; Wang, C.-Q.; Fan, Y.-Q.; Xie, Y.-S.; Yin, Z.-F.; Xu, Z.-J.; Zhang, H.-L.; Cao, J.-T.; Wang, Y. Application of chrysophanol in zebrafish to reduce dietary introduced lipid and its possible mechanism. Int. J. Clin. Exp. Med. 2015, 8, 10558–10567. [Google Scholar]
- Park, S.; Lim, W.; Song, G. Chrysophanol selectively represses breast cancer cell growth by inducing reactive oxygen species production and endoplasmic reticulum stress via AKT and mitogen-activated protein kinase signal pathways. Toxicol. Appl. Pharmacol. 2018, 360, 201–211. [Google Scholar] [CrossRef]
- Kaschula, C.H.; Hunter, R.; Cotton, J.; Tuveri, R.; Ngarande, E.; Dzobo, K.; Schäfer, G.; Siyo, V.; Lang, D.; Kusza, D.A.; et al. The garlic compound ajoene targets protein folding in the endoplasmic reticulum of cancer cells. Mol. Carcinog. 2016, 55, 1213–1228. [Google Scholar] [CrossRef]
- Siyo, V.; Schäfer, G.; Hunter, R.; Grafov, A.; Grafova, I.; Nieger, M.; Katz, A.A.; Parker, M.I.; Kaschula, C.H. The Cytotoxicity of the Ajoene Analogue BisPMB in WHCO1 Oesophageal Cancer Cells Is Mediated by CHOP/GADD153. Molecules 2017, 22, 892. [Google Scholar] [CrossRef]
- Petrovic, V.; Nepal, A.; Olaisen, C.; Bachke, S.; Hira, J.; Søgaard, C.; Røst, L.; Misund, K.; Andreassen, T.; Melø, T.; et al. Anti-Cancer Potential of Homemade Fresh Garlic Extract Is Related to Increased Endoplasmic Reticulum Stress. Nutrients 2018, 10, 450. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.-C.; Lai, K.-H.; Su, J.-H.; Wu, Y.-J.; Sheu, J.-H. 7-Acetylsinumaximol B Induces Apoptosis and Autophagy in Human Gastric Carcinoma Cells through Mitochondria Dysfunction and Activation of the PERK/eIF2α/ATF4/CHOP Signaling Pathway. Mar. Drugs 2018, 16, 104. [Google Scholar] [CrossRef] [PubMed]
- Alves-Fernandes, D.K.; Oliveira, É.A.; Faião-Flores, F.; Alicea-Rebecca, G.; Weeraratna, A.T.; Smalley, K.S.M.; de Moraes Barros, S.B.; Maria-Engler, S.S. ER stress promotes antitumor effects in BRAFi/MEKi resistant human melanoma induced by natural compound 4-nerolidylcathecol (4-NC). Pharmacol. Res. 2018, 141, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.-X.; Wang, X.-N.; Tang, Y.-Y.; Cen, W.-J.; Li, Z.-H.; Wang, G.-C.; Jiang, J.-W.; Wang, X.-C. PP-22 promotes autophagy and apoptosis in the nasopharyngeal carcinoma cell line CNE-2 by inducing endoplasmic reticulum stress, downregulating STAT3 signaling, and modulating the MAPK pathway. J. Cell. Physiol. 2019, 234, 2618–2630. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Huang, T.; Shen, Y.; Liu, Y.; Zhou, F.; Jin, Y.; Sattar, H.; Wei, Y. Reactive Oxygen Species-Mediated Tumor Microenvironment Transformation: The Mechanism of Radioresistant Gastric Cancer. Oxid. Med. Cell. Longev. 2018, 2018, 5801209. [Google Scholar] [CrossRef] [PubMed]
- Nunes, T.; Hamdan, D.; Leboeuf, C.; El Bouchtaoui, M.; Gapihan, G.; Nguyen, T.T.; Meles, S.; Angeli, E.; Ratajczak, P.; Lu, H.; et al. Targeting Cancer Stem Cells to Overcome Chemoresistance. Int. J. Mol. Sci. 2018, 19, 4036. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Vila, M.; Takahashi, R.; Usuba, W.; Kohama, I.; Ochiya, T. Drug Resistance Driven by Cancer Stem Cells and Their Niche. Int. J. Mol. Sci. 2017, 18, 2574. [Google Scholar] [CrossRef]
