Jumping on the Bandwagon: A Review on the Versatile Applications of Gold Nanostructures in Prostate Cancer
Abstract
:1. Introduction
2. Nanotechnology and Its Importance in Theranostics
3. Attractive Properties of Gold Nanoparticles
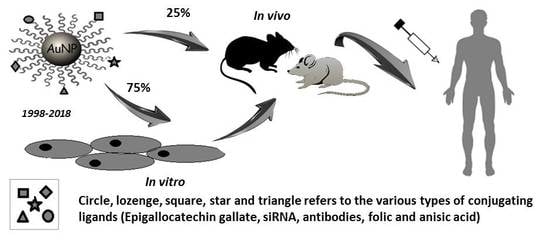
4. Arena of Gold Nanoparticles in PCa
4.1. Gold Nanoparticles for Diagnosis of PCa
4.1.1. In Vitro Applications
4.1.2. In Vivo Applications
4.2. Gold Nanoparticles for the Treatment of PCa
4.2.1. In Vitro Applications
4.2.2. In Vivo Applications
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Kumar, V.L.; Majumder, P.K. Prostate Gland: Structure, Functions and Regulation. Int. Urol. Nephrol. 1995, 27, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.L.; Wang, S.C.; Ho, C.J.; Kao, Y.L.; Hsieh, T.Y.; Chen, W.J.; Chen, C.J.; Wu, P.R.; Ko, J.L.; Lee, H.; et al. Prostate Cancer Mortality-To-Incidence Ratios Are Associated with Cancer Care Disparities in 35 Countries. Sci. Rep. 2017, 7, 40003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bubendorf, L.; Schöpfer, A.; Wagner, U.; Sauter, G.; Moch, H.; Willi, N.; Gasser, T.C.; Mihatsch, M.J. Metastatic patterns of prostate cancer: An autopsy study of 1,589 patients. Hum. Pathol. 2000, 31, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Mottet, N.; Bellmunt, J.; Briers, E.; Bergh, R.; Bolla, M.; Casteren, N.; Cornford, P.; Culine, S.; Joniau, S.; Lam, T. Guidelines on Prostate Cancer; European Association of Urology: Arnhemthe, The Netherlands, 2015; pp. 1–137. [Google Scholar]
- Eton, D.T.; Lepore, S.J. Prostate cancer and health-related quality of life: A review of the literature. Psychooncology 2002, 11, 307–326. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhu, X.; Lu, P.Y.; Rosato, R.R.; Tan, W.; Zu, Y. Oligonucleotide aptamers: New tools for targeted cancer therapy. Mol. Ther. Nucleic Acids 2014, 3, e182. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Gao, D.W.; Feng, J.; He, J.; Seo, Y.; Tedesco, J.; Wolodzko, J.G.; Hasegawa, B.H.; Franc, B.L. Biodistributions of 177Lu- and 111In-labeled 7E11 antibodies to prostate-specific membrane antigen in xenograft model of prostate cancer and potential use of 111In-7E11 as a pre-therapeutic agent for 177Lu-7E11 radioimmunotherapy. Mol. Imaging Biol. 2009, 11, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Van Rij, C.M.; Frielink, C.; Goldenberg, D.M.; Sharkey, R.M.; Lutje, S.; McBride, W.J.; Oyen, W.J.; Boerman, O.C. Pretargeted Radioimmunotherapy of Prostate Cancer with an Anti-TROP-2xAnti-HSG Bispecific Antibody and a (177)Lu-Labeled Peptide. Cancer Biother. Radiopharm. 2014, 29, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Elgqvist, J. Nanoparticles as Theranostic Vehicles in Experimental and Clinical Applications-Focus on Prostate and Breast Cancer. Int. J. Mol. Sci. 2017, 18, 1102. [Google Scholar] [CrossRef] [PubMed]
- Sanna, V.; Sechi, M. Nanoparticle therapeutics for prostate cancer treatment. Nanomedicine 2012, 8 (Suppl. 1), S31–S36. [Google Scholar] [CrossRef] [PubMed]
- European Commission; Health & Consumer Protection Directorate-General; Scientific Committee on Health and Environmental Risks. The Appropriateness of Existing Methodologies to Assess the Potential Risks Associated with Engineered and Adventitious Products of Nanotechnologies; Scientific Committee on Emerging and Newly Identified Health Risks, Directorate-General for Health & Consumers: Brussels, Belgium, 2006; Volume 2, p. 13. [Google Scholar]
- Thambiraj, S.; Hema, S.; Shankaran, D.R. An Overview on Applications of Gold Nanoparticle for Early Diagnosis and Targeted Drug Delivery to Prostate Cancer. Recent Pat. Nanotechnol. 2018, 12, 110–131. [Google Scholar] [CrossRef] [PubMed]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases 2007, 2, MR17–MR71. [Google Scholar] [CrossRef]
- Ahmed, N.; Fessi, H.; Elaissari, A. Theranostic applications of nanoparticles in cancer. Drug Discov. Today 2012, 17, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, L.Y.; Theek, B.; Storm, G.; Kiessling, F.; Lammers, T. Recent progress in nanomedicine: Therapeutic, diagnostic and theranostic applications. Curr. Opin. Biotechnol. 2013, 24, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Langut, Y.; Talhami, A.; Mamidi, S.; Shir, A.; Zigler, M.; Joubran, S.; Sagalov, A.; Flashner-Abramson, E.; Edinger, N.; Klein, S.; et al. PSMA-targeted polyinosine/polycytosine vector induces prostate tumor regression and invokes an antitumor immune response in mice. Proc. Natl. Acad. Sci. USA 2017, 114, 13655–13660. