Combining Mechanochemistry and Spray Congealing for New Praziquantel Pediatric Formulations in Schistosomiasis Treatment
Abstract
:1. Introduction
2. Results and Discussion
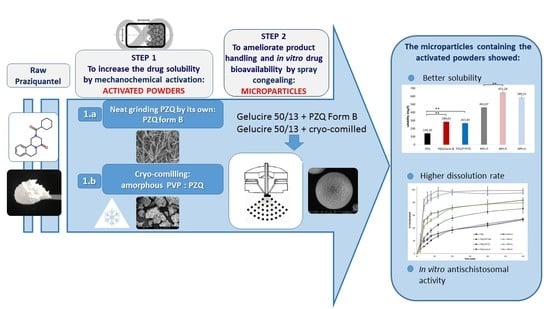
2.1. Analysis of the Activated Materials
2.2. Evaluation of the Activated PZQ-Loaded Spray-Congealed Microparticles
3. Material and Methods
3.1. Materials
3.2. Preparation of Activated Materials by Neat Grinding
3.3. Preparation of Microparticles by Spray Congealing
3.4. HPLC Analysis
3.5. Solubility and Dissolution Studies
3.6. Wettability Studies
3.7. Viscosity Measurements
3.8. Scanning Electron Microscopy (SEM)
3.9. Environmental Scanning Electron Microscopy (ESEM)
3.10. Particle Size Analysis
3.11. Differential Scanning Calorimetry (DSC) Studies
3.12. Hot Stage Microscopy (HSM) Analysis
3.13. X-Ray Powder Diffraction Studies (PXRD)
3.14. Fourier Transform-Infrared Spectra (FT-IR) Analysis
3.15. Physical Stability During Storage
3.16. In Vitro Studies on S. Mansoni
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bergquist, R.; Utzinger, J.; Keiser, J. Controlling schistosomiasis with praziquantel: How much longer without a viable alternative? Infect. Dis. Poverty 2017, 6, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, L.D.P.; Fontes, D.A.F.; Aguilera, C.S.B.; Timóteo, T.R.R.; Ângelos, M.A.; Silva, L.C.P.B.B.; de Melo, C.G.; Rolim, L.A.; da Silva, R.M.F.; Neto, P.J.R. Schistosomiasis: Drugs used and treatment strategies. Acta Trop. 2017, 176, 179–187. [Google Scholar] [CrossRef]
- Utzinger, J.; N’Goran, E.K.; Caffrey, C.R.; Keiser, J. From innovation to application: Social-ecological context, diagnostics, drugs and integrated control of schistosomiasis. Acta Trop. 2011, 120, S121–S137. [Google Scholar] [CrossRef] [PubMed]
- Colley, D.G.; Bustinduy, A.L.; Secor, W.E.; King, C.H. Human schistosomiasis. Lancet 2014, 383, 2253–2264. [Google Scholar] [CrossRef] [Green Version]
- da Silva, V.B.R.; Campos, B.R.K.L.; de Oliveira, J.F.; Decout, J.L.; do Carmo Alves de Lima, M. Medicinal chemistry of antischistosomal drugs: Praziquantel and Oxamniquine. Bioorg. Med. Chem. 2017, 25, 3259–3277. [Google Scholar] [CrossRef] [PubMed]
- WHO. Model List of Essential Medicines for Children, 6th ed. March 2017. Available online: http://www.who.int/medicines/publications/essentialmedicines/6th_EMLc2017_FINAL_amendedAug2017.pdf?ua=1 (accessed on 11 June 2018).
- Trastullo, R.; Dolci, L.S.; Passerini, N.; Albertini, B. Development of flexible and dispersible oral formulations containing praziquantel for potential schistosomiasis treatment of pre-school age children. Int. J. Pharm. 2015, 495, 536–550. [Google Scholar] [CrossRef] [PubMed]
- Olliaro, P.L.; Vaillant, M.; Hayes, D.J.; Montresor, A.; Chitsulo, L. Practical dosing of praziquantel for schistosomiasis in preschool-aged children. TMIH 2013, 18, 1085–1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Geng, Y.; Li, H.; Zhang, Y.; You, J.; Chang, Y. Enhancement the oral bioavailability of praziquantel by incorporation into solid lipid nanoparticles. Pharmazie 2009, 64, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Chaud, M.V.; Lima, A.C.; Vila, M.M.D.C.; Paganelli, M.O.; Paula, F.C.; Pedreiro, L.N.; Gremião, M.P.D. Development and evaluation of praziquantel solid dispersions in sodium starch glycolate. Trop. J. Pharm. Res. 2013, 12, 163–168. [Google Scholar] [CrossRef]
- Perissutti, B.; Passerini, N.; Trastullo, R.; Keiser, J.; Zanolla, D.; Zingone, G.; Voinovich, D.; Albertini, B. An explorative analysis of process and formulation variables affecting comilling in a vibrational mill: The case of praziquantel. Int. J. Pharm. 2017, 533, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Zanolla, D.; Perissutti, B.; Passerini, N.; Chierotti, M.R.; Hasa, D.; Voinovich, D.; Gigli, L.; Demitri, N.; Geremia, S.; Keiser, J.; et al. A new soluble and bioactive polymorph of praziquantel. Eur. J. Pharm. Biopharm. 2018, 127, 19–28. [Google Scholar] [CrossRef]
- Zanolla, D.; Perissutti, B.; Passerini, N.; Invernizzi, S.; Voinovich, D.; Bertoni, S.; Melegari, C.; Millotti, G.; Albertini, B. Milling and comilling Praziquantel at cryogenic and room temperatures: Assessment of the process-induced effects on drug properties. J. Pharm. Biomed. Anal. 2018, 153, 82–89. [Google Scholar] [CrossRef]
- EMA Guideline on Pharmaceutical Development of Medicines for Paediatric Use. 2013. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-pharmaceutical-development-medicines-paediatric-use_en.pdf (accessed on 6 March 2019).