- Tang, L.; Wei, F.; Wu, Y.; He, Y.; Shi, L.; Xiong, F.; Gong, Z.; Guo, C.; Li, X.; Deng, H.; et al. Role of metabolism in cancer cell radioresistance and radiosensitization methods. J. Exp. Clin. Cancer Res. 2018, 37, 87. [Google Scholar] [CrossRef] [Green Version]
- Kesharwani, S.S.; Kaur, S.; Tummala, H.; Sangamwar, A.T. Multifunctional approaches utilizing polymeric micelles to circumvent multidrug resistant tumors. Colloids Surf. B. Biointerfaces 2019, 173, 581–590. [Google Scholar] [CrossRef]
- Belli, C.; Trapani, D.; Viale, G.; D’Amico, P.; Duso, B.A.; Della Vigna, P.; Orsi, F.; Curigliano, G. Targeting the microenvironment in solid tumors. Cancer Treat. Rev. 2018, 65, 22–32. [Google Scholar] [CrossRef]
- Chou, C.-K.; Liu, W.; Hong, Y.-J.; Dahms, H.-U.; Chiu, C.-H.; Chang, W.-T.; Chien, C.-M.; Yen, C.-H.; Cheng, Y.-B.; Chiu, C.-C. Ethyl acetate extract of Scindapsus cf. hederaceus exerts the inhibitory bioactivity on human non-small cell lung cancer cells through modulating ER stress. Int. J. Mol. Sci. 2018, 19, 1832. [Google Scholar] [CrossRef]
- Martin, S.; Lamb, H.K.; Brady, C.; Lefkove, B.; Bonner, M.Y.; Thompson, P.; Lovat, P.E.; Arbiser, J.L.; Hawkins, A.R.; Redfern, C.P.F. Inducing apoptosis of cancer cells using small-molecule plant compounds that bind to GRP78. Br. J. Cancer 2013, 109, 433–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virrey, J.J.; Dong, D.; Stiles, C.; Patterson, J.B.; Pen, L.; Ni, M.; Schonthal, A.H.; Chen, T.C.; Hofman, F.M.; Lee, A.S. Stress Chaperone GRP78/BiP Confers Chemoresistance to Tumor-Associated Endothelial Cells. Mol. Cancer Res. 2008, 6, 1268–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ermakova, S.P.; Kang, B.S.; Choi, B.Y.; Choi, H.S.; Schuster, T.F.; Ma, W.-Y.; Bode, A.M.; Dong, Z. (-)-Epigallocatechin gallate overcomes resistance to etoposide-induced cell death by targeting the molecular chaperone glucose-regulated protein 78. Cancer Res. 2006, 66, 9260–9269. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, P.; Liu, Y.; Yang, Y.; Ye, X.; Zhang, F.; Huang, H. Isoalantolactone induces apoptosis through ROS-mediated ER stress and inhibition of STAT3 in prostate cancer cells. J. Exp. Clin. Cancer Res. 2018, 37, 309. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zheng, Y.; Gu, J.; Wang, S.; Wang, N.; Yang, B.; Zhang, F.; Wang, D.; Fu, W.; Wang, Z. Betulinic acid chemosensitizes breast cancer by triggering ER stress-mediated apoptosis by directly targeting GRP78. Cell Death Dis. 2018, 9, 636. [Google Scholar] [CrossRef]
- Zhang, Q.-Y.; Wang, F.-X.; Jia, K.-K.; Kong, L.-D. Natural Product Interventions for Chemotherapy and Radiotherapy-Induced Side Effects. Front Pharmacol. 2018, 9, 1253. [Google Scholar] [CrossRef]
- Mazumder, A.; Cerella, C.; Diederich, M. Natural scaffolds in anticancer therapy and precision medicine. Biotechnol. Adv. 2018, 36, 1563–1585. [Google Scholar] [CrossRef] [PubMed]
- Vengoji, R.; Macha, M.A.; Batra, S.K.; Shonka, N.A. Natural products: A hope for glioblastoma patients. Oncotarget 2018, 9, 22194–22219. [Google Scholar] [CrossRef]
- Kumar, M.S.; Adki, K.M. Marine natural products for multi-targeted cancer treatment: A future insight. Biomed. Pharmacother. 2018, 105, 233–245. [Google Scholar] [CrossRef]
- Rietjens, I.M.C.M.; Louisse, J.; Beekmann, K. The potential health effects of dietary phytoestrogens. Br. J. Pharmacol. 2017, 174, 1263–1280. [Google Scholar] [CrossRef] [PubMed]