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Wang, H.; He, B.; Zeng, L.; Tan, T.; Cao, H.; He, X.; Zhang, Z.; Guo, S.; Li, Y. Current Approaches of Photothermal Therapy in Treating Cancer Metastasis with Nanotherapeutics. Theranostics 2016, 6, 762–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Zhen, Z.; Todd, T.; Chu, P.K.; Xie, J. Nanoparticles for Improving Cancer Diagnosis. Mater. Sci. Eng. R. Rep. 2013, 74, 35–69. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.; Saini, S.; Sharma, S. Nanotechnology: The future medicine. J. Cutan. Aesthet. Surg. 2010, 3, 32–33. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Rahman, T. The difficulties in cancer treatment. ecancermedicalscience 2012, 6, ed16. [Google Scholar] [PubMed]
- Patri, A.K.; Myc, A.; Beals, J.; Thomas, T.P.; Bander, N.H.; Baker, J.R., Jr. Synthesis and in Vitro Testing of J591 Antibody-Dendrimer Conjugates for Targeted Prostate Cancer Therapy. Bioconj. Chem. 2004, 15, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Kaittanis, C.; Bolaender, A.; Yoo, B.; Shah, N.; Ouerfelli, O.; Grimm, J. Targetable Clinical Nanoparticles for Precision Cancer Therapy Based on Disease-Specific Molecular Inflection Points. Nano Lett. 2017, 17, 7160–7168. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.R.; Singh, B.N.; Khan, W.; Singh, H.B.; Naqvi, A.H. ROS-mediated apoptotic cell death in prostate cancer LNCaP cells induced by biosurfactant stabilized CdS quantum dots. Biomaterials 2012, 33, 5753–5767. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, P.; Roberts, A.M.; Khan, S.; Hafeez, B.B.; Chauhan, S.C.; Jaggi, M.; Yallapu, M.M. Magnetic nanoformulations for prostate cancer. Drug Discov. Today 2017, 22, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Mijnendonckx, K.; Leys, N.; Mahillon, J.; Silver, S.; Van Houdt, R. Antimicrobial silver: Uses, toxicity and potential for resistance. Biometals 2013, 26, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Carabineiro, S.A.C. Applications of Gold Nanoparticles in Nanomedicine: Recent Advances in Vaccines. Molecules 2017, 22, 857. [Google Scholar] [CrossRef] [PubMed]
- Dykman, L.A.; Khlebtsov, L.G. Gold Nanoparticles in Biology and Medicine: Recent Advances and Prospects. Acta Nat. 2011, 3, 34–55. [Google Scholar]
- Yeh, Y.C.; Creran, B.; Rotello, V.M. Gold nanoparticles: Preparation, properties, and applications in bionanotechnology. Nanoscale 2012, 4, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Male, K.B.; Lachance, B.; Hrapovic, S.; Sunahara, G.; Luong, J.H. Assessment of cytotoxicity of quantum dots and gold nanoparticles using cell-based impedance spectroscopy. Anal. Chem. 2008, 80, 5487–5493. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Pelaz, B.; del Pino, P.; Carril, M.; Escudero, A.; Parak, W.J.; Soliman, M.G.; Zhang, Q.; Carrillo-Carrion, C. Gold-Based Nanomaterials for Applications in Nanomedicine. Top. Curr. Chem. 2016, 370, 169–202. [Google Scholar] [PubMed]
- Shuklaa, R.; Chandaa, N.; Kumar, S.N.; Kani, P.; Caldwellk, C.; Zambrea, A.; Casteele, S.W.; Engelbrechtg, H.; Upendranb, A.; Smitha, C.J.; et al. Laminin receptor specific therapeutic gold nanoparticles (198AuNP-EGCg) show efficacy in treating prostate cancer. Proc. Natl. Acad. Sci. USA 2012, 109, 12426–12431. [Google Scholar] [CrossRef] [PubMed]
- Malam, Y.; Loizidou, M.; Seifalian, A.M. Liposomes and nanoparticles: Nanosized vehicles for drug delivery in cancer. Trends Pharmacol. Sci. 2009, 30, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Cao-Milan, R.; Liz-Marzan, L.M. Gold nanoparticle conjugates: Recent advances toward clinical applications. Expert Opin. Drug Deliv. 2014, 11, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Delong, R.K.; Reynolds, C.M.; Malcolm, Y.; Schaeffer, A.; Severs, T.; Wanekaya, A. Functionalized gold nanoparticles for the binding, stabilization, and delivery of therapeutic DNA, RNA, and other biological macromolecules. Nanotechnol. Sci. Appl. 2010, 3, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.K.; Rashid, R.; Murtaza, G.; Zahra, A. Gold Nanoparticles: Synthesis and Applications in Drug Delivery. Trop. J. Pharm. Res. 2014, 13, 1169. [Google Scholar] [CrossRef] [Green Version]
- Mie, G. Beitrage zur Optik triiber Medien. Ann. Phys. 1908, 330, 377–445. [Google Scholar] [CrossRef]