- Passerini, N.; Perissutti, B.; Albertini, B.; Franceschinis, E.; Lenaz, D.; Hasa, D.; Locatelli, I.; Voinovich, D. A new approach to enhance oral bioavailability of Silybum Marianum dry extract: Association of mechanochemical activation and spray congealing. Phytomed 2012, 19, 160–168. [Google Scholar] [CrossRef]
- Albertini, B.; Di Sabatino, M.; Melegari, C.; Passerini, N. Formulation of spray congealed microparticles with self-emulsifying ability for enhanced glibenclamide dissolution performance. J. Microencapsul. 2015, 32, 181–192. [Google Scholar] [CrossRef]
- Perissutti, B.; Rubessa, F.; Princivalle, F. Solid dispersions of carbamazepine with Gelucire 44/14 and 50/13. S.T.P. Pharma Sci. 2000, 10, 479–484. [Google Scholar]
- Albertini, B.; Di Sabatino, M.; Melegari, C.; Passerini, N. Formulating SLMs as oral pulsatile system for potential delivery of melatonin to pediatric population. Int. J. Pharm. 2014, 469, 67–79. [Google Scholar] [CrossRef]
- Qi, S.; Marchaud, D.; Craig, D.Q.M. An investigation into the mechanism of dissolution rate enhancement of poorly water-soluble drugs from spray chilled gelucire 50/13 microspheres. J. Pharm. Sci. 2010, 99, 262–274. [Google Scholar] [CrossRef]
- Šagud, I.; Zanolla, D.; Perissutti, B.; Passerini, N.; Škorić, I. Identification of degradation products of praziquantel during the mechanochemical activation. J. Pharm. Biomed. Anal. 2018, 159, 291–295. [Google Scholar] [CrossRef]
- Hasa, D.; Voinovich, D.; Perissutti, B.; Grassi, G.; Fiorentino, S.; Farra, R.; Abrami, M.; Colombo, I.; Grassi, M. Reduction of melting temperature and enthalpy of drug crystals: Theoretical aspects. Eur. J. Pharm. Sci. 2013, 50, 17–28. [Google Scholar] [CrossRef]
- Borrego-Sánchez, A.; Hernández-Laguna, A.; Sainz-Díaz, C.I. Molecular modeling and infrared and Raman spectroscopy of the crystal structure of the chiral antiparasitic drug Praziquantel. J. Mol. Model. 2017, 23, 106. [Google Scholar] [CrossRef]
- Espinosa-Lara, J.C.; Guzman-Villanueva, D.; Arenas-García, I.J.; Herrera-Ruiz, D.; Rivera-Islas, J.; Román-Bravo, P.; Morales-Rojas, H.; Höpfl, H. Cocrystals of Active Pharmaceutical Ingredients—Praziquantel in Combination with Oxalic, Malonic, Succinic, Maleic, Fumaric, Glutaric, Adipic, And Pimelic Acids. Cryst. Growth Des. 2013, 13, 169–185. [Google Scholar] [CrossRef]
- Costa, E.D.; Priotti, J.; Orlandi, S.; Leonardi, D.; Lamas, M.C.; Nunes, T.G.; Diogo, H.P.; Salomon, C.J.; Ferreira, M.J. Unexpected solvent impact in the crystallinity of praziquantel/poly (vinylpyrrolidone) formulations. A solubility, DSC and solid-state NMR study. Int. J. Pharm. 2016, 511, 983–993. [Google Scholar] [CrossRef]
- Brubach, J.B.; Ollivon, M.; Jannin, V.; Mahler, B.; Bourgaux, C.; Lesieur, P.; Roy, P. Structural and thermal characterization of mono- and diacyl polyoxyethylene glycol by infrared spectroscopy and X-ray diffraction coupled to differential calorimetry. J. Phys. Chem. B 2004, 108, 17721–17729. [Google Scholar] [CrossRef]
- Sun, Y.; Bu, S. Simple, cheap and effective high-performance liquid chromatographic method for determination of praziquantel in bovine muscle. J. Chromatogr. B 2012, 899, 160–162. [Google Scholar] [CrossRef]
- Praziquantel. In European Pharmacopoeia 8.0; Directorate for the Quality of Medicines of the Council of Europe (EDQM): Strasbourg, France, 2014; pp. 3086–3087.