- Chikara, S.; Nagaprashantha, L.D.; Singhal, J.; Horne, D.; Awasthi, S.; Singhal, S.S. Oxidative stress and dietary phytochemicals: Role in cancer chemoprevention and treatment. Cancer Lett. 2018, 413, 122–134. [Google Scholar] [CrossRef] [PubMed]

| Compound | Tumor Type/Cell Line | ER Stress Signaling | Reference |
|---|---|---|---|
| Curcumin (Curcuma longa) | Human prostate cancer PC3 | ↑ IRE1α; BiP; PDI; calreticulin | [59] |
| Curcumin (Curcuma longa) | Murine Myeloma WEHI-3 | ↑ ATF6; CHOP; IRE1α; caspase-12 | [60] |
| Curcumin + sildenafil (Curcuma longa) | Human gastric, colon, liver cancer HC116; HT29; HEPG2 | ↑ pEIF2α; CHOP | [61] |
| Curcumin + irinotecan (Curcuma longa) | Human colorectal cancer HT29; LoVo | ↑ CHOP; PDI; BiP | [62] |
| Bisdemethoxycurcumin | Human lung cancer NCI H460 | ↑ BiP; IRE 1(α and β); CHOP; ATF6(α and β); caspase-4 | [63] |
| Demetoxycurcumin | Human lung cancer NCI H460 | ↑ BiP; IRE 1β; CHOP; ATF6(α and β); caspase-4 | [64] |
| B63 analogue of curcumin | Human colon cancer SW620 | ↑ ER stress markers | [65] |
| B19 analogue of curcumin | Human ovarian cancer A2780; CP70 | ↑ ROS; p-PERK; pEIF2α; CHOP | [66] |
| WZ35 analogue of curcumin | Human lung cancer HI975 | ↑ pEIF2α; ATF4; CHOP | [67] |
| MTH-3 analogue of curcumin | Human breast cancer MDA-MB-231 | ↑ CHOP; ERO1; PDI; PERK; calnexin ↓BiP | [68] |
| Compound | Tumor Type/Cell Line | ER Stress Signaling | Reference |
|---|---|---|---|
| Resveratrol | Human multiple myeloma ANBL-6; | ↑ IE1α; CHOP; JNK activation ↓XBP1s | [71] |
| Resveratrol | Human melanoma A375SM | ↑ pEIF2α; CHOP | [72] |
| Resveratrol + palmitate | Human hepatoblastoma HepG2 | ↑ ATF4; CHOP | [73] |
| Resveratrol | Human lung cancer NCI-H460 | ↑ CHOP; BiP | [74] |
| Resveratrol | Human nasopharyngeal cancer NPC-TW076; NPC-TW039 | ↑ IRE 1α; CHOP; ATF6α; p-PERK | [75] |
| Resveratrol | Human ovarian cancer Pa-1; MDAH2774; SKOV3 | ↑ PERK;CHOP; IRE 1α; ATF6α; BiP | [77] |
| Resveratrol + arsenic trioxide | Human lung cancer A549 | ↑ BiP; CHOP; caspase-12 | [76] |
| RES006 Resveratrol analog | Human hepatoblastoma HepG2 | ↑ pEIF2α; ATF4; CHOP | [81] |
| TMS Resveratrol analog | Human lung cancer A579; H1975 | ↑ pEIF2α; p-PERK CHOP; IRE1α; p-JNK | [79] |
| Compound | Tumor Type/Cell Line | ER Stress Signaling | Reference |
|---|---|---|---|
| Polyphenon E® | Human prostate cancer PC3 | ↑ CHOP | [84] |
| EGCG | Human mesothelioma MM98 | ↑ BiP; CHOP; ATF4; XBP1 | [86] |
| JP8 EGCG analog | Melanoma B16 | ↑ ATF4; CHOP | [87] |
| EGCG | Human bladder carcinoma T24/83 | ↑ Binding to BiP | [88] |
| Compound | Tumor Type/Cell Line | ER Stress Signaling | Reference |
|---|---|---|---|
| α-Tocopheryl succinate | Human gastric cancer SGC-7901 | ↑ BiP; CHOP; caspase-4; | [89] |
| γ-tocotrienol | Malignant +SA mammary epithelial cell line | ↑ p-PERK; p-EIF2α; ATF4; CHOP | [92] |
| γ-tocotrienol | Human breast cancer MDA-MB-231 | ↑ BiP; ATF4; CHOP; XBP1 | [93] |
| γ-tocotrienol | Human breast cancer MCF-7; MDA-MB-231 | ↑ PERK; p-EIF2α; ATF4; CHOP | [94] |
| γ-tocotrienol | Human breast cancer MCF-7; MDA-MB-231; MCF10A | ↑ p-PERK; p-EIF2α; ATF4; CHOP; TRB3 | [95] |
| γ-tocotrienol δ-tocotrienol | Human cervical and breast cancer HeLa; MCF-7 | ↑ p-IRE1α; XBP1s; CHOP; caspase-12 | [96] |
| δ-tocotrienol | Human melanoma BLM; A375 | ↑ BiP; CHOP; PERK; IRE1α; p-EIF2α; ATF4; CHOP; caspase-4 | [97] |