- Eustis, S.; El-Sayed, M.A. Why gold nanoparticles are more precious than pretty gold: Noble metal surface plasmon resonance and its enhancement of the radiative and nonradiative properties of nanocrystals of different shapes. Chem. Soc. Rev. 2006, 35, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Review of Some Interesting Surface Plasmon Resonance-enhanced Properties of Noble Metal Nanoparticles and Their Applications to Biosystems. Plasmonics 2007, 2, 107–118. [Google Scholar] [CrossRef]
- Paul, B.; Tiwari, A. A Brief Review on the Application of Gold Nanoparticles as Sensors in Multi Dimensional Aspects. IOSR J. Environ. Sci. Toxicol. Food Technol. (IOSR-JESTFT) 2015, 1, 1–7. [Google Scholar]
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated Absorption and Scattering Properties of Gold Nanoparticles of Different Size, Shape, and Composition: Applications in Biological Imaging and Biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahan, M.M.; Doiron, A.L. Gold Nanoparticles as X-Ray, CT, and Multimodal Imaging Contrast Agents: Formulation, Targeting, and Methodology. J. Nanomater. 2018, 2018, 1–15. [Google Scholar] [CrossRef] [Green Version]
- El-Sayed, M.A. Some Interesting Properties of Metals Confined in Time and Nanometer Space of Different Shapes. Acc. Chem. Res. 2001, 34, 257–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nolsøe, C.P.; Torp-Pedersen, S.; Burcharth, F.; Horn, T.; Pedersen, S.; Christensen, N.E.; Lorentzen, T. Interstitial hyperthermia of colorectal liver metastases with a US-guided Nd-YAG laser with a diffuser tip: A pilot clinical study. Radiol. Radiol. 1993, 187, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Lilja, H.; Ulmert, D.; Vickers, A.J. Prostate-specific antigen and prostate cancer: Prediction, detection and monitoring. Nat. Rev. Cancer 2008, 8, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.; Torres, C.V.; Ahumada, O.; Cebrián, V.; Gómez-Abad, C.; Díaz, A.; Manso, S.M. Gold nanoparticle triggered dual optoplasmonic-impedimetric sensing of prostate-specific antigen on interdigitated porous silicon platforms. Sens. Actuators B Chem. 2018, 267, 559–564. [Google Scholar] [CrossRef]
- Barbosa, A.I.; Wichers, J.H.; van Amerongen, A.; Reis, N.M. Towards One-Step Quantitation of Prostate-Specific Antigen (PSA) in Microfluidic Devices: Feasibility of Optical Detection with Nanoparticle Labels. BioNanoSci 2017, 7, 718–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pal, M.; Khan, K. Detection of prostate cancer risk factor immunosensor based deposition of graphene layer decorated gold nanoparticles. Anal. Biochem. 2017. [Google Scholar] [CrossRef] [PubMed]
- Suresh, L.; Brahman, P.K.; Reddy, K.R.; Bondili, J.S. Development of an electrochemical immunosensor based on gold nanoparticles incorporated chitosan biopolymer nanocomposite film for the detection of prostate cancer using PSA as biomarker. Enzym. Microb Technol. 2018, 112, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Vural, T.; Yaman, Y.T.; Ozturk, S.; Abaci, S.; Denkbas, E.B. Electrochemical immunoassay for detection of prostate specific antigen based on peptide nanotube-gold nanoparticle-polyaniline immobilized pencil graphite electrode. J. Colloid Interface Sci. 2018, 510, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, M.; Nirala, N.R.; Srivastava, S.K.; Prakash, R. A comparative Study of Aptasensor Vs Immunosensor for Label-Free PSA Cancer Detection on GQDs-AuNRs Modified Screen-Printed Electrodes. Sci. Rep. 2018, 8, 1923. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Reekmans, G.; Saerens, D.; Friedt, J.M.; Frederix, F.; Francis, L.; Muyldermans, S.; Campitelli, A.; Van Hoof, C. Prostate-specific antigen immunosensing based on mixed self-assembled monolayers, camel antibodies and colloidal gold enhanced sandwich assays. Biosens. Bioelectron. 2005, 21, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Harmsen, S.; Wall, M.A.; Huang, R.; Kircher, M.F. Cancer imaging using surface-enhanced resonance Raman scattering nanoparticles. Nat. Protocol. 2017, 12, 1400–1414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sattarahmady, N.; Rahi, A.; Heli, H. A signal-on built in-marker electrochemical aptasensor for human prostate-specific antigen based on a hairbrush-like gold nanostructure. Sci. Rep. 2017, 7, 11238. [Google Scholar] [CrossRef] [PubMed]
- Kasten, B.B.; Liu, T.; Nedrow-Byers, J.R.; Benny, P.D.; Berkman, C.E. Targeting prostate cancer cells with PSMA inhibitor-guided gold nanoparticles. Bioorg. Med. Chem. Lett. 2013, 23, 565–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norouzi, M.; Nazari, B.; Miller, D.W. Electrospun-Based Systems in Cancer Therapy; Woodhead Publishing: Sawston, UK, 2017; pp. 337–356. [Google Scholar]