- Kim, M.S.; Kim, J.S.; Hwang, S.J. Enhancement of wettability and dissolution properties of cilostazol using the supercritical antisolvent process: Effect of various additives. Chem. Pharm. Bull. 2010, 58, 230–233. [Google Scholar] [CrossRef]
- Lombardo, F.C.; Pasche, V.; Panic, G.; Endriss, Y.; Keiser, J. Life cycle maintenance and drug-sensitivity assays for early drug discovery in Schistosoma mansoni. Nat. Protoc. 2019, 14, 461–481. [Google Scholar] [CrossRef]











| Samples | HPLC Assay: Impurity Retention Time (min) (Content, %) | PZQ Recovery (%) | Solubility (mg/L) | Endothermic Peak (°C) | Residual Crystallinity (%) | ||
|---|---|---|---|---|---|---|---|
| Impurity A | Impurity B | Impurity X | |||||
| PZQ | - * | - * | - * | 100 | 140.30 ± 9.26 | 143.51 ± 0.35 | 100 |
| PZQ-PVP PM | - * | - * | - * | 99.98 ± 0.04 | 151.78 ± 27.22 | 142.31 ± 0.22 | 100 |
| PZQ-PVP CC | 3.4–3.5 (0.18) | - * | 4.0–4.2 (0.93) | 98.89 ± 0.08 | 267.40 ± 11.00 | 129.36 ± 0.43 | 27.70 |
| PZQ Form B | - * | - * | - * | 99.55 ±0.05 | 284.61 ± 4.67 | 112.50 ± 0.32 | n.a. ** |
| Samples (Abbreviation) | Composition (%, w/w) | Real Drug Content (%) | Encapsulation Efficiency (%) | |||||
|---|---|---|---|---|---|---|---|---|
| PZQ | PVP | Gelucire 50/13 | ||||||
| Powders | Activated materials | PZQ:PVP cryocomilled | (PZQ:PVP CC) | 50 | 50 | - | 46.0 ± 1.2 | - |
| Milled PZQ (Form B) | (PZQ Form B) | 100 | - | 100 | - | |||
| Raw materials | PZQ:PVP physical mixture | (PZQ:PVP PM) | 50 | 50 | - | 46 | - | |
| Raw PZQ | (PZQ) | 100 | - | - | 100 | - | ||
| Microparticles | MPs | PZQ:PVP cryo-comilled | (MPsA) | 15 | 15 | 70 | 13.75 ± 0.14 | 91.7 |
| Milled PZQ_Form B | (MPsB) | 15 | - | 85 | 13.54 ± 0.83 | 90.3 | ||
| PZQ:PVP physical mixture | (MPsC) | 15 | 15 | 70 | 14.35 ± 0.18 | 95.7 | ||
| Raw PZQ | (MPsD) | 15 | - | 85 | 15.16 ± 0.11 | 101.0 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albertini, B.; Perissutti, B.; Bertoni, S.; Zanolla, D.; Franceschinis, E.; Voinovich, D.; Lombardo, F.; Keiser, J.; Passerini, N. Combining Mechanochemistry and Spray Congealing for New Praziquantel Pediatric Formulations in Schistosomiasis Treatment. Int. J. Mol. Sci. 2019, 20, 1233. https://doi.org/10.3390/ijms20051233
Albertini B, Perissutti B, Bertoni S, Zanolla D, Franceschinis E, Voinovich D, Lombardo F, Keiser J, Passerini N. Combining Mechanochemistry and Spray Congealing for New Praziquantel Pediatric Formulations in Schistosomiasis Treatment. International Journal of Molecular Sciences. 2019; 20(5):1233. https://doi.org/10.3390/ijms20051233
Chicago/Turabian StyleAlbertini, Beatrice, Beatrice Perissutti, Serena Bertoni, Debora Zanolla, Erica Franceschinis, Dario Voinovich, Flavio Lombardo, Jennifer Keiser, and Nadia Passerini. 2019. "Combining Mechanochemistry and Spray Congealing for New Praziquantel Pediatric Formulations in Schistosomiasis Treatment" International Journal of Molecular Sciences 20, no. 5: 1233. https://doi.org/10.3390/ijms20051233
APA StyleAlbertini, B., Perissutti, B., Bertoni, S., Zanolla, D., Franceschinis, E., Voinovich, D., Lombardo, F., Keiser, J., & Passerini, N. (2019). Combining Mechanochemistry and Spray Congealing for New Praziquantel Pediatric Formulations in Schistosomiasis Treatment. International Journal of Molecular Sciences, 20(5), 1233. https://doi.org/10.3390/ijms20051233