| Compound | Tumor Type /Cell Line | ER Stress Signalling | Reference |
|---|---|---|---|
| α-Mangosteen | Pheochromocytoma PC12 | ↓ Ca2+ ATPase activity; ↑ JNK | [100] |
| Mangosteen fruit extract | Human prostate cancer LNCaP; 22RV1 | ↑ BiP; PERK; IRE1α calnexin CHOP; caspase-4 | [102] |
| Garcinone-E | Human ovarian cancer HEY; A2780 | ↑ BiP; IRE1α; XBP1; CHOP; caspase-12 | [103] |
| Gartanin | Human prostate cancer LNCaP; 22RV1 | ↑ CHOP | [105] |
| Garcinol | Human hepatocellular carcinoma Hep3B | ↑ CHOP | [106] |
| Gambogic acid | Human cervical carcinoma HeLa | ↑ BiP; XBP1s; CHOP; GADD34; JNK | [109] |
| Compound | Tumor Type /Cell Line | ER Stress Signaling | Reference |
|---|---|---|---|
| Pimpinelol (Pimpinella haussknechtii) | Human breast cancer MCF-7 | ↑ ATF4; CHOP; GADD34; TRIB3 | [110] |
| Pristimerin (Maytenus sp) | Human breast cancer MCF-7 | ↑ ATF4; CHOP; IRE1α; pEIF2α | [111] |
| Cnidium officinale Makino | Human myeloid lymphoma U937; U266 | ↑ p-PERK; pEIF2α; ATF4; CHOP | [56] |
| Salvia Miltiorrhiza | Human myeloid lymphoma U937; U266 | ↑ p-PERK; pEIF2α, ATF4; CHOP | [112] |
| Protodioscin (Dioscoreae rhizome) | Human cervical cancer HeLa; C33A | ↑ BiP; p-PERK; pEIF2α, ATF4; CHOP; JNK | [114] |
| Paenia suffruticosa | Human pancreatic cancer PANC1; AsPC1; BxPC3 | ↑ DAPK3 | [115] |
| Clinacanthus nutans | Human lymphoma and leukemia SUP-T1; MOLT-4 | ↑ IRE1α; CHOP | [116] |
| Chrysophanol | Human breast cancer MCF-7; BT-474 | ↑ ROS; p-PERK; pEIF2α; CHOP | [118] |
| Garlic extract | Human multiple myeloma and human prostate cancer RPMI-8226; DU145 | ↑ BiP; MAPK kinases; RBX1; SKP1 | [121] |
| Ajoene (allyl sulfur compound from garlic) | Human breast cancer and human esophageal cancer MDA-MB-231; WHC1O | ↑ BiP; CHOP | [120] |
| 7-Acetylsinumaximol B (Sinularia sandensis) | Human gastric cancer NCI-N87 | ↑ p-PERK; pEIF2α; ATF4; CHOP; p-ATF6 | [122] |
| 4-nerolidylcatechol (Pothomorphe umbellata L) | Human melanoma SK-MEL-28; BRAi/MEKi SK-MEL-28 | ↑ p-PERK; IRE1α; BiP; ATF4; CHOP | [123] |
| PP-22 (Paris polyphilla) | Human nasopharyngeal carcinoma CNE-2 | ↑ PERK; CHOP; BiP; PDI; ERO-LA; IRE-LA | [124] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Limonta, P.; Moretti, R.M.; Marzagalli, M.; Fontana, F.; Raimondi, M.; Montagnani Marelli, M. Role of Endoplasmic Reticulum Stress in the Anticancer Activity of Natural Compounds. Int. J. Mol. Sci. 2019, 20, 961. https://doi.org/10.3390/ijms20040961
Limonta P, Moretti RM, Marzagalli M, Fontana F, Raimondi M, Montagnani Marelli M. Role of Endoplasmic Reticulum Stress in the Anticancer Activity of Natural Compounds. International Journal of Molecular Sciences. 2019; 20(4):961. https://doi.org/10.3390/ijms20040961
Chicago/Turabian StyleLimonta, Patrizia, Roberta M. Moretti, Monica Marzagalli, Fabrizio Fontana, Michela Raimondi, and Marina Montagnani Marelli. 2019. "Role of Endoplasmic Reticulum Stress in the Anticancer Activity of Natural Compounds" International Journal of Molecular Sciences 20, no. 4: 961. https://doi.org/10.3390/ijms20040961
APA StyleLimonta, P., Moretti, R. M., Marzagalli, M., Fontana, F., Raimondi, M., & Montagnani Marelli, M. (2019). Role of Endoplasmic Reticulum Stress in the Anticancer Activity of Natural Compounds. International Journal of Molecular Sciences, 20(4), 961. https://doi.org/10.3390/ijms20040961