- Oh, M.H.; Yu, J.H.; Kim, I.; Nam, Y.S. Genetically Programmed Clusters of Gold Nanoparticles for Cancer Cell-Targeted Photothermal Therapy. ACS Appl. Mater. Interfaces 2015, 7, 22578–22586. [Google Scholar] [CrossRef] [PubMed]
- Mayle, K.M.; Dern, K.R.; Wong, V.K.; Chen, K.Y.; Sung, S.; Ding, K.; Rodriguez, A.R.; Knowles, S.; Taylor, Z.; Zhou, Z.H. Engineering A11 Minibody-Conjugated,Polypeptide-Based Gold Nanoshells for Prostate Stem Cell Antigen (PSCA)–Targeted Photothermal Therapy. J. Lab. Autom. 2016, 22, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Tsai, L.-C.; et al. EGCG/gelatin-doxorubicin gold nanoparticles enhance therapeutic efficacy of doxorubicin for prostate cancer treatment. Nanomedicine (Lond.) 2016, 11, 9–30. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; O’Driscoll, C.M.; Holmes, J.D.; Rahme, K. Bioconjugated gold nanoparticles enhance cellular uptake: A proof of concept study for siRNA delivery in prostate cancer cells. Int. J. Pharm. 2016, 509, 16–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitzgerald, K.A.; Rahme, K.; Guo, J.; Holmes, J.D.; O’Driscoll, C.M. Anisamide-targeted gold nanoparticles for siRNA delivery in prostate cancer—Synthesis, physicochemical characterisation and in vitro evaluation. J. Mater. Chem. B 2016, 4, 2242–2252. [Google Scholar] [CrossRef]
- hukla, S.; MacLennan, G.T.; Fu, P.; Patel, J.; Marengo, S.R.; Resnick, M.I.; Gupta, S. Nuclear factor-kappaB/p65 (Rel A) is constitutively activated in human prostate adenocarcinoma and correlates with disease progression. Neoplasia 2004, 6, 390–400. [Google Scholar]
- Gannon, P.O.; Lessard, L.; Stevens, L.M.; Forest, V.; Begin, L.R.; Minner, S.; Tennstedt, P.; Schlomm, T.; Mes-Masson, A.M.; Saad, F. Large-scale independent validation of the nuclear factor-kappa B p65 prognostic biomarker in prostate cancer. Eur. J. Cancer 2013, 49, 2441–2448. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Na, K.Y.; Lee, K.H.; Lee, H.W.; Lee, J.K.; Kim, K.T. Selective uptake of epidermal growth factor-conjugated gold nanoparticle (EGF-GNP) facilitates non-thermal plasma (NTP)-mediated cell death. Sci. Rep. 2017, 7, 10971. [Google Scholar] [CrossRef] [PubMed]
- Al-Yasiri, A.Y.; Khoobchandani, M.; Cutler, C.S.; Watkinson, L.; Carmack, T.; Smith, C.J.; Kuchuk, M.; Loyalka, S.K.; Lugao, A.B.; Katti, K.V. Mangiferin functionalized radioactive gold nanoparticles (MGF-(198)AuNPs) in prostate tumor therapy: Green nanotechnology for production, in vivo tumor retention and evaluation of therapeutic efficacy. Dalton Trans. 2017, 46, 14561–14571. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Li, K.; Mao, Y.-H.; Qu, H.; Yao, B.; Zhong, W.-W.; Ma, B.; Wang, Z.-Y. Gold-chrysophanol nanoparticles suppress human prostate cancer progression through inactivating AKT expression and inducing apoptosis and ROS generation in vitro and in vivo. Int. J. Oncol. 2017, 51, 1089–1103. [Google Scholar] [CrossRef] [PubMed]
- Lechtman, E.; Pignol, J.P. Interplay between the gold nanoparticle sub-cellular localization, size, and the photon energy for radiosensitization. Sci. Rep. 2017, 7, 13268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khoo, A.M.; Cho, S.H.; Reynoso, F.J.; Aliru, M.; Aziz, K.; Bodd, M.; Yang, X.; Ahmed, M.F.; Yasar, S.; Manohar, N.; et al. Radiosensitization of Prostate Cancers In Vitro and In Vivo to Erbium-filtered Orthovoltage X-rays Using Actively Targeted Gold Nanoparticles. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Giljohann, D.A.; Seferos, D.S.; Daniel, W.L.; Massich, M.D.; Patel, P.C.; Mirkin, C.A. Gold nanoparticles for biology and medicine. Angew. Chem. Int. Ed. Engl. 2010, 49, 3280–3294. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Kuang, H.; Zhang, W.; Aguilar, Z.P.; Wei, H.; Xu, H. Comparisons of the biodistribution and toxicological examinations after repeated intravenous administration of silver and gold nanoparticles in mice. Sci. Rep. 2017, 7, 3303. [Google Scholar] [CrossRef] [PubMed]
- Gaillet, S.; Rouanet, J.M. Silver nanoparticles: Their potential toxic effects after oral exposure and underlying mechanisms—A review. Food Chem. Toxicol. 2015, 77, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Johnston, H.J.; Hutchison, G.; Christensen, F.M.; Peters, S.; Hankin, S.; Stone, V. A review of the in vivo and in vitro toxicity of silver and gold particulates: Particle attributes and biological mechanisms responsible for the observed toxicity. Crit. Rev. Toxicol. 2010, 40, 328–346. [Google Scholar] [CrossRef] [PubMed]
- Bednarski, M.; Dudek, M.; Knutelska, J.; Nowinski, L.; Sapa, J.; Zygmunt, M.; Nowak, G.; Luty-Blocho, M.; Wojnicki, M.; Fitzner, K.; et al. The influence of the route of administration of gold nanoparticles on their tissue distribution and basic biochemical parameters: In vivo studies. Pharmacol. Rep. 2015, 67, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.P.M.; Chen, A.L.; Foster, A.; Drezek, R. In vivo biodistribution of nanoparticles. Nanomedicine 2011, 6, 815–835. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S. Nanoparticles Types, Classification, Characterization, Fabrication Methods and Drug Delivery Applications; Springer: Berlin, Germnay, 2016; pp. 33–93. [Google Scholar]
- Yu, Q.; Xiong, X.Q.; Zhao, L.; Xu, T.T.; Bi, H.; Fu, R.; Wang, Q.H. Biodistribution and Toxicity Assessment of Superparamagnetic Iron Oxide Nanoparticles In Vitro and In Vivo. Curr. Med. Sci. 2018, 38, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.L.; Kai, M.P.; DuRoss, A.N.; Sahay, G.; Sun, C. Biodistribution and Toxicity of Micellar Platinum Nanoparticles in Mice via Intravenous Administration. Nanomaterials (Basel) 2018, 8, 410. [Google Scholar] [CrossRef] [PubMed]
- E Elder, A.; Yang, H.; Gwiazda, R.; Teng, X.; Thurston, S.; He, H.; Oberdörster, G. Testing Nanomaterials of Unknown Toxicity: An Example Based on Platinum Nanoparticles of Different Shapes. Adv. Mater. 2007, 19, 3124–3129. [Google Scholar] [CrossRef]
- Gelli, K.; Porika, M.; Anreddy, R.N. Assessment of pulmonary toxicity of MgO nanoparticles in rats. Environ. Toxicol. 2015, 30, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Kovriznych, J.A.; Sotnikova, R.; Zeljenkova, D.; Rollerova, E.; Szabova, E.; Wimmerova, S. Acute toxicity of 31 different nanoparticles to zebrafish (Danio rerio) tested in adulthood and in early life stages—Comparative study. Interdiscip. Toxicol. 2013, 6, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Mangalampalli, B.; Dumala, N.; Perumalla Venkata, R.; Grover, P. Genotoxicity, biochemical, and biodistribution studies of magnesium oxide nano and microparticles in albino wistar rats after 28-day repeated oral exposure. Environ. Toxicol. 2018, 33, 396–410. [Google Scholar] [CrossRef] [PubMed]
- Wissing, S.A.; Kayser, O.; Müller, R.H. Solid lipid nanoparticles for parenteral drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 1257–1272. [Google Scholar] [CrossRef] [PubMed]
- Fundaro, A.; Cavalli, R.; Bargoni, A.; Vighetto, D.; Zara, G.P.; Gasco, M.R. Non-stealth and stealth solid lipid nanoparticles (SLN) carrying doxorubicin: Pharmacokinetics and tissue distribution after i.v. administration to rats. Pharmacol. Res. 2000, 42, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Firme, C.P., 3rd; Bandaru, P.R. Toxicity issues in the application of carbon nanotubes to biological systems. Nanomedicine 2010, 6, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Johnston, H.J.; Hutchison, G.R.; Christensen, F.M.; Peters, S.; Hankin, S.; Aschberger, K.; Stone, V. A critical review of the biological mechanisms underlying the in vivo and in vitro toxicity of carbon nanotubes: The contribution of physico-chemical characteristics. Nanotoxicology 2010, 4, 207–246. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Diagaradjane, P.; Cho, S.H. Nanoparticle-mediated thermal therapy: Evolving strategies for prostate cancer therapy. Int. J. Hyperth. 2010, 26, 775–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Q.; Zhang, Z.; Gao, F.; Li, Y.; Shi, J. In vivo biodistribution and urinary excretion of mesoporous silica nanoparticles: Effects of particle size and PEGylation. Small 2011, 7, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Liong, M.; Sherman, S.; Xia, T.; Kovochich, M.; Nel, A.E.; Zink, J.I.; Tamanoi, F. Mesoporous Silica Nanoparticles for Cancer Therapy: Energy-Dependent ellular Uptake and Delivery of Paclitaxel to Cancer Cells. Nanobiotechnology 2007, 3, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Liu, T.; Li, L.; Liu, H.; Chen, D.; Tang, F. The absorption, distribution, excretion and toxicity of mesoporous silica nanoparticles in mice following different exposure routes. Biomaterials 2013, 34, 2565–2575. [Google Scholar] [CrossRef] [PubMed]
- Alexis, F.; et al. Factors Affecting the Clearance and Biodistribution of Polymeric Nanoparticles. Mol. Pharm. 2019, 5, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Alexis, F.; Pridgen, E.M.; Langer, R.; Farokhzad, O.C. Nanoparticle Technologies for Cancer Therapy. In Handbook of Experimental Pharmacology; Springer: Berlin, Germany, 2010; pp. 55–86. [Google Scholar]
- Zheng, T.; Pierre-Pierre, N.; Yan, X.; Huo, Q.; Almodovar, A.J.; Valerio, F.; Rivera-Ramirez, I.; Griffith, E.; Decker, D.D.; Chen, S.; et al. Gold nanoparticle-enabled blood test for early stage cancer detection and risk assessment. ACS Appl. Mater. Interfaces 2015, 7, 6819–6827. [Google Scholar] [CrossRef] [PubMed]
- Butterworth, K.T.; Nicol, J.R.; Ghita, M.; Rosa, S.; Chaudhary, P.; McGarry, C.K.; McCarthy, H.O.; Jimenez-Sanchez, G.; Bazzi, R.; Roux, S. Preclinical evaluation of gold-DTDTPA nanoparticles as theranostic agents in prostate cancer radiotherapy. Nanomedicine 2016, 11, 2035–2047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangadlao, J.D.; Wang, X.; McCleese, C.; Escamilla, M.; Ramamurthy, G.; Wang, Z.; Govande, M.; Basilion, J.P.; Burda, C. Prostate-Specific Membrane Antigen Targeted Gold Nanoparticles for Theranostics of Prostate Cancer. ACS Nano 2018, 12, 3714–3725. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.; Gerlach, W.; Ghandehari, H. Comparative effect of gold nanorods and nanocages for prostate tumor hyperthermia. J. Control. Release 2015, 220 Pt A, 245–252. [Google Scholar] [CrossRef]
- Agarwal, A.; Huang, S.W.; O’Donnell, M.; Day, K.C.; Day, M.; Kotov, N.; Ashkenazi, S. Targeted gold nanorod contrast agent for prostate cancer detection by photoacoustic imaging. J. Appl. Phys. 2007, 102, 064701. [Google Scholar] [CrossRef]
- Min, K.H.; Kim, Y.H.; Wang, Z.; Kim, J.; Kim, J.S.; Kim, S.H.; Kim, K.; Kwon, I.C.; Kiesewetter, D.O.; Chen, X. Engineered Zn(II)-Dipicolylamine-Gold Nanorod Provides Effective Prostate Cancer Treatment by Combining siRNA Delivery and Photothermal Therapy. Theranostics 2017, 7, 4240–4254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avvakumova, S.; Galbiati, E.; Sironi, L.; Locarno, S.A.; Gambini, L.; Macchi, C.; Pandolfi, L.; Ruscica, M.; Magni, P.; Collini, M.; et al. Theranostic Nanocages for Imaging and Photothermal Therapy of Prostate Cancer Cells by Active Targeting of Neuropeptide-Y Receptor. Bioconj. Chem. 2016, 27, 2911–2922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, S.A.; Kanchanapally, R.; Fan, Z.; Beqa, L.; Singh, A.K.; Senapati, D.; Ray, P.C. A gold nanocage-CNT hybrid for targeted imaging and photothermal destruction of cancer cells. Chem. Commun. (Camb.) 2012, 48, 6711–6713. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.M.; Stanfield, J.; Kabbani, W.; Hsieh, J.T.; Cadeddu, J.A. Selective prostate cancer thermal ablation with laser activated gold nanoshells. J. Urol. 2008, 179, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Gobin, A.M.; Moon, J.J.; West, J.L. EphrinA1-targeted nanoshells for photothermal ablation of prostate cancer cells. Int. J. Nanomed. 2008, 3, 351–358. [Google Scholar]
- Stern, J.M.; Kibanov Solomonov, V.V.; Sazykina, E.; Schwartz, J.A.; Gad, S.C.; Goodrich, G.P. Initial Evaluation of the Safety of Nanoshell-Directed Photothermal Therapy in the Treatment of Prostate Disease. Int. J. Toxicol. 2016, 35, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.; Youn, H.; Lee, S.; Ban, C. Ultra-effective photothermal therapy for prostate cancer cells using dual aptamer-modified gold nanostars. J. Mater. Chem. B 2014, 2, 4862–4867. [Google Scholar] [CrossRef] [Green Version]
- Espinosa, A.; Silva, A.K.; Sanchez-Iglesias, A.; Grzelczak, M.; Pechoux, C.; Desboeufs, K.; Liz-Marzan, L.M.; Wilhelm, C. Cancer Cell Internalization of Gold Nanostars Impacts Their Photothermal Efficiency In Vitro and In Vivo: Toward a Plasmonic Thermal Fingerprint in Tumoral Environment. Adv. Healthc. Mater. 2016, 5, 1040–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raghavan, V.; O’Flatharta, C.; Dwyer, R.; Breathnach, A.; Zafar, H.; Dockery, P.; Wheatley, A.; Keogh, I.; Leahy, M.; Olivo, M. Dual plasmonic gold nanostars for photoacoustic imaging and photothermal therapy. Nanomedicine (Lond.) 2017, 12, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Singh, A.K.; Khan, S.A.; Senapati, D.; Yu, H.; Ray, P.C. Gold nano-popcorn-based targeted diagnosis, nanotherapy treatment, and in situ monitoring of photothermal therapy response of prostate cancer cells using surface-enhanced Raman spectroscopy. J. Am. Chem. Soc. 2010, 132, 18103–18114. [Google Scholar] [CrossRef] [PubMed]





| Type of NP | Biodistribution and Accumulation | Toxicity | Efficacy of Treatment on PCa | References |
|---|---|---|---|---|
| Silver | Heart, Lung, Kidney, Liver and Spleen | Size Dependent: dose <10 mg kg−1 is safe, while it is toxic when a dose over 20 mg kg−1 is adminstered | Moderately to Highly effective (depends on the coating and targeting ligand) | [69,70,71] |
| Gold | Kidney, Heart, Brain (Size dependant <20nm), Spleen, and Liver (Highest accumulation) | Size, Shape, and Surface coating Dependent for ex: < 50nm and Neutral charged colloidal AuNPs are non-toxic | Highly Effective (Many Modalities exist to induce targeted killing with little or no side effects) | [69,72,73] |
| Quantum Dots | Kidney, Liver, and Spleen | Low Toxicity (due to incorporation of heavy metals) | Not Applicable (mostly used as biosensors) | [73,74] |
| Iron Oxide | Liver and Spleen | Low to non-toxic (based on surface charge and coating) | Moderately Effective | [73,75] |
| Platinum | Liver, Spleen, Kidney, and Lungs | Non- toxic | Not Applicable (only for PSA quantification) | [76,77] |
| Magnesium Oxide | Liver, Spleen, Stomach, Kidney Brain | Dose-dependent toxicity | Not Applicable | [78,79,80] |
| Solid lipid | Liver, Heart, Kidney, and Spleen | Non-toxic (must be stabilized by surfactants to form administrable emulsions) | Moderately Effective | [32,81,82] |
| Carbon | Liver, Spleen, bladder, Intestine | Dose and Route of Administration dependent toxicity | Moderately to Highly Effective (depends on method of treatment) | [83,84,85] |
| Mesoporous Silica | Liver and Spleen | Non-toxic | Moderately Effective | [86,87,88] |
| Polymer-based | Liver, Spleen, and Kidney | Low toxicity (based on surface charge) | Highly Effective | [74,89,90] |
 Various shapes of gold nanostructures used in prostate cancer, some examples are listed below | |||
| Gold Nanostructures/Formulation | Size | Application in Prostate Cancer | Reference |
| AuNPs-Citrate nanospheres | 100 nm | Early stage detection in blood | [91] |
| AuNPs-Epigallocatechin gallate and gelatin doxorubicin | 10–85 nm | DOX release and fluorescence imaging | [58] |
| AuNPs-Dithiolated diethylenetriamine pentaacetic acid | 5.37 nm | Radiotherapy | [92] |
| AuNPs-PEI-siRNA-Anisamide | 8–50 nm | Targeting and gene knockdown | [60] |
| AuNP-5kPEG-PSMA-1-Pc4 | 5–25 nm | Targeting and fluorescent photodynamic therapy (PDT) | [93] |
| AuNRs-Polyethyleneglycol 5KD | 60 × 14.8 nm | Plasmonic photothermal therapy (PPTT) | [94] |
| AuNRs-antibody (Ab-17) | 45 × 15 nm | detection by photoacoustic imaging | [95] |
| AuNRs-Zn(II)-Dipicolylamine-siRNA | 84.1 ± 8.6 nm | siRNA Delivery and PPTT | [96] |
| AuNcgs-Polyethyleneglycol 5KD | 50 nm | Plasmonic PPTT | [94] |
| AuNcgs-Peptides | 40-50 nm | Imaging and PPTT | [97] |
| AuNcgs-CNT hybrid-para-aminothiophenol | — | targeted imaging and PPTT | [98] |
| AuNShs-Polyethyleneglycol | 110 nm silica–10 nm Au shell | Thermal ablation with laser | [99] |
| AuNShs-PEG-EphrinA1 | 98–112 nm silica 2–4 nm Au shell | targeted PPTT | [100] |
| AuNShs | — | Clinical safety profile in human patients | [101] |
| AuNS-PEG-A10-DUP-1 aptamers | 61.9 nm | Ultra-Effective Photothermal Therapy | [102] |
| AuNS- citrate- polyvinylpyrrolidone | 25, 85, 150 nm | Photothermal Therapy | [103] |
| AuNS-PEG and AuNS@SiO2 | 100 nm | photoacoustic imaging and PPTT | [104] |
| Nano-popcorn -shaped AuNPs. | 4.3–28 nm | diagnosis in LNCaP by surface-enhanced Raman scattering (SERS) an PPTT | [105] |
| Type of AuNPs | Application | Study Type | Diagnosis/Treatment | Target | Result | Reference |
|---|---|---|---|---|---|---|
| anti-PSA camel antibody coated to streptavidin coated AuNPs | A PSA sandwich modified biosensor was used and quantification was done using a surface plasmon resonance instrument. | In vitro | Diagnosis | Not applicable | Major enhancement in sensitivity of PSA detection was observed with a limit of detection as low as 1 ng/mL. | [51] |
| (EGCg) tagged 198AuNPs | PC3-xenograft SCID mice /Intratumorally | In vivo | Treatment | Laminin receptors | 80% reduction of tumor volumes after 28 days | [31] |
| AuNP- biotin-PEG12-CTT54 inhibitor | Prostate cancer cells were targeted with PSMA inhibitor (CTT54)-guided gold NPs. | In vitro | Treatment | PSMA receptor | Higher and selective binding to LNCaP cells compared to control non-targeted AuNPs in a time-dependent manner. | [39] |
| Phage-AuNP | PC3-cells | In vitro | Treatment | PSMA receptors | Target specific photothermal therapy | [56] |
| AuNPs-PEG-Tf/AuNPs-PEI-FA.siRNA | LNCap cells /PC3-cells | In vitro | Treatment | Transferrin and Folate receptors | Cellular uptake and non-cytotoxicity of the AuNPs-PEG-Tf was observed. RelA gene silencing after 24 h was observed for AuNPs-PEI-FA.siRNA. | [59] |
| EGCG-AuNPs.DOX | PC3-cells | In vitro | Treatment | Laminin Receptors | Enhanced receptor mediated endocytosis and induction of apoptosis after 24 h | [58] |
| Au@DTDTPA | CT-contrast imaging and radiotherapy in PC3, DU 145, PNT2-C2 cells, and Human PC3 xenograft tumor models. | In vitro | Treatment and Diagnosis | Not applicable | 10 % CT imaging enhancement, increased cytotoxicity after 24 h exposure to the NPs, and tumor growth delay of 17 days. | [92] |
| A11 minibody-conjugated to a gold nanoshell | Photothermal therapy on PSCA-transfected 22Rv1 prostate cancer cells | In vitro | Treatment | PSCA receptor | Enhanced localized killing of prostate cancer cells compared to nontargeted gold nanoshells. | [57] |
| GF- 198AuNP | CF-1 mice/intratumoral | In vivo | Treatment | Laminin receptors | 80% retention of the injected dose (ID) in prostate tumors after 24 h. By three weeks post treatment, over 5 fold reduction of tumor | [64] |
| Chrysophanol-AuNPs | LNCap/PC3/DU 145 | In vitro | Treatment | Not Applicable | Inactivating AKT expression and inducing apoptosis and ROS generation. | [65] |
| Silver enhanced AuNPs | microfluidic immunoassay precoated with CapAband layered with immobilized gold NPs. | In vitro | Diagnosis | Not applicable | PSA limit of detection range from 10 to 100 ng/mL. | [46] |
| Au-GrO | Au-GrO on platinum electrode, immobilized with anti PSA | In vitro | Diagnosis | Not applicable | Immunosensor had a PSA limit of detection of 0.24 fg/mL. | [47] |
| AuNPs encapsulated with a silica shell | Injected intratumorally in Hi-Myc mouse | In vivo | Diagnosis | Not applicable | Highly sensitive tumor detection with contrast-enhanced raman imaging | [52] |
| Hairbrush-like gold nanostructure | NPs as transducers to fabricate a signal-on built in-marker electrochemical aptasensor for the detection of PSA | In vitro | Diagnosis | Not applicable | The aptasensor detected PSA with a limit of detection of 50 pg mL−1. | [53] |
| EGF-GNP | DU 145 cells | In vitro | Treatment | EGFR receptor | NTP irradiation showed selective apoptosis of cells that have undergone receptor mediated endocytosis. | [63] |
| gGNRs | Radiotherapy to X-rays using actively targeted gGNRs; applied to mice bearing PC3-xenograft tumors and to PC3 cells | In vitro | Treatment | Not applicable | 50% reduction in tumor volume after 2 months of treatment. | [67] |
| Chitosan-AuNP | A sandwich-type electrochemical immunosensor using anti-PSA was designed for detecting PSA. | In vitro | Diagnosis | Not applicable | The fabricated immunosensor demonstrated excellent sensitivity, stability, and a detection limit of 0.001 ng/mL. | [48] |
| PANI/AuNP-PNT | Anti-PSA Ab immobilized on modified PANI/AuNP-PNT pencil graphite electrode with HRP-anti PSA antibody to form sandwich immunoassay | In vitro | Diagnosis | Not applicable | Limit of detection was found out to be 0.68 ng/mL | [49] |
| PSi-GNP | PSA was immobilized at different concentrations on the surface of the sandwich bioassay (NiCr electrode). | In vitro | Diagnosis | Not applicable | Enhanced PSA sensitivity with a limit of detection at 1 ng/mL | [45] |
| GQDs-AuNRs | Standard PSA solutions were used. NPs immobilized on electrodes tested for efficiency (Anti-PSA.GQDs-AuNRs vs. aptamer-GQDs-AuNRs). | In vitro | Diagnosis | Not applicable | Both had same limit of detection (LOD) of 0.14 ng/mL. The aptasensor advantages over the immunosensor were the stability, simplicity, cost effectiveness. | [50] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarkis, M.; Ghanem, E.; Rahme, K. Jumping on the Bandwagon: A Review on the Versatile Applications of Gold Nanostructures in Prostate Cancer. Int. J. Mol. Sci. 2019, 20, 970. https://doi.org/10.3390/ijms20040970
Sarkis M, Ghanem E, Rahme K. Jumping on the Bandwagon: A Review on the Versatile Applications of Gold Nanostructures in Prostate Cancer. International Journal of Molecular Sciences. 2019; 20(4):970. https://doi.org/10.3390/ijms20040970
Chicago/Turabian StyleSarkis, Monira, Esther Ghanem, and Kamil Rahme. 2019. "Jumping on the Bandwagon: A Review on the Versatile Applications of Gold Nanostructures in Prostate Cancer" International Journal of Molecular Sciences 20, no. 4: 970. https://doi.org/10.3390/ijms20040970
APA StyleSarkis, M., Ghanem, E., & Rahme, K. (2019). Jumping on the Bandwagon: A Review on the Versatile Applications of Gold Nanostructures in Prostate Cancer. International Journal of Molecular Sciences, 20(4), 970. https://doi.org/10.3390/ijms20